Abstract
Objective:
To evaluate cognitive-behavior therapy plus bright light therapy (CBT plus BLT) for adolescents diagnosed with delayed sleep phase disorder (DSPD).
Design:
Randomized controlled trial of CBT plus BLT vs. waitlist (WL) control with comparisons at pre- and post-treatment. There was 6-month follow-up for the CBT plus BLT group only.
Setting:
Flinders University Child & Adolescent Sleep Clinic, Adelaide, South Australia.
Patients:
49 adolescents (mean age 14.6 ± 1.0 y, 53% males) diagnosed with DSPD; mean chronicity 4 y 8 months; 16% not attending school. Eighteen percent of adolescents dropped out of the study (CBT plus BLT: N = 23 vs WL: N = 17).
Interventions:
CBT plus BLT consisted of 6 individual sessions, including morning bright light therapy to advance adolescents' circadian rhythms, and cognitive restructuring and sleep education to target associated insomnia and sleep hygiene.
Measurements and Results:
DSPD diagnosis was performed via a clinical interview and 7-day sleep diary. Measurements at each time-point included online sleep diaries and scales measuring sleepiness, fatigue, and depression symptoms. Compared to WL, moderate-to-large improvements (d = 0.65-1.24) were found at post-treatment for CBT plus BLT adolescents, including reduced sleep latency, earlier sleep onset and rise times, total sleep time (school nights), wake after sleep onset, sleepiness, and fatigue. At 6-month follow-up (N = 15), small-to-large improvements (d = 0.24-1.53) continued for CBT plus BLT adolescents, with effects found for all measures. Significantly fewer adolescents receiving CBT plus BLT met DPSD criteria at post-treatment (WL = 82% vs. CBT plus BLT = 13%, P < 0.0001), yet 13% still met DSPD criteria at the 6-month follow-up.
Conclusions:
CBT plus BLT for adolescent DSPD is effective for improving multiple sleep and daytime impairments in the immediate and long-term. Studies evaluating the treatment effectiveness of each treatment component are needed.
Clinical Trial Information:
Australia – New Zealand Trials Registry Number: ACTRN12610001041044.
Citation:
Gradisar M; Dohnt H; Gardner G; Paine S; Starkey K; Menne A; Slater A; Wright H; Hudson JL; Weaver E; Trenowden S. A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. SLEEP 2011;34(12):1671-1680.
Keywords: Delayed sleep phase disorder, cognitive-behavior therapy, bright light therapy, adolescents, sleepiness, insomnia
INTRODUCTION
Delayed sleep phase disorder (DSPD) is a circadian rhythm disorder comprising an endogenous “clock” that is delayed later in relation to the individual's desired bed and rise times.1 This mistimed scheduling significantly impairs an important aspect of their functioning.2,3 Due to the delayed sleep timing, the individual experiences chronic difficulty falling asleep. Indeed, individuals with DSPD may inadvertently attempt sleep during peak alertness of their circadian rhythm.4,5 Eventual sleep onset often occurs at a very late time (e.g., 01:00-06:00)2 and can be associated with a learned or conditioned insomnia.2,4,6,7 The development of this insomnia is associated with circadian phase misalignment; thus the DPSD diagnosis takes precedence over an insomnia disorder diagnosis. Once asleep, sleep duration and any awakenings are considered normal.2 However, difficulties arise if the individual is required to rise at a socially conventional time (e.g., for work or school). These social commitments often occur earlier than their endogenous sleep offset time. Accordingly, the DSPD individual experiences reduced sleep duration and associated daytime impairment. Thus, the individual with DSPD who needs to conform to the 9-to-5 society can experience long sleep latencies, late sleep onset, reduced sleep, difficulty rising, and impaired functioning during weekdays. However, they may “catch up” on weekends and/or holidays by a later scheduling of their rise time, resulting in extended sleep.8 Such a scenario is not uncommon in the adolescent (11-18 y) attending school.9,10
Although the prevalence of DSPD is stated to be 7% to 16%, and more common in adolescents,2 survey studies show rates between 0.4% and 7%.11–13 Taking all these figures into account, this equates to, for example, 50,000-190,000 Australian adolescents,14 or possibly 1,000,000+ adolescents in the USA7 suffering from the effects of DSPD. The social and economic cost of adolescent DSPD to industrialized societies is currently unknown; however the potential impact on the individual is considerable when considering the host of negative sequelae due to sleep loss common in this age group, including poor school performance,15–18 mood disturbances,8 and in extreme cases heightened accident risk18,19 and suicidal ideation and attempts.20–22 With such societal and individual costs during a sensitive developmental period that prepares adolescents for entry into adulthood,23 it is warranted that evidence-based treatments be investigated.
Recommended treatments for DSPD include chronotherapy, melatonin administration, or timed light exposure; however, the studies reviewed in these papers primarily used adults.24,25 Studies reporting treatment effects for adolescent DSPD have used pharmacological approaches, including high-dose methylcobalamin,26–28 triazolam,26 zolpidem, and trazodone.29 In some cases, studies have involved inpatient settings to provide intensive social structuring.27,30 However, limitations of these studies are low sample size27 and, importantly, lack of control comparison.27,28,30 Furthermore, pharmacological approaches can produce side effects,27 relapse by virtue of removing the treatment (i.e., cease medication),27 and have ongoing financial costs. Relapse also occurs after discharge from hospitalization in some adolescent patients.27,30 An alternative approach is to use a treatment consisting of cognitive and behavioral techniques as well as bright light therapy. In a recent example, Bootzin and Stevens31 enlisted 55 adolescents (mean age = 16.1, range 13-19 y, 62% males) with a history of substance abuse in a trial of group multicomponent sleep therapy. Although not formally diagnosed with DSPD, some adolescents in the treatment nonetheless reported symptoms akin to a delayed sleep pattern (i.e., sleep latency = 36.7 min, sleep duration = 7.3 h, “late group” DLMO = 23:00).32 The therapy consisted of cognitive-behavior elements, including stimulus control, sleep hygiene education, cognitive restructuring, and mindfulness-based stress reduction to address sleep latency, and morning bright light therapy to advance adolescents' delayed circadian rhythms. For adolescents who completed treatment (4-6 sessions), significant improvements were obtained, including a reduction in sleep latency (to 17 min), and an increase in sleep duration (to 8.4 h). We speculate improvements (at least in the “late DLMO group”) may have partially been due to an advance in their circadian rhythm, such that the discrepancy between their endogenous sleep timing and their social timing was reduced.
Despite the advantages of such an intervention, the evidence base for adolescents with DSPD is very limited. Not only can modification of sleep times with bright light therapy address the circadian timing, but cognitive and behavioral techniques can address the associated insomnia and assist in the maintenance of regular sleep schedules. However, in order for an intervention to be considered well established, probably efficacious, or promising, independent studies are needed, either replicating the same methodology or preferably using more controlled designs.7,33 Thus, the purpose of the present study was to build upon previous work28,31,34 by evaluating cognitive-behavior therapy plus bright light therapy (CBT plus BLT) for adolescent DSPD using a randomized-controlled design. Evidence from adult DSPD patients shows an adolescent onset of their condition,1,35–38 suggesting DSPD is a chronic sleep disorder that remains stable or worsens. Thus, we assumed that DSPD adolescents in a waitlist control condition (WL) would demonstrate relative stability in their condition compared to DSPD adolescents undergoing CBT plus BLT. On this assumption, we predicted adolescents in the CBT plus BLT condition would obtain improvements in sleep latency, sleep onset and rise times, sleep duration, and daytime functioning at post-treatment relative to WL. We further predicted that improvements at post-treatment would remain after 6 months, given adolescents completed treatment with an improved skill set to address future sleep disturbance.
METHODS
Participants
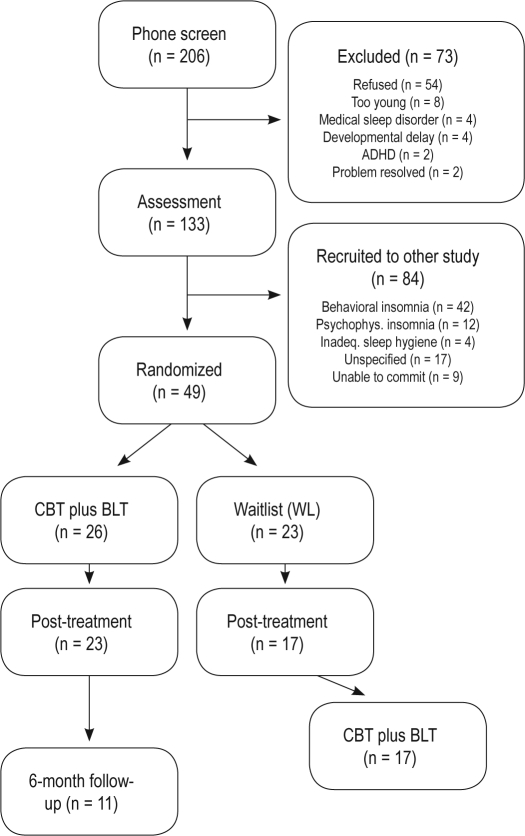
Two hundred six families contacted our Child & Adolescent Sleep Clinic at Flinders University, South Australia, in response to advertisements in school newsletters and local newspapers (see Figure 1). As part of a larger study, 73 participants were excluded, leaving 133 school-aged children who were assessed. Of these, 65 were adolescents, and 49 met criteria for DSPD as per the International Classification of Sleep Disorders, 2nd edition (ICSD-2; see Measures).2 DSPD adolescents were aged between 11-18 y (mean = 14.6 ± 1.0 y, 53% males) and often attended with 1 parent/caregiver (mean = 45.3 ± 5.2 y). Twenty-six adolescents were assigned to the CBT plus BLT condition, and 23 were assigned to the WL condition. Three adolescents ceased CBT plus BLT after the first treatment session, and 6 dropped out from the WL. This resulted in 23 adolescents (age = 14.7 ± 1.7 y) in the CBT plus BLT condition and 17 in the WL condition (age = 14.7 ± 1.8 y). Adolescents who dropped out were more likely to be male, χ2(1, N = 49) = 5.68, P = 0.02, and obtained more total sleep time on weekends, t45 = 2.37, P = 0.02. One hundred percent of adolescents reported the onset of their sleep problem was gradual, with mean chronicity of 4 y 8 months (mean onset ∼ 9 y 11 mo). Twenty-three percent (11/49) reported being on medication. These medications were used to treat asthma (when required; N = 4), acne (N = 1), or sleep (N = 4, temazepam, promethazine, amitriptyline). Only 1 adolescent in the CBT plus BLT group used sleep medication (promethazine – see footnote A at end of paper), and ceased this during and after treatment. All waitlist adolescents continued to use their medication when required (N = 8). Fourteen percent (7/49) of adolescents reported social substance use (alcohol/marijuana), with 5 of these adolescents dropping out of the study. Two adolescents had a previously diagnosed mood disorder, and 4 had a suspected emotional disorder (unipolar depression, social anxiety disorder). These adolescents were evenly spread across the CBT plus BLT, WL, and drop-out groups. Of the 49 families interviewed, most were predominantly from middle (55%) and high (39%) income socioeconomic backgrounds, with 68% of mothers and 64% of fathers with tertiary-level qualifications. Ninety-one percent of parents were married or in a marriage-like relationship.
Figure 1.
Participant flow through the randomized controlled procedure.
Sixteen percent of adolescents (8/49) reported not attending school. Of the 41 teens attending school, 24% were frequently 5-10 min late, 24% reported arriving 10+ min late everyday, 5% reported missing entire days due to their sleep disturbance, 24% reported receiving detention, exclusion, or expulsion for arriving late to school, and 5% of parents reported being late to work due to difficulties getting their adolescent to school. One of the diagnostic criteria for DSPD is that it significantly impacts an important area of the individual's life; all teens reported their sleep disturbance affected their schooling (e.g., late/no attendance, poor grades), and thus differentiated them from the typical sleep patterns found during adolescence.9 The study was granted ethics approval from the Flinders University Social and Behavioral Ethics Committee.
Design
A randomized, mixed-model design was employed, with a 2 × between-groups factor (group: CBT plus BLT vs. WL) and 2 × within-groups factor (time: pre-treatment, post-treatment). For the CBT plus BLT group only, a 6-month follow-up was also performed. Sleep diary and questionnaires were completed online at each time-point. Our previous work has demonstrated online surveys are a valid method of data collection from adolescents.39,40
Measures
Sleep diary
A 7-day sleep diary was completed by adolescents at each time point of the study. All adolescents reported that they completed the diary themselves, or required little help from parents. Adolescents completed details of their previous night's sleep (i.e., bedtime, lights out, sleep latency, sleep onset time, number of awakenings, wake after sleep onset [WASO], sleep duration, rise time, naps) each morning (see footnote B at end of paper). These values were averaged for school nights (Sun to Thurs) and weekend nights (Fri and Sat). For teens not attending school, school nights were calculated if the teen needed to rise (e.g., for an appointment). Where daily diary entries were incomplete (predominantly school nights), sleep data were averaged over these nights (e.g., school week sleep over 4 nights). Sleep diaries provide more accurate estimates of self-reported sleep than surveys, due to reduced recall bias,7,41 and show good correlations with objective measures of sleep (e.g., wrist actigraphy).42 A week of sleep monitoring with a sleep diary or wrist actigraphy (accompanied by a sleep diary) is a criterion for a diagnosis of DSPD,2 and both were used to diagnose DSPD in the present study. However, due to inconsistent wearing of wrist monitors and occasional loss of this equipment following treatment, there were insufficient data (i.e., low power) to analyze, and be representative, for post-treatment and 6-month follow-up. Of all the sleep variables measured by the sleep diary, the process variables of most interest were sleep onset and rise times, as these reflect the primary changes for normalizing DSPD. The primary outcome measures from the sleep diary include sleep onset latency, as a common aspect of DSPD is difficulty fall asleep; the discrepancy in rise times, which reflects a match between the endogenous and societal clocks; and total sleep time, which is predicted to increase with improvements in the abovementioned process and outcome variables.
Sleepiness
The Pediatric Daytime Sleepiness Scale (PDSS) was used to assess changes in daytime sleepiness inherent in patients with restricted sleep.43 The PDSS is an 8-item scale with items targeting aspects of daytime sleepiness relevant for adolescents (e.g., “How often do you have trouble getting out of bed in the morning?,” “How often do you fall asleep or feel drowsy in class?” Responses range from 0 – Never, to 4 – Always. Item 3, “Are you usually alert during the day?” was reverse scored. Total scores range from 0 to 32. The PDSS is a psychometrically sound instrument,43 with adequate reliability demonstrated in the current study (Cronbach α = 0.78). As daytime sleepiness, particularly morning sleepiness, is a classic feature of DSPD, this is one of the primary outcome measures of the study.
Fatigue
The Flinders Fatigue Scale (FFS) was used to assess changes in daytime fatigue experienced over the previous 2 weeks,44 with previous reports indicating adolescent DSPD patients experience fatigue.27 The FFS comprises 7 items, including “Was fatigue a problem for you?” and “How much was your fatigue caused by poor sleep?” Six items were in Likert format, with responses ranging from 0 – Not at all to 4 – Extremely. One item, “At what time(s) did you experience fatigue,” was a multiple checklist with possible responses ranging from 0 to 7. Total scores ranged from 0 to 31, with higher scores indicating greater fatigue. The FFS has shown good validity and treatment sensitivity with insomnia patients.44 Cronbach α in the current study was 0.89. As sleepiness is more often reported in adolescent sleep studies than fatigue, fatigue in this study is considered a secondary outcome measure.
Depression symptoms
The Short Mood and Feelings Questionnaire (MFQ)45 was used to measure depression symptoms, due to the association of depression with sleep loss and DSPD in this population.7,46 The MFQ is a 13-item self-report measure, with each item rated on a 3-point Likert scale, ranging from 0 – Not true to 2 – True. Total scores range from 0 to 26, with higher scores indicating greater frequency of depression symptoms. The MFQ possesses good psychometric properties in school-aged children.45 Cronbach α for the present study was 0.86. Unlike many depression scales, the short version of the MFQ does not contain any sleep items, and thus was not confounded by treatment. As depression coincides with sleep disturbance, depression symptoms are considered a secondary outcome variable.
Clinical sleep history interview
A semi-structured, clinical sleep history interview (CSHI) was developed by the authors to help diagnose pediatric sleep disorders. The CSHI contains questions targeting ICSD-2 insomnia and circadian rhythm disorder criteria. Questions address usual sleep patterns, history of the sleep problem, bedtime routine, insomnia symptoms, sleep hygiene behaviors, sleep-onset cues, and limit-setting behavior, differential diagnosis, and adolescent and parent treatment goals. Opportunities were provided for the adolescent to answer sensitive questions (e.g., substances used) with the parent out of the interview room, and visa versa. For the DSPD diagnosis, the CSHI assisted in collecting information pertinent to Criterion A (chronic difficulty initiating sleep and difficulty rising) and D (differential diagnosis).2
Procedure
Figure 1 presents a diagram of participant entry into the study. Recruitment was conducted from July 2006 to October 2009, and treatment completed by November 2009. In response to advertisements that were directed at DSPD symptoms, adolescents' parents underwent a brief phone screen. Parents who confirmed a pattern of difficulty initiating sleep and rising, as well as some form of impaired functioning, collected details for accessing password-protected sleep diaries and questionnaires and a wrist activity monitor. Excluded participants were referred to an appropriate health service.
After completing the sleep diary and questionnaires, adolescents and at least 1 parent underwent an initial clinical sleep history interview with trained sleep clinicians. Therapists were registered psychologists (authors HD, KS, AS, HW), trainee psychologists (authors GG, SP, AM) undergoing postgraduate clinical psychology training, or the clinic supervisor (author MG) who had 5 years' experience in the area of behavioral sleep medicine. Adolescents were diagnosed for DSPD at a consensus meeting of therapists and the clinic supervisor. Following diagnosis, group allocation (CBT plus BLT vs. WL) was determined based on a computer-generated permuted block schedule.
CBT plus BLT comprised six 45- to 60-min sessions with the adolescent and at least 1 parent. The first 4 sessions were held weekly, and the remaining 2 held biweekly. The first session consisted of sleep education (sleep architecture, circadian rhythms, sleep homeostasis), sleep hygiene aimed at evening de-arousal (reduce evening light, avoid late caffeine, physical exercise, electronic media use), and plans for morning bright light therapy to advance sleep timing.4,7,47–49 The light source was natural sunlight (when available); otherwise, a broad-spectrum light lamp was provided (∼1,000 lux).48 Adolescents were instructed to begin light exposure at their natural wake-up time on day 1, with ≥ 30 min of light (max 2 h).47 Day 1 was often begun on the weekend (Saturday),48 and despite the mean weekend rise time of 09:30 (Table 2), we found adolescents commonly slept in on day 1 until after 10:30. Thereafter, they were instructed to begin light exposure 30 min earlier each day48 until they reached a target time of 06:00, which is the earliest time light exposure has occurred in previous studies.34 Sleep diaries were provided each session to check adolescents complied with instructions. Reviewing and monitoring light therapy progress was continued until session 3 to 5, depending upon the degree of initial phase delay. After adolescents reached their target time, they ceased light therapy and attempted to maintain a regular rise time (06:30-07:30), consistent with stimulus control recommendations used previously with adolescents.31 Furthermore, they were instructed to go to bed when they felt sleepy and to avoid napping.
Table 2.
Pre- and post-treatment mean ± SDs of sleep, sleepiness, and depression symptoms between DSPD adolescents undergoing CBT plus BLT (N = 23) or in a Waitlist (WL) control condition (N = 17)
| Pre-treatment |
Post-treatment |
Effect size (d) | |||
|---|---|---|---|---|---|
| CBT plus BLT | WL | CBT plus BLT | WL | ||
| Sleep onset latency (min) | |||||
| school* | 78.1 ± 41.0 | 78.8 ± 31.3 | 22.2 ± 12.8 | 65.3 ± 42.0 | 1.13 |
| weekend* | 68.5 ± 42.3 | 62.7 ± 37.2 | 26.1 ± 18.7 | 52.6 ± 43.7 | 0.65 |
| Sleep onset time (clock time) | |||||
| school* | 00:06 ± 67.2min | 23:48 ± 69.4min | 23:28 ± 58.8min | 00:10 ± 65.3min | 0.89 |
| weekend* | 00:56 ± 84.6min | 00:09 ± 62.1min | 23:45 ± 60.0min | 00:37 ± 78.9min | 1.24 |
| Total sleep time (h) | |||||
| school* | 7.1 ± 1.3 | 6.9 ± 1.4 | 8.1 ± 0.6 | 6.9 ± 1.1 | 0.81 |
| weekend | 8.3 ± 1.5 | 8.4 ± 1.6 | 8.4 ± 1.0 | 8.1 ± 1.7 | 0.22 |
| WASO (min)* | 26.6 ± 27.5 | 14.6 ± 22.6 | 4.6 ± 7.2 | 16.1 ± 19.2 | 1.14 |
| Rise time (clock time) | |||||
| school* | 07:32 ± 33.0min | 07:22 ± 28.9min | 07:06 ± 27.5min | 07:29 ± 40.1min | 0.92 |
| weekend*a | 09:28 ± 81.6min | 09:22 ± 63.2min | 08:42 ± 72.8min | 09:33 ± 56.8min | 0.92 |
| Discrepant rise time (h) | 1.8 ± 1.2 | 2.0 ± 1.1 | 1.3 ± 1.0 | 2.1 ± 1.0 | 0.45 |
| Daytime sleepiness (0-32)* | 20.4 ± 4.8 | 18.1 ± 4.4 | 14.0 ± 3.6 | 16.0 ± 3.7 | 0.79 |
| Fatigue (0-31)* | 17.2 ± 1.2 | 16.6 ± 1.3 | 10.2 ± 1.2 | 14.9 ± 1.3 | 0.75 |
| Depression symptoms (0-26) | 7.6 ± 3.6 | 7.4 ± 5.5 | 3.9 ± 2.8 | 6.2 ± 4.5 | 0.71 |
significant interaction (i.e., significant improvement in CBT plus BLT group vs. WL group) with P < 0.05; effect sizes are: d < 0.20 = small, d < 0.50 = moderate, d < 0.80 = large;
despite the mean weekend rise time of ∼09:30, many parents placed some limits on their adolescents' sleep-in, and when adolescents slept-in on their first day of light therapy they often slept till after 10:30. Process variables = sleep onset time and rise time; Primary outcome variables = sleep onset latency, total sleep time, daytime sleepiness; Secondary outcome variables = fatigue, depression symptoms.
Session two introduced the rationale for cognitive therapy in addressing associated insomnia associated with their circadian disturbance (i.e., identify automatic thoughts about sleep).7 These automatic thoughts were monitored from sessions 3 to 5. Session 3 introduced the evaluation of these thoughts, including the generation of alternative thinking. Alternative thinking was monitored and discussed in sessions 4 and 5. The sixth (final) session assessed whether treatment goals were achieved, assessed adolescents' knowledge of sleep and circadian rhythms, as well as relapse prevention.
Post-treatment measures using a sleep diary and questionnaires were completed by adolescents in the CBT plus BLT condition. All adolescents attending school completed post-treatment and follow-up evaluations during school terms. Adolescents in the WL condition provided the same information at an equivalent time-point (after an 8-week duration), and were then provided CBT plus BLT. Follow-up measures were conducted 6 months after treatment ceased for the CBT plus BLT group only. Post-treatment and follow-up diagnosis was based on quantitative criteria, including sleep timing between the school week and weekend (< 2 h difference from sleep diary data), daytime sleepiness levels (PDSS total score < 20), plus questions directed at the impact of their sleep on functioning (i.e., adolescent self-report of no impact on important areas of functioning [e.g., school, family]). Follow-ups were conducted by authors MG and HD, who were not blind to group allocation.
Statistical Analyses
Despite intending to use an intention-to-treat analysis, no data following randomization for drop-outs could be obtained.50 Due to reservations about data imputation (i.e., last observation carried forward method),51 and few meaningful differences between drop-outs and completers, we opted for a complete-case analysis. One drawback of complete-case analysis is reduced power; however, this was overcome in the present study by the use of effect sizes (see below). Using a series of independent samples t-tests, no significant differences were found between the 2 groups at pre-treatment for any variables (all P > 0.05) with the exception of gender, t39 = 2.36, P = 0.02. Analyses were performed with and without gender as a covariate, and no difference was found between the 2 sets of results. To test whether CBT plus BLT provided greater improvements than WL control from pre- to post-treatment, a 2-way mixed model ANOVA was employed. Significant interactions found from these mixed-model analyses would confirm our predictions. To test the long-term effects of CBT plus BLT, a one-way repeated measures ANOVA was performed on time (pre-treatment, post-treatment, and 6-month follow-up) for the CBT plus BLT group only. Fifteen of the 23 adolescents from the CBT plus BLT group provided 6-month follow-up data, and independent t-tests showed that these adolescents differed significantly from those who did not participate in the 6-month follow-up only in the chronicity of their sleep problem (t21 = 2.46, P = 0.02), with participating adolescents experiencing their sleep problem more than twice as long (6 y 7 mo vs. 2 y 6 mo). Due to the relatively small sample size and multiple analyses performed, interaction effect sizes for repeated measures are reported (d). Effect sizes are not affected to the same extent as are inferential statistics by the chances of a type I or II error occurring. We therefore encourage the reader to focus on interpreting the Cohen's ds in Tables 2 and 3. These effect sizes are calculated as the difference in change scores for each group (e.g., Δ WASOCBT - Δ WASOWL) divided by the pooled SDs in change scores for each group (e.g., [SD Δ WASOCBT + SDΔ WASOWL] / 2). For the within-subjects comparisons for the CBT plus BLT group only, effect sizes (d) were calculated as the difference in mean scores divided by the pooled SDs and corrected for the dependence between the means.52 Chi-square analyses were employed when testing for independence between categorical data.
Table 3.
Pre-treatment, post-treatment, and 6-month follow-up mean ± SDs of sleep, sleepiness, and depression symptoms for DSPD adolescents undergoing CBT plus BLT (N = 15)
| Pre-treatment | Post-treatment | Pre- Post-treatment effect size (d) | 6-mo follow-up | Pre- Follow-up effect size (d) | |
|---|---|---|---|---|---|
| Sleep onset latency (min) | |||||
| school*^ | 70.8 ± 44.9 | 23.8 ± 12.6 | 1.03 | 32.5 ± 21.8 | 0.72 |
| weekend*^ | 68.3 ± 45.4 | 22.5 ± 11.3 | 1.06 | 23.8 ± 19.3 | 0.88 |
| Sleep onset time (clock time) | |||||
| school* | 00:03 ± 80.9min | 23:08 ± 55.0min | 1.33 | 23:14 ± 36.1min | 0.55 |
| weekend*^ | 01:10 ± 88.8min | 23:37 ± 56.8min | 1.02 | 23:46 ± 73.2min | 0.90 |
| Total sleep time (h) | |||||
| school* | 7.3 ± 1.4 | 8.1 ± 0.6 | 0.72 | 7.9 ± 0.6 | 0.46 |
| weekend | 8.4 ± 1.3 | 8.3 ± 1.2 | 0.06 | 8.9 ± 1.5 | 0.34 |
| WASO (min)*^ | 23.1 ± 24.2 | 5.5 ± 22.6 | 0.72 | 4.2 ± 6.6 | 1.01 |
| Rise time (clock time) | |||||
| school | 07:28 ± 32.6min | 07:12 ± 24.7min | 0.58 | 07:32 ± 23.8min | 0.24 |
| weekend* | 09:29 ± 73.8min | 08:32 ± 75.5min | 0.76 | 08:59 ± 69.6min | 0.54 |
| Discrepant rise time (h)*^ | 2.1 ± 1.1 | 1.3 ± 1.2 | 0.69 | 1.4 ± 1.3 | 0.75 |
| Daytime sleepiness (0-32)*^ | 19.7 ± 5.4 | 13.6 ± 3.3 | 0.93 | 12.1 ± 3.7 | 1.31 |
| Fatigue (0-31)*^ | 16.8 ± 4.3 | 9.1 ± 4.9 | 0.96 | 8.1 ± 3.5 | 1.53 |
| Depression symptoms (0-26)*^ | 7.3 ± 3.1 | 3.7 ± 2.4 | 1.20 | 3.3 ± 3.9 | 0.86 |
significant change (i.e., significant improvement) from pre- to post-treatment;
significant change (i.e., significant improvement) from pre-treatment to 6-month follow-up; effect sizes are: d > 0.20 = small, d > 0.50 = moderate, d > 0.80 = large; Process variables = sleep onset time and rise time; Primary outcome variables = sleep onset latency, total sleep time, daytime sleepiness; Secondary outcome variables = fatigue, depression symptoms.
RESULTS
Clinical Features of DSPD Adolescents at Assessment
As treatment studies of adolescent DSPD samples are limited, we describe here some sleep characteristics of our sample. Although these adolescents primarily experienced a delay in their sleep timing, there was also sufficient evidence of associated insomnia symptoms, daytime impairment, and poor sleep hygiene. Table 1 presents the percentages of adolescents who confirmed these symptoms and other behaviors during the CSHI. In terms of sleep complaints, difficulty falling asleep and waking in the morning were the most common. One hundred percent of teens reported at least one form of daytime dysfunction associated with their sleep problem. Many of these nighttime and daytime complaints are experienced by adults with insomnia.2 Poor sleep hygiene comprised mainly of electronic media in their bedroom (mean = 2.4 items). Coping with sleep loss took the form of approximately one-third of the sample reporting frequent napping (2.9 naps/wk, 103.3 ± 60.2 min, at 15:40 ± 35min), and consuming caffeine (coffee, tea, chocolate). Interestingly, three-quarters of adolescents reported feeling “flat” after school yet alert after their dinner meal (∼18:30-18:50),36 which may be representative of a delayed “post-lunch dip” and “wake-maintenance zone,” respectively. Despite indications of insomnia and poor sleep hygiene, our differential diagnosis indicated DSPD to be the primary sleep disorder.2 Nevertheless, the levels of insomnia and poor sleep hygiene validated the use of the respective cognitive techniques and sleep education (described above in Procedure) to address these factors in therapy.
Table 1.
Prevalence of insomnia symptoms, poor sleep hygiene, and miscellaneous sleep behaviors
| Insomnia Symptoms |
||||||||
|---|---|---|---|---|---|---|---|---|
| Primary Sleep Problema | Daytime Impairmentsb | Sleep Hygienec | Miscellaneous Sleep Behaviors | |||||
| Difficulty waking | (45%) | Daytime sleepiness | (90%) | Electronic media in bedroom | (89%) | Mornings | ||
| Difficulty falling asleep | (33%) | Fatigue/tired | (88%) | Cell phone | (66%) | Avoid morning light | (85%) | |
| Daytime sleepiness | (13%) | Inattention/concentration | (88%) | TV/DVD | (32%) | Parent ask teen to risea | (80%) | |
| Unrefreshing sleep | (9%) | Moody/irritable | (75%) | Computer | (30%) | Teen wake by self (weekends) | (65%) | |
| Low motivation/energy | (75%) | Caffeine after noon | (35%) | Teen wake by self (school mornings) | (9%) | |||
| Secondary Sleep Problem | Somatic complaints | (50%) | Frequently napped | (33%) | ||||
| Difficulty waking | (26%) | Problems socializing | (20%) | Alcohol | (12%) | Afternoon/Evening | ||
| Difficulty falling asleep | (48%) | Risk of accidents | (13%) | Nicotine | (10%) | “Flat” after school | (75%) | |
| Daytime sleepiness | (4%) | Alert after dinner | (75%) | |||||
| Unrefreshing sleep | (22%) | Cognitions | ||||||
| Racing thoughts in bed | (89%) | |||||||
| Worried about sleep | (68%) | |||||||
| Anxious about sleep | (58%) | |||||||
Adolescents were asked to state their primary and secondary sleep complaint: these total 100%, respectively;
adolescents could confirm < 1 insomnia-related daytime impairment (from the ICSD-2 General Insomnia criteria): these exceed 100%;
adolescents could nominate < 1 form of electronic media present in their bedroom (total > 100%);
this item confirmed by parents if they needed to repeatedly ask their adolescent to get out of bed ≥ 3 times.
Comparison of CBT plus BLT versus Waitlist on Sleep and Functioning
Descriptive statistics (means, SDs) and effect sizes are presented in Table 2. Data for school night sleep parameters and weekend sleep parameters are presented separately. Relative to adolescents in the WL control group, adolescents in the CBT plus BLT group at post-treatment demonstrated significant improvements on school nights for sleep onset latency (F1,34 = 10.57, P = 0.003), sleep onset time (F1,36 = 11.78, P = 0.002), total sleep time (F1,32 = 5.78, P = 0.02), and rise time (F1,32 = 7.29, P = 0.01). Although individuals experiencing DSPD are not known for significant awakenings during sleep, Table 2 shows our sample of adolescents did experience wake after sleep onset (WASO), with 30% reporting ≥ 30 min at pre-treatment. Analysis showed that overall WASO decreased in response to CBT plus BLT (F1,37 = 12.16, P = 0.001).
Improvements were also seen for the weekend sleep patterns of adolescents in the CBT plus BLT group, including sleep onset latency (F1,37 = 4.22, P = 0.046), sleep onset time (F1,37 = 14.48, P = 0.001), and rise time (F1,35 = 7.85, P = 0.008). However, no improvements were found for total sleep time on weekends (F1,36 = 0.46, P > 0.05), suggesting the significant advances in weekend sleep onset time and rise time were relatively uniform for the CBT plus BLT group, thus not providing a greater opportunity for sleep on weekends. However, despite significant advances in rise times for the school week and weekend for the CBT plus BLT group relative the to the control group, the discrepancy in rise times showed no significant interaction from pre- to post-treatment, F1,31 = 1.71, P > 0.05. Analyses showed the CBT plus BLT group continued to sleep-in on weekends at post-treatment (relative to their school week rise time), t16 = 1.52, P < 0.0001. A likely contributor to these continued weekend sleep-ins is that adolescents who underwent CBT plus BLT still reported later bedtimes on weekends relative to their school-night bedtime, t20 = 5.47, P < 0.0001.
For functioning measures, significant interactions were found for daytime sleepiness, F1,29 = 4.85, P = 0.04, and fatigue, F1,33 = 5.26, P = 0.03. The interaction for depression symptoms was not significant, F1,28 = 4.08, P = 0.05, yet a moderate-large effect exists (see Table 2). Improvements in sleepiness, however, were not associated with increases in school-night total sleep time, rs(16) = −0.17, P > 0.05, but rather advances in sleep timing (as indicated by a change in sleep onset time on school nights), rs(16) = 0.60, P = 0.01. Despite small-moderate correlation coefficients, changes in depression symptoms were not associated with improvements in sleep onset timing, rs(16) = 0.46, P = 0.07, or total sleep time on school nights, rs(16) = −0.33, P = 0.23. Changes in fatigue were not directly related to changes in total sleep time, rs(18) = −0.11, P = 0.67, or sleep timing, rs(18) = 0.10, P = 0.70.
Long-Term Effects of CBT Plus BLT on Sleep and Functioning
Table 3 presents the descriptives (means, SDs) and effect sizes for the DSPD adolescents assigned to CBT plus BLT, and who provided 6-month follow-up data (N = 15). Repeated measures analysis showed several of these measures remained significant at the 6-month follow-up compared to pre-treatment. These include sleep onset latency on school nights (F1,13 = 6.77, P = 0.02) and weekends (F1,14 = 10.24, P = 0.006); sleep onset time on weekends (F1,14 = 11.91, P = 0.004); WASO (F1,14 = 9.92, P = 0.007); discrepancy in rise times (F1,11 = 8.17, P = 0.016); daytime sleepiness (F1,11 = 17.70, P = 0.001), fatigue (F1,11 = 29.45, P < 0.0001), and depression symptoms (F1,11 = 9.51, P = 0.01). Although significance was not obtained for sleep onset time on school nights, F1,12 = 3.92, P = 0.07, a moderate effect was found (d = 0.55). Furthermore, despite the lack of significant findings in weekend total sleep time and school morning rise time, small effects were nonetheless found (d = 0.34 and d = 0.24, respectively).
Does CBT Plus BLT Resolve Adolescent DPSD Diagnosis?
Equivalent post-treatment data between the CBT plus BLT and WL groups were used to ascertain whether adolescents met criteria for DSPD as per the ICSD-2 diagnostic criteria.2 Specifically, evidence for a delayed sleep timing resulting in chronic difficulty falling asleep and waking in the morning, with associated insomnia or daytime sleepiness that affect the adolescent's life confirm a DSPD diagnosis. At post-treatment, 87% adolescents who received CBT plus BLT did not meet diagnostic criteria compared to 13% who did, whereas 18% in the WL condition did not meet DSPD criteria vs. 82% who did, χ2(1, N = 40) = 19.22, P < 0.0001. For adolescents in the CBT plus BLT group who still met criteria, 2 were not attending school and were not able to sufficiently advance their sleep timing to align with school start times, and the remaining subject's DSPD condition improved after treatment (54-min phase advance in school sleep onset time), but still qualified for mild DSPD (i.e., school sleep onset time = 00:16). Of the 3 adolescents who improved in the Waitlist condition, all began to rise earlier on weekends. Despite not qualifying for a DSPD diagnosis at post-treatment, all 3 WL adolescents opted for CBT plus BLT due to residual symptoms. At the 6-month follow-up point, 2 of the 15 CBT plus BLT adolescents (13%) qualified for a DSPD diagnosis. Both adolescents' relapses were due to a combination of late weekend sleep onset and rise times that occurred during holidays.
DISCUSSION
DSPD is a chronic and disruptive sleep disorder reported to be more common during adolescence.2 In the present study, adolescents with DSPD typically experienced lengthy sleep latencies, late sleep onsets, and restricted nocturnal sleep, and elevated levels of daytime dysfunction (i.e., sleepiness and fatigue). Insomnia and poor sleep hygiene were common features, and all reported poor school performance and/or attendance. These constellations of symptoms were experienced for a mean of almost 5 years. However, the findings from the present study provide evidence that 8 weeks of CBT plus BLT can help to alleviate DSPD for adolescents in the immediate term, and to some extent, the longer-term.
The Contribution of CBT Plus BLT to Healthier Sleep Patterns
Adolescents in the present study underwent morning bright light therapy, whereby their rise time gradually advanced each day in combination with bright light. This resulted in significantly earlier rise times at post-treatment. Researchers suggest one contribution to the development of DSPD may be the avoidance of morning light.4,29 We note that the vast majority of adolescents in the present study claimed to avoid morning bright light (85%). This was likely the case on weekends, as adolescents' rise times were significantly later than school week rise times, thus denying them sufficient light in the morning. This neglect of morning light disallows any resetting or phase advancing of the endogenous clock.53 In response to this, one of this study's treatment techniques was morning bright light therapy—a technique often recommended for the treatment of DSPD4,7,24,47,48 despite a minimal evidence base,7,53 especially in youth.33 In one adult study, Rosenthal et al.34 provided 20 DSPD patients with 2 h of 2,500 lux of full-spectrum light between 06:00-09:00, with subjective improvements in sleep-onset time that coincided with improvements in objective morning sleepiness. Morning light therapy has also been used with sleep-disturbed adolescents with a history of substance abuse31 and inpatient and outpatient adolescents diagnosed with DSPD.27,28,30 However, we cannot conclusively attribute the earlier rise times to a circadian phase advance without an objective measure (e.g., dim light melatonin onset, core body temperature nadir).
Coincident with the earlier rise times, adolescents in the CBT plus BLT condition also demonstrated significantly earlier sleep onset times. Adolescents exposed to evening bright light have shown later sleep onsets than adolescents living in homes without electricity,54 suggesting another potential precipitating and/or maintaining factor for adolescent DSPD. Evening dim light has been a common component in the treatment of DSPD34 and was instructed during the CBT plus BLT program. Dim light could alleviate the maintenance of a phase delay53 and be a possible contributing factor to earlier sleep onset times. As previously stated, without an objective assessment of circadian phase, the causation between evening dim light and earlier sleep onset times via a phase advance is not conclusive. It does appear, though, that the change in the process variables of sleep onset time and rise times assisted in the beneficial primary outcomes of reduced sleep latency and increased sleep duration due to CBT plus BLT.
Aside from a delay in adolescents' sleep timing, there may be additional factors contributing to their DSPD. As multiple groups have suggested, associated insomnia may accompany DSPD.2,4,6,7 Indeed, insomnia symptoms were common in this sample of DSPD adolescents (e.g., difficulty falling asleep, mind racing in bed). CBT plus BLT included techniques to target this insomnia. Of these techniques, cognitive therapy directly targeted unhelpful cognitions about sleep, particularly the catastrophizations around a lengthy sleep latency and next day functioning. However, the extent to which cognitive therapy reduced insomnia symptoms and reduced sleep latency is not known without a standardized assessment of changes in adolescents' sleep cognitions. Another component in CBT plus BLT was sleep hygiene education, which aimed to help reduce nighttime arousal. Behaviors promoting nighttime arousal were predominantly around the limiting of electronic media use prior to bed. Previous work with nonclinical adolescent samples suggests evening electronic media use is associated with delayed bedtime, longer sleep latency, next-day impairment, and weekend sleep-ins.55,56–58 We note that compared to previous reports, we found minimal evidence of substance use and mood disorders contributing to adolescents' DSPD (see reviews),7,9,49 possibly suggesting recent increases in technology use may be an emerging risk factor moving more typical adolescent sleep patterns into the pathological range.9,49 However, a casual link between electronic media reduction and improved sleep outcomes in clinical trials is desired.
Barriers to Successful Treatment Outcomes
Notwithstanding the benefits received by many adolescents from CBT plus BLT, there were still significant barriers to treatment and subsequent maintenance of healthy sleep patterns. Previous reports indicate the school attendance of adolescents with DSPD can be jeopardized, although these data derive from case studies or reviews.26,29,59,60 Sixteen percent (N = 8) of our 49 DSPD adolescents were not attending school when enrolling in our study. Treatment assisted 2 out of 4 adolescents in the CBT plus BLT group to return to school. Non-school attendance appears to be an influential factor in the recovery from DSPD.30 Our clinical notes from 2 of these adolescents show 1 was motivated to return to school for the face-to-face social interaction, yet their sleep timing did not advance enough at post-treatment to align with their school start time. The other adolescent was phase advancing well during treatment; however, the prospect of engaging with school friends and the workload after missing school for over 1 year appeared too daunting. Future interventions may need to provide added treatment components for this subpopulation to ensure better outcomes.
A second barrier may be delaying sleep patterns during weekends and holiday periods. Okawa and Uchiyama49 suggest DSPD may be triggered by vacation periods, and it was our experience that relapses occurred due to later sleep scheduling during school holidays. These relapses occurred despite our relapse prevention. Such non-adherence indicates low motivation to maintain regular sleep patterns, which is likely akin to the motivation of our adolescents who dropped out after 1 treatment session at which the morning bright light therapy plan was outlined. Indeed, in a previous adolescent sleep treatment study, non-completers attended 0-1 of 6 sessions.31 It therefore remains a challenge for future research to develop a sleep treatment package that can address motivational issues to engage and retain adolescents during treatment and maintain benefits in the longer term,40 which do seem possible.61
Limitations and Future Directions
Although multiple subjective benefits were found in the present study, objective verification of a circadian phase advance (e.g., dim light melatonin onset, core body temperature) would assist in the validation of the evening dim light and morning bright light therapy components, as has been found in previous studies.34 In practical applications this can be difficult due to time and financial barriers7,47–49; yet given the virtually nonexistent evidence for morning light therapy for adolescent DSPD,33 controlled evaluations are nonetheless desired. Similarly, associated insomnia was proposed to accompany adolescent DSPD, and indeed such clinical features were present at pre-treatment (i.e., nighttime sleep disturbance and associated daytime impairment). However, systematic pre- and post-treatment evaluations of insomnia were not performed. This is necessary to help determine if various treatment components help to reduce the associated insomnia. This leads to another limitation: despite many improvements in sleep and daytime functioning, we cannot uniquely contribute these benefits to any one particular treatment component. Future dismantling studies are required to determine the therapeutic efficacy of the various treatment components contained in CBT plus BLT. Furthermore, although CBT plus BLT was manualized and consistently supervised, no formal measures of treatment integrity (e.g., independent ratings of the proximity of treatment delivered against the manual) were taken. Finally, although our DSPD sample qualified for the disorder, several cases were relatively mild in nature. For instance, numerous case reports and studies in the literature are of adolescents with sleep onset times in the suggested 01:00 to 06:00 range.2,27,47,59 Coupled with the mid-high SES level of our sample, the benefits reported here may not generalize to severe populations and/or to lower SES groups.62
CONCLUSIONS
DPSD is the most common sleep disorder assessed and treated among adolescents in our clinic, yet controlled evaluations of treatments are limited. The present study found CBT plus BLT, which combines phase advancing techniques to correct a delayed circadian rhythm, and cognitive and behavioral techniques to address associated insomnia, the regularization of sleep schedules, and sleep hygiene, produced subjective benefits in sleep latency, sleep onset time, total sleep time, rise times, daytime sleepiness, and fatigue compared to controls. Non-school attendance, holiday periods, and low motivation to change may be barriers to treatment. Given adolescent DSPD is being treated by clinicians worldwide, the field requires reports of controlled (and possibly even uncontrolled) treatments to support recommendations for alleviating this chronic and often debilitating sleep disorder.7
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
FOOTNOTES
A. In Australia, promethazine is an over-the-counter antihistamine often prescribed as a pharmacological agent for sleeplessness in children and adolescents.
B. Unfortunately, no data were collected to ensure teens complied with sleep diary instructions.
ACKNOWLEDGMENTS
The authors wish to thank Nathan Weber, Steve Butler, and Ben Maddock for their ICT assistance in developing the online questionnaires and sleep diary, and the teenagers and their families who participated in this project. This project was supported by the Channel 7 Childrens' Research Foundation of South Australia, and the Flinders University Faculty of Social and Behavioural Sciences.
ABBREVIATIONS
- CBT plus BLT
cognitive-behavior therapy plus bright light therapy
- WL
waitlist
- DSPD
delayed sleep phase disorder
- ICSD-2
International Classification of Sleep Disorders, Second Edition
- PDSS
Pediatric Daytime Sleepiness Scale
- FFS
Flinders Fatigue Scale
- MFQ
Short Mood and Feelings Questionnaire
- CSHI
Clinical Sleep History Interview
- WASO
wake after sleep onset
- SES
socioeconomic status
REFERENCES
- 1.Weitzman ED, Czeisler CA, Coleman RM, Spielman AJ, Zimmerman JC, Dement W, Richardson G, Pollak CP. Delayed sleep phase syndrome. A chronobiological disorder with sleep-onset insomnia. Arch Gen Psychiatry. 1981;38:737–46. doi: 10.1001/archpsyc.1981.01780320017001. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine. The International classification of sleep disorders, 2nd ed.: Diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed., text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 4.Barion A, Zee PC. A clinical approach to circadian rhythm sleep disorders. Sleep Med. 2007;8:566–77. doi: 10.1016/j.sleep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferber R. Circadian rhythm sleep disorders in childhood. In: Ferber R, Kryger MH, editors. Principles and practices of sleep medicine in the child. Oxford, UK: Elsevier Health Sciences; 1995. [Google Scholar]
- 6.Bootzin RR. Is bright light exposure an effective therapy for insomnia? Comment on Lack L; Wright H; Gibbon S, et al. The treatment of early-morning awakening insomnia with 2 evenings of bright light. Sleep. 2005;28:540–1. doi: 10.1093/sleep/28.5.616. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt JK. Delayed sleep phase syndrome: pathophysiology and treatment options. Sleep. 2004;27:1195–203. doi: 10.1093/sleep/27.6.1195. [DOI] [PubMed] [Google Scholar]
- 8.Thorpy MJ, Korman E, Spielman AJ, Glovinsky PB. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1988;9:22–27. doi: 10.1016/0197-0070(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 9.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed sleep phase in adolescence. Sleep Med. 2007;8:602–12. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region and sleep. Sleep Med. doi: 10.1016/j.sleep.2010.11.008. in press. [DOI] [PubMed] [Google Scholar]
- 11.Johnson EO, Roth T, Schultz L, Breslau N. Epidemiology of DSM-IV insomnia in adolescence: Lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117:247–56. doi: 10.1542/peds.2004-2629. [DOI] [PubMed] [Google Scholar]
- 12.Ohayon MM, Roberts RE, Zulley J, Smirne S, Priest RG. Prevalence and patterns of problematic sleep among older adolescents. J Am Child Adolesc Psychiatry. 2000;39:1549–56. doi: 10.1097/00004583-200012000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Pelayo RP, Thorpy MJ, Glovinsky P. Prevalence of delayed and advanced sleep phase syndrome among adolescents. Sleep Res. 1988;17:392. [Google Scholar]
- 14.Australian Bureau of Statistics. Population by age, sex, Australian States and Territories (Catalogue no. 3201.0) Canberra: Australian Bureau of Statistics; 2009. [Google Scholar]
- 15.Curcio G, Ferrara M, de Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Med Rev. 2006;10:323–37. doi: 10.1016/j.smrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Gradisar M, Terrill G, Johnston A, Douglas P. Adolescent sleep and working memory performance. Sleep Biol Rhythms. 2008;6:146–54. [Google Scholar]
- 17.Dewald JF, Meijer AM, Ooort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010;14:179–89. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 18.National Sleep Foundation. 2006 Sleep in America poll. Washington, DC: National Sleep Foundation; 2006. [Google Scholar]
- 19.Danner F, Phillips B. Adolescent sleep, school start times, and teen motor vehicle crashes. J Clin Sleep Med. 2008;4:533–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Wong MM, Brower KJ, Zucker RA. Sleep problems, suicidal ideation, and self-harm behaviors in adolescence. J Psychiatric Res. doi: 10.1016/j.jpsychires.2010.09.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wojnar M, Ilgen MA, Wojnar J, McCammon RJ, Valenstein M, Brower KJ. Sleep problems and suicidality in the National Comorbidity Survey Replication. J Psychiatric Res. 2009;43:526–31. doi: 10.1016/j.jpsychires.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X. Sleep and adolescent suicidal behavior. Sleep. 2004;27:1351–8. doi: 10.1093/sleep/27.7.1351. [DOI] [PubMed] [Google Scholar]
- 23.Santrock JW. Life-span development. 12th ed. Columbus, OH: McGraw-Hill; 2008. [Google Scholar]
- 24.Sack R, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: Part II: advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder and irregular sleep-wake rhythm. Sleep. 2007;30:1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgenthaler TI, Lee-Choing T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. Sleep. 2007;30:1445–59. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ando K, Hayakawa T, Ohta T, Kayukawa Y, Ito A, Iwata T, Okada T. Long-term follow-up study of 10 adolescent patients with sleep-wake schedule disorders. Japan J Psych Neurol. 1994;48:37–41. doi: 10.1111/j.1440-1819.1994.tb02994.x. [DOI] [PubMed] [Google Scholar]
- 27.Ohta T, Ando K, Iwata T, et al. Treatment of persistent sleep-wake schedule disorders in adolescents with methylcobalamin (Vitamin B12) Sleep. 1991;14:414–8. [PubMed] [Google Scholar]
- 28.Okawa M, Uchiyama M, Ozaki S, Shibui K, Ichikawa H. Circadian rhythm sleep disorders in adolescents: clinical trials of combined treatments based on chronobiology. Psychiatry Clin Neurosci. 2002;52:483–90. doi: 10.1046/j.1440-1819.1998.00449.x. [DOI] [PubMed] [Google Scholar]
- 29.Tatman J. A parent's nightmare. J Clin Sleep Med. 2005;1:427–8. [PubMed] [Google Scholar]
- 30.Iwamitsu Y, Ozeki Y, Konishi M, Murakami J, Kimura S, Okawa M. Psychological characteristics and the efficacy of hospitalization treatment on delayed sleep phase syndrome patients with school refusal. Sleep Biol Rhythms. 2007;5:15–22. [Google Scholar]
- 31.Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clin Psychol Rev. 2005;25:629–644. doi: 10.1016/j.cpr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Hasler BP, Bootzin RR, Cousins JC, Fridel K, Wenk GL. Circadian phase in sleep-disturbed adolescents with a history of substance abuse: a pilot study. Behav Sleep Med. 2008;6:55–73. doi: 10.1080/15402000701796049. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn BR, Elliot AJ. Treatment efficacy in behavioral pediatric sleep medicine. J Psychosom Res. 2003;54:587–97. doi: 10.1016/s0022-3999(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, et al. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. 1990;13:354–61. [PubMed] [Google Scholar]
- 35.Alvarez B, Dahlitz MJ, Vignau J, Parkes JD. The delayed sleep phase syndrome: clinical and investigative findings in 14 subjects. J Neurol Neurosurg Psychiatry. 1992;55:665–70. doi: 10.1136/jnnp.55.8.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dagan Y, Eisenstein M. Circadian rhythm sleep disorders: toward a more precise definition and diagnosis. Chronobiol Int. 1999;16:213–22. doi: 10.3109/07420529909019087. [DOI] [PubMed] [Google Scholar]
- 37.Ohta T. Circadian rhythm sleep disorders: a brief review with special reference to long-term follow-up. Nagoya J Med Sci. 1995;58:83–93. [PubMed] [Google Scholar]
- 38.Yamadera H, Takahashi K, Okawa M. A multicenter study of sleep-wake rhythm disorders: clinical features of sleep-wake rhythm disorders. Psychiatry Clin Neurosci. 1996;50:195–201. doi: 10.1111/j.1440-1819.1996.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 39.Moseley L, Gradisar M. Evaluation of a school-based sleep intervention for adolescent sleep problems. Sleep. 2009;32:334–41. doi: 10.1093/sleep/32.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cain N, Gradisar M, Moseley L. A motivational school-based intervention for adolescent sleep problems. Sleep Med. doi: 10.1016/j.sleep.2010.06.008. in press. [DOI] [PubMed] [Google Scholar]
- 41.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 42.Wolfson AR, Carskadon MA, Acebo C, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–6. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- 43.Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The Pediatric Sleepiness Scale (PDSS): Sleep habits and school outcomes in middle-school children. Sleep. 2003;26:455–8. [PubMed] [Google Scholar]
- 44.Gradisar M, Lack L, Richards H, et al. The Flinders Fatigue Scale: preliminary psychometric properties and clinical sensitivity of a new scale for measuring daytime fatigue associated with insomnia. J Clin Sleep Med. 2007;3:722–8. [PMC free article] [PubMed] [Google Scholar]
- 45.Angold A, Costello EJ, Messer SC. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Methods Psychiatric Res. 1995;5:237–49. [Google Scholar]
- 46.Shirayama M, Shirayama Y, Iida H, et al. The psychological aspects of patients with delayed sleep phase syndrome (DSPD) Sleep Med. 2003;4:427–33. doi: 10.1016/s1389-9457(03)00101-1. [DOI] [PubMed] [Google Scholar]
- 47.Bjorvatn B, Pallesen S. A practical approach to circadian rhythm sleep disorders. Sleep Med Rev. 2009;13:47–60. doi: 10.1016/j.smrv.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Lack L, Wright HR. Clinical management of delayed sleep phase disorder. Behav Sleep Med. 2007;5:57–76. doi: 10.1207/s15402010bsm0501_4. [DOI] [PubMed] [Google Scholar]
- 49.Okawa M, Uchiyama M. Circadian rhythm sleep disorders: characteristics and entrainment pathology in delayed sleep phase and non-24 sleep-wake syndrome. Sleep Med Rev. 2007;11:485–96. doi: 10.1016/j.smrv.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319:670–4. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane P. Handling drop-out in longitudinal clinical trials: a comparison of the LOCF and MMRM approaches. Pharm Stat. 2008;7:93–106. doi: 10.1002/pst.267. [DOI] [PubMed] [Google Scholar]
- 52.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–25. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 53.Shirani A, Louis EK., St. Illuminating rationale and uses for light therapy. J Clin Sleep Med. 2009;5:155–63. [PMC free article] [PubMed] [Google Scholar]
- 54.Peixoto CAT, da Silva AGT, Carskadon MA, Louzada FM. Adolescents living in homes without electric lighting have earlier sleep times. Behav Sleep Med. 2009;7:73–80. doi: 10.1080/15402000902762311. [DOI] [PubMed] [Google Scholar]
- 55.National Sleep Foundation. 2011 Sleep in America Poll: Communications Technology and Sleep. Washington, DC: National Sleep Foundation; 2011. [Google Scholar]
- 56.Eggermont S, Van den Bulck J. Nodding off or switching off? The use of popular media as a sleep aid in secondary-school children. J Pediatr Child Health. 2006;42:428–33. doi: 10.1111/j.1440-1754.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 57.Shochat T, Flint-Bretler O, Tzischinsky O. Sleep patterns, electronic media exposure and daytime sleep-related behaviours among Israeli adolescents. Acta Paediatr. 2010;99:1396–400. doi: 10.1111/j.1651-2227.2010.01821.x. [DOI] [PubMed] [Google Scholar]
- 58.Weaver E, Gradisar M, Dohnt H, Lovato N, Douglas P. The effect of presleep video-game playing on adolescent sleep. J Clin Sleep Med. 2010;6:184–9. [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia J, Rosen G, Mahowald M. Circadian rhythms and circadian rhythm disorders in children and adolescents. Semin Ped Neurol. 2001;8:229–40. doi: 10.1053/spen.2001.29044. [DOI] [PubMed] [Google Scholar]
- 60.Ichikawa H, Sato T, Takahashi K. Sleep-waking rhythm disorders observed in five school refusers. Jpn J Psychiatry Neurol. 1990;44:188. [Google Scholar]
- 61.Ando K, Hayakawa T, Ohta T, et al. Long-term follow-up study of 10 adolescent patients with sleep-wake schedule disorders. Jpn J Psychiatry Neurol. 1994;48:37–41. doi: 10.1111/j.1440-1819.1994.tb02994.x. [DOI] [PubMed] [Google Scholar]
- 62.Tikotzky L, Sadeh A. The role of cognitive behavioural therapy in behavioural childhood insomnia. Sleep Med. 2010;11:686–91. doi: 10.1016/j.sleep.2009.11.017. [DOI] [PubMed] [Google Scholar]