Abstract
Introduction:
This study was conducted to evaluate the therapeutic performance of a new auto Servo Ventilation device (Philips Respironics autoSV Advanced) for the treatment of complex central sleep apnea (CompSA). The features of autoSV Advanced include an automatic expiratory pressure (EPAP) adjustment, an advanced algorithm for distinguishing open versus obstructed airway apnea, a modified auto backup rate which is proportional to subject's baseline breathing rate, and a variable inspiratory support. Our primary aim was to compare the performance of the advanced servo-ventilator (BiPAP autoSV Advanced) with conventional servo-ventilator (BiPAP autoSV) in treating central sleep apnea (CSA).
Study Design:
A prospective, multicenter, randomized, controlled trial.
Setting:
Five sleep laboratories in the United States.
Participants:
Thirty-seven participants were included.
Measurements and Results:
All subjects had full night polysomnography (PSG) followed by a second night continuous positive airway pressure (CPAP) titration. All had a central apnea index ≥ 5 per hour of sleep on CPAP. Subjects were randomly assigned to 2 full-night PSGs while treated with either the previously marketed autoSV, or the new autoSV Advanced device. The 2 randomized sleep studies were blindly scored centrally. Across the 4 nights (PSG, CPAP, autoSV, and autoSV Advanced), the mean ± 1 SD apnea hypopnea indices were 53 ± 23, 35 ± 20, 10 ± 10, and 6 ± 6, respectively; indices for CSA were 16 ± 19, 19 ± 18, 3 ± 4, and 0.6 ± 1. AutoSV Advanced was more effective than other modes in correcting sleep related breathing disorders.
Conclusions:
BiPAP autoSV Advanced was more effective than conventional BiPAP autoSV in the treatment of sleep disordered breathing in patients with CSA.
Citation:
Javaheri S; Goetting MG; Khayat R; Wylie PE; Goodwin JL; Parthasarathy S. The performance of two automatic servo-ventilation devices in the treatment of central sleep apnea. SLEEP 2011;34(12):1693-1698.
Keywords: Bilevel positive pressure ventilation, servo-ventilation, central sleep apnea, auto EPAP, pressure support
INTRODUCTION
Central sleep apnea (CSA) occurs in a variety of conditions.1 CSA may occur in patients with obstructive sleep apnea (OSA) when continuous positive airway pressure (CPAP) is commenced.2–6 This condition has been called complex sleep apnea (CompSA)2 or CPAP-emergent central apneas and constitutes about 6% to 20% of patients with OSA.2–6 CSA and Hunter-Cheyne-Stokes (HCSB) breathing occur in patients with heart failure7–9 and is associated with increased likelihood of death.7,10,11 CSA is also reported in patients using opioids.12,13
A new generation of positive airway pressure devices termed “adaptive servo-ventilation” has been successfully used in a number of studies both for CompSA,6 and CSA associated with systolic heart failure,14–21 opioids,12 and idiopathic periodic breathing.22 In such devices there are multiple settings to consider. The expiratory positive airway pressure (EPAP) is titrated manually to eliminate obstructive disordered breathing events. The pressure support is variable, increasing with hypopnea and decreasing during hyperventilation. The backup rate is set either by clinical judgment or automatically, and, if spontaneous breathing does not occur within that specific time frame, a mandated breath is delivered to abort any impending apnea.
In the present study, we evaluated a new generation advanced adaptive-servo ventilator (BiPAP autoSV Advanced, Philips Respironics) in which the EPAP is adjusted automatically by algorithms aimed at correcting obstructive disordered breathing events in addition to other features aimed at treating CSA.
Our primary aim was to compare the performance of the advanced servo-ventilator (BiPAP autoSV Advanced) with conventional servo-ventilator (BiPAP autoSV) in treating CSA. In order to address this aim we performed a randomized, double-blind, crossover study. The preliminary results of this study have been published in the abstract form.23
METHODS
This was a prospective, multicenter, randomized, controlled trial. The trial was overseen and approved by an accredited IRB and all consents were obtained for each enrolled subject in the trial. In addition the trial was registered with Clinical Trials and can be found under the registration number NCT00720213.
The study involved 37 consecutive eligible patients in whom a non-blinded CPAP titration study had demonstrated the presence of a central apnea index (CAI) ≥ 5/h of sleep. All of these patients had undergone full-night attended diagnostic polysomnography (PSG) and had moderate to severe sleep disordered breathing (SDB) with an apnea hypopnea index (AHI) ≥ 15/h. Full-night CPAP titration study followed the diagnostic study. Most patients had already been treated with CPAP > 4 weeks and continued to demonstrate persistent central apnea, with CAI ≥ 5/h.
The study enrolled 5 females and 32 males with mean age of 63 ± 11 and a BMI of 31 ± 6 kg/m2. Sixteen subjects were on medications for systemic hypertension, 6 had atrial fibrillation, 6 had history of coronary artery disease, 2 had congestive heart failure, 2 had received a pacemaker, and 6 had diabetes mellitus.
Upon confirmation that CPAP was unsuccessful in treating their SDB, the patients were randomized to 2 consecutive attended titration PSGs with either BiPAP autoSV Advanced or conventional BiPAP autoSV. Participants were blinded to which device they were treated with during their study nights (the 2 randomized BiPAP studies were scored blindly at a central location).
Operation of BiPAP autoSV Advanced
To determine inspiratory positive airway pressure (IPAP), the BiPAP autoSV Advanced algorithm monitors the average peak flow using an internal pneumotachograph. The average peak flow is monitored during a 4-min moving window and an average, target peak flow is determined. If the peak flow diminishes below this target, the pressure support (the pressure above the prevailing expiratory pressure) increases. The maximum inspiratory support level is up to 30 cm H2O minus the expiratory pressure. In contrast, if the patient's average peak flow increases above the desired target, then the pressure support decreases. With sufficient patient breathing effort, the pressure support is capable of going down to the level of expiratory pressure, i.e., zero pressure support. Thus the support is variable and may change on a breath to breath basis.
To determine the expiratory positive airway pressure (EPAP) level, the BiPAP autoSV Advanced analyzes airflow and snoring signals to assess and preserve airway patency. The airflow is measured by the pneumotachograph and EPAP is automatically increased with the evidence of airway obstruction in a manner similar to an automatic CPAP device. The expiratory pressure automatically adjusts up (Figure 1) and down within the available range (4 cm H2O to 25 cm H2O). Expiratory pressures can also be set at fixed levels if desired.
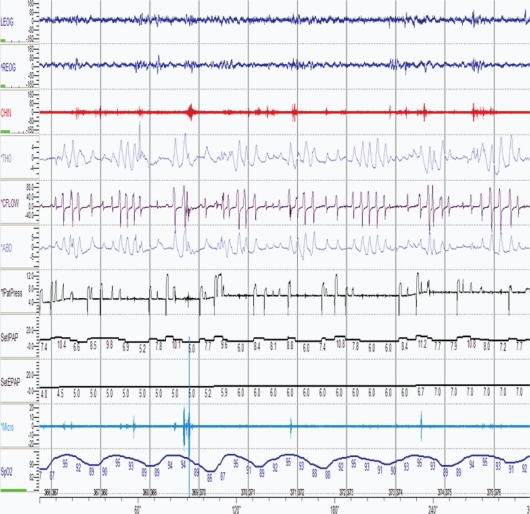
Figure 1.
A 5-min epoch of a polysomnogram showing EPAP pressure automatically titrating in response to partially closed airway. LEOG, Left Eye; REOG, Right Eye; CHIN, CHIN EMG; THO, Thoracic Belt; CFLOW, Patient Flow; ABD, Abdominal Belt; PatPress, Patient Pressure; SetIPAP, IPAP pressure; SetEPAP, EPAP pressure; Micro, Snore Microphone; SpO2, Oxygen Saturation.
With episodes of upper airway obstruction (obstructive apnea and hypopnea), the expiratory pressure increases progressively by increments of 1 cm H2O. Each pressure increment occurs over a 15-second period. In order for EPAP to increase, typically 2 SDB events, apneas, hypopneas, or a combination thereof must occur.
The algorithm's automatic backup rate is based on calculations performed on a moving window of the last 12 spontaneous breaths. Two calculated values are made with the first being proportional to the time of exhalation and the second value based on the overall breath period. The device monitors the immediate exhalation time and spontaneous breath period. A mandatory breath is delivered if a spontaneous breath does not occur within the calculated parameters. A minimum breath rate is enforced, ranging from 8 to 10 breaths per minute.
The algorithm of BiPAP autoSV Advanced differs from that of the previous generation BiPAP autoSV in 2 ways. First, with the previous generation of the BiPAP autoSV, the EPAP had to be manually titrated in order to eliminate OSA events. Second, the algorithm for the automatic backup rate was not proportional to baseline breathing rate but instead, constant values were added to the total breath period or to the expiratory time for the initiation of a mandatory breath. The BiPAP auto SV Advanced should be set up using the manufacturing settings.
Device Detection of Sleep Disordered Breathing (SDB) Events
The BiPAP autoSV Advanced identifies and responds to breathing events defined by the following criteria. Apnea is recognized by cessation or a decrease in airflow ≥ 80%. Hypopnea is a decrease in airflow of 40% but < 80%.
Obstructed airway and clear airway events are distinguished from each other based on flow response to a machine triggered breath. If peak flow decreases by > 80% (apnea) and if there is no airflow measured in response to a mandatory machine triggered breath, the event is characterized as obstructive in nature. If airflow is detected in response to a mandatory machine triggered breath, the event is characterized as a clear airway apnea. With obstructed airway events, the EPAP increases.
Initiation of ASV Treatment during PSG
On the study nights with the previous generation BiPAP autoSV, the expiratory pressure was set to the level equivalent to the CPAP titration prescription pressure that had eliminated all obstructive events. If required, the EPAP could be increased in order to eliminate any residual obstructive events. However, this was not necessary for these patients.
With the BiPAP autoSV Advanced, the starting expiratory pressure was set at 2 cm H2O below the CPAP titration prescription pressure but did not go below the device minimum EPAP of 4 cm H2O. This was chosen because the expiratory pressure could automatically increase in order to eliminate obstructive events and to allow the device to search for lower expiratory pressures as needed.
The mandatory breath rate was set to the automatic mode on both machines. The algorithm for determining IPAP is identical between the two devices and the maximum pressure of 30 cm H2O was available.
Scoring of PSG Studies
All of the randomized studies were blinded and centrally scored. Sleep stages and SDB events were classified according to ASSM 2007 recommended criteria.24 An apnea was defined as cessation of airflow > 90% for ≥ 10 sec. Obstructive apnea was defined as the absence of airflow associated with continued thoracoabdominal excursions. Hypopneas were defined as a reduction in airflow and/or thoracoabdominal excursions ≥ 30% and associated with ≥ 4% drop in arterial oxygen saturation.
Statistical Analysis
Our primary aim was to demonstrate non-inferiority comparison of the performance of the advanced servo-ventilator (BiPAP autoSV Advanced) versus conventional servo-ventilator (BiPAP autoSV) in treating CSA. Because of the asymmetric distributions of the endpoints, the nonparametric Friedman analysis of variance was used to compare the related values in the 4 PSGs of the study, including diagnostic PSG, CPAP, BiPAP autoSV, and BiPAP autoSV Advanced. Post hoc pair-wise comparisons were done with the Wilcoxon Signed Ranks test, with Bonferroni correction. P values < 0.05 were considered significant. Descriptive statistics include the mean, standard deviation, and median values. All analyses were completed in SPSS 15.0 (Chicago, IL).
RESULTS
The sleep architecture and sleep related breathing disorders across 4 nights of sleep studies are shown in Tables 1 and 2. When compared to the diagnostic night, during therapy with BiPAP autoSV and BiPAP autoSV Advanced, stage 2 sleep diminished, whereas REM sleep increased (Table 1). Furthermore, the arousal index decreased significantly with all positive airway pressure devices compared to the diagnostic PSG. Periodic leg movement index (PLMI) during sleep did not change significantly, although the index was lower while receiving servo-ventilation (Table 1).
Table 1.
Sleep architecture across the four polysomnography nights
| Variable | Diagnostic | CPAP titration | Auto SV | AutoSV Advanced | P-value* |
|---|---|---|---|---|---|
| Sleep Efficiency (%) | 73 (73 ± 15) 28–99 | 76 (77 ± 13) 48–97 | 80 (77 ± 13) 44–99 | 77 (76 ± 12) 52–96 | 0.49 |
| N 1 (% TST) | 15 (19 ± 14) 4–57 | 16 (16 ± 7) 5–31 | 14 (17 ± 12) 4–54 | 13 (16 ± 9) 4–41 | 0.63 |
| N 2 (% TST) | 69 (67 ± 15) 35–91 | 68 (66 ± 11) 41–86 | 56 (55 ± 12)a,b 23–74 | 56 (55 ± 12)a,b 21–74 | < 0.001 |
| N 3 (% TST) | 8 (13 ± 11) 1–40 | 2 (6 ± 7) 0–21 | 14 (13 ± 10)b 0–44 | 9 (12 ± 10)b 0–46 | 0.005 |
| REM (% TST) | 8 (11 ± 9) 0–38 | 17 (17 ± 7)a 0–34 | 16 (16 ± 6)a 0–28 | 18 (17 ± 7)a 0–33 | 0.003 |
| Arousal Index, n/h | 31 (36 ± 23) 7–104 | 13 (19 ± 14)a 1–51 | 24 (26 ± 11) 8–48 | 21 (24 ± 11)a 7–49 | 0.008 |
| PLMI, n/h | 1 (8 ± 17) 0–91 | 3 (18 ± 42) 0–193 | 2 (3 ± 3) 0–12 | 2 (3 ± 2) 0–12 | 0.43 |
CPAP, continuous positive airway pressure; PLMI, Periodic leg movements index during sleep.
Friedman Test comparing all 4 nights.
Significant vs. Diagnostic; Bonferroni-adjusted P-values for pairwise comparisons were P ≤ 0.048.
Significant vs. CPAP; Bonferroni-adjusted P-values for pairwise comparisons were P ≤ 0.014. Values are median (mean ± SD), followed by minimum–maximum on second line.
Table 2.
Respiratory indices across the four polysomnography nights
| Variable | Diagnostic | CPAP titration | Auto SV | AutoSV Advanced | P-value* |
|---|---|---|---|---|---|
| Apnea Hypopnea Index, n/h | 51 (53 ± 23) 17–93 | 29 (35 ± 20)a 11–94 | 6 (10 ± 10)a,b 0–40 | 5 (6 ± 6)a,bc 0–27 | < 0.001 |
| Central Apnea Index, n/h | 9 (16 ± 19) 0–72 | 10 (19 ± 18) 5–75 | 1 (3 ± 4)a,b 0–14 | 0.3 (0.6 ± 1)a,b,c 0–3 | < 0.001 |
| Obstructive Apnea Index, n/h | 6 (12 ± 17) 0–73 | 0.4 (1 ± 1)a 0–6 | 1 (2 ± 2)a,b 0–13 | 1 (1 ± 2)a,c 0–9 | < 0.001 |
| Hypopnea Index, n/h | 19 (21 ± 14) 1–55 | 12 (15 ± 12) 1–41 | 2 (5 ± 6)a,b 0–29 | 2 (4 ± 5)ab 0–21 | < 0.001 |
| Mixed Apnea Index, n/h | 0.5 (4 ± 9) 0–49 | 0 (0.4 ± 1)a 0–6 | 0.2 (0.4 ± 1) 0–4 | 0 (0.2 ± 0.4)a 0–2 | 0.002 |
| Baseline SpO2, % | 95 (95 ± 2) 91–98 | 96 (96 ± 2) 90–99 | 96 (96 ± 1) 94–99 | 96 (96 ± 1) 93–100 | 0.02‡ |
| Min SpO2, % | 81 (79 ± 10) 52–93 | 86 (84 ± 10) 43–93 | 89 (87 ± 9)a 53–95 | 88 (88 ± 5)a 74–97 | < 0.001 |
CPAP, continuous positive airway pressure.
Friedman Test comparing all 4 nights.
Significant vs. Diagnostic; Bonferroni-adjusted P-values for pairwise comparisons were P ≤ 0.016.
Significant vs. CPAP; Bonferroni-adjusted P-values for pairwise comparisons were P ≤ 0.001.
Significant vs. Auto SV; Bonferroni-adjusted P-values for pairwise comparisons were P ≤ 0.035. Values are median (mean ± SD), followed by minimum–maximum on second line.
For Baseline SpO2, pairwise comparisons were not significant after Bonferroni adjustment.
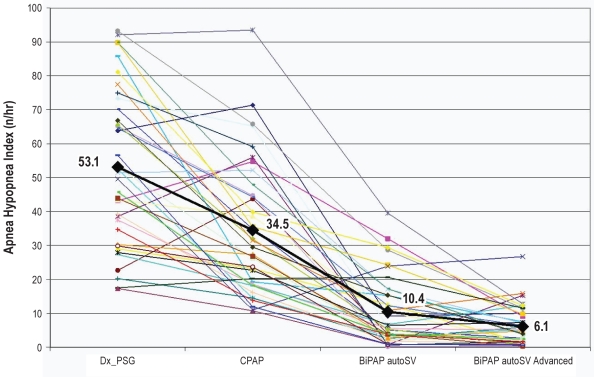
The CAI was significantly lower during treatment nights with positive airway pressure therapy when compared to the diagnostic sleep study night (Table 2). However, across the 4 nights, the AHI during BiPAP autoSV Advanced was significantly lower than AHI during BiPAP autoSV and the CPAP nights. The CAI decreased significantly during BiPAP autoSV Advanced night when compared to BiPAP autoSV (Table 2). The reduction in AHI was associated with improvement in oxygen saturation. The individual values for AHI across 4 nights of sleep studies are shown in Figure 2. In 4 subjects with the most severe sleep apnea, the AHI decreased considerably though remained elevated on BiPAP autoSV Advanced (Figure 2).
Figure 2.
Shows the individual apnea-hypopnea index comparing diagnostic PSG, CPAP titration study, BiPAP autoSV, and BiPAP autoSV Advanced. There is a significant reduction in the apnea-hypopnea index with either the previous generation of BiPAP autoSV or BiPAP autoSV Advanced (P < 0.001). However, there is also a significant further reduction in AHI with BiPAP autoSV compared to BiPAP autoSV Advanced (P = 0.0354).
When compared to the BiPAP autoSV, the BiPAP autoSV Advanced did not show significant difference between pressures (Table 3). However BiPAP autoSV Advanced does provide more breaths than the BiPAP autoSV, which could have contribute to better improvement.
Table 3.
Pressure data
| Variable | Device | Mean | Median | Std. Dev. | Min | Max |
|---|---|---|---|---|---|---|
| EPAP 5th percentile | Auto SV | 9 | 9 | 2 | 5 | 12 |
| Auto SV Advanced | 7 | 7 | 2 | 4 | 10 | |
| EPAP 90th percentile | Auto SV | 9 | 9 | 2 | 5 | 12 |
| Auto SV Advanced | 10 | 10 | 3 | 5 | 16 | |
| EPAP mean | Auto SV | 9 | 9 | 2 | 5 | 12 |
| Auto SV Advanced | 8 | 9 | 2 | 5 | 13 | |
| Pressure support 5th percentile | Auto SV | 0.2 | 0.1 | 0.4 | 0.0 | 2.0 |
| Auto SV Advanced | 0.2 | 0.0 | 0.3 | 0.0 | 1.2 | |
| Pressure support 90th percentile | Auto SV | 6 | 5 | 4 | 1 | 15 |
| Auto SV Advanced | 9 | 8 | 4 | 3 | 19 | |
| Pressure support mean | Auto SV | 3 | 2 | 2 | 1 | 7 |
| Auto SV Advanced | 4 | 3 | 2 | 1 | 7 | |
| Mean leak | Auto SV | 41 | 38 | 10 | 26 | 68 |
| Auto SV Advanced | 40 | 42 | 9 | 25 | 62 | |
| Number of machine breaths | Auto SV | 283 | 180 | 238 | 32 | 985 |
| Auto SV Advanced | 834 | 706 | 531 | 103 | 2193 |
DISCUSSION
The results of this study show the new BiPAP autoSV Advanced leads to elimination and successful reduction of the spectrum of events, including obstructive and central apneas and hypopneas. The results with BiPAP autoSV Advanced are superior to previous generation BiPAP autoSV. These results confirm those of a smaller study21 involving 10 patients with mixed pattern of breathing events showing the efficacy of this device.
The new device differs from previous conventional generation device in that the expiratory pressure is automatically titrated and the backup rate automatically changes based on the patient's intrinsic breathing rate. We speculate that both of these features contributed to the superior performance of the BiPAP autoSV Advanced when compared to the conventional BiPAP autoSV. It should be noted that both CAI and OAI, which are responsive to the back-up rate and automatic EPAP, respectively were lower with BiPAP autoSV Advanced (Table 2). The automated EPAP determination feature in the BiPAP autoSV Advanced should reduce the need for clinical decision making in choosing an appropriate EPAP setting.
While the results of the present study show that the automatic algorithm of BiPAP autoSV Advanced is statistically superior to the manual titration using the previous generation BiPAP autoSV, the clinical significance is unclear because the long term affects were not studied.
As noted previously, all patients had a CAI ≥ 5 on CPAP, and most of these patients demonstrated persistent CSA after several weeks of CPAP therapy. The BiPAP autoSV Advanced was effective in decreasing the CAI from 16 ± 19/h to 0.6 ± 1/h. The reduced variability in the CAI reflected by the small scatter of the number of events during BiPAP autoSV Advanced suggest that the device is a reliable means of controlling events (Figure 2). The smaller variation was achieved despite the heterogeneity of the patients studied. CompSA was the predominant breathing pattern in all 37 patients, and HCSB pattern was seen in 10 of the 37 patients. One patient was not treated sufficiently on both nights with auto SV therapy. On both these nights manual therapy adjustments were not made per protocol. Optimal patient management requires careful supervision and appropriate intervention.
The differences between BiPAP autoSV Advanced and previous BiPAP autoSV device are that in BiPAP autoSV Advanced, the expiratory pressure is automatically titrated and the mandatory breath rate more closely tracks patient breathing. For EPAP, the algorithm dictates continuous searching for the most ideal but minimal expiratory pressure and the corresponding IPAP level. The inspiratory support could be equal to EPAP and the EPAP could be as low as the device minimum of 4 cm of H2O. This is an important feature, because in patients with CompSA, opioid-associated CSA, and heart failure there are periods of the night when patients' intrinsic breathing pattern is normal. Central apneas are rare during REM sleep, and in NREM sleep, there are periods when disordered breathing events are absent. During periods of normal breathing, pressure can be minimized and minimal inspiratory and expiratory pressure could reduce the hemodynamic burden of increased intrathoracic pressure from positive airway pressure on the cardiovascular system.11
CONCLUSION
In this short-term, randomized, crossover, single-night, efficacy study involving patients with CSA, BiPAP autoSV Advanced resulted in more effective treatment of both central and obstructive events. We speculate that both the automated backup rate and the automated EPAP determination features conferred such superiority to conventional servo-ventilation.
Long-term cardiovascular or mortality event driven studies are needed to determine the impact of such new technology on quality of life, morbidity, and mortality.
DISCLOSURE STATEMENT
This study was funded by Respironics, Inc. Drs. Javaheri and Parthasarathy have received research support from Respironics, Inc. The other authors have indicated no other financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the following individuals from Philips Respironics for their support in the successful execution of this study: Jeremy Powers for his study management, Mike Kane for his engineering support, Jeff Jasko for his statistical support, Bill Hardy and Gary Lotz for their valuable and constructive insights on this manuscript.
ABBREVIATIONS
- PSG
polysomnogram
- PAP
positive airway pressure
- EPAP
expiratory PAP
- IPAP
inspiratory PAP
- CPAP
continuous PAP
- BiPAP
bilevel PAP
- SDB
sleep disordered breathing
- OSA
obstructive sleep apnea
- CSA
central sleep apnea
- HCSB
Hunter-Cheyne-Stokes breathing
- AHI
apnea hypopnea index
- CompSA
complex sleep apnea
Footnotes
A commentary on this article appears in this issue on page 1625.
REFERENCES
- 1.Javaheri S. Central sleep apnea. In: Lee-Chiong T, editor. Sleep medicine essentials. Hoboken, NJ: Wiley-Blackwell; 2009. pp. 81–9. [Google Scholar]
- 2.Morgenthaler TI, Kagramanov V, Hanak V, et al. Complex sleep apnea syndrome: Is it a unique clinical syndrome? Sleep. 2006;29:1203–9. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 3.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009;5:205–11. [PMC free article] [PubMed] [Google Scholar]
- 4.Dernaika T, Tawk M, Nazir S, et al. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night sleep studies. Chest. 2007;132:81–7. doi: 10.1378/chest.06-2562. [DOI] [PubMed] [Google Scholar]
- 5.Lehman S, Antic NA, Thompson C, et al. Central sleep apnea on commencement of continuous positive airway pressure in patients with a primary diagnosis of obstructive sleep apnea-hypopnea. J Clin Sleep Med. 2007;3:462–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Allam JS, Olson EJ, Gay PC, et al. Efficacy of adaptive servo-ventilation in treatment of complex and central sleep apnea syndromes. Chest. 2007;132:1839–46. doi: 10.1378/chest.07-1715. [DOI] [PubMed] [Google Scholar]
- 7.Javaheri S, Shukla R, Zeigler H, et al. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–34. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 8.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 9.Sin DD, Fitzgerald F, Parker JD, et al. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 10.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 11.Javaheri S. CPAP should not be used for central sleep apnea in congestive heart failure patients. J Clin Sleep Med. 2006;2:399–402. [PubMed] [Google Scholar]
- 12.Javaheri S, Malik A, Smith J, et al. Adaptive pressure support servo-ventilation: A novel treatment for sleep apnea associated with use of opioids. J Clin Sleep Med. 2008;4:305–10. [PMC free article] [PubMed] [Google Scholar]
- 13.Farney RJ, Walker JM, Boyle KM, et al. Adaptive servo-ventilation in patients with sleep disordered breathing associated with chronic opioid medications for non-malignant pain. J Clin Sleep Med. 2008;4:311–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Teschler H, Dohring J, Wang YM, et al. Adaptive pressure support servo-ventilation: A novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–9. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 15.Pepperell JC, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 16.Szollosi I, O'Driscoll DM, Dayer MJ, et al. Adaptive servo-ventilation and dead space: Effects on central sleep apnoea. J Sleep Res. 2006;15:199–205. doi: 10.1111/j.1365-2869.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 17.Kasai T, Narui K, Dohi T, et al. First experience of using new adaptive servo-ventilation device for Cheyne-Stokes respiration with central sleep apnea among japanese patients with congestive heart failure: Report of 4 clinical cases. Circ J. 2006;70:1148–54. doi: 10.1253/circj.70.1148. [DOI] [PubMed] [Google Scholar]
- 18.Fietze I, Blau A, Glos M, et al. Bi-level positive pressure ventilation and adaptive servo ventilation in patients with heart failure and Cheyne-Stokes respiration. Sleep Med. 2008;9:652–9. doi: 10.1016/j.sleep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Oldenburg O, Schmidt A, Lamp B, et al. Adaptive servo-ventilation improves cardiac function in patients with chronic heart failure and Cheyne-Stokes respiration. Eur J Heart Fail. 2008;10:581–6. doi: 10.1016/j.ejheart.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Arzt M, Wensel R, Montalvan S, et al. Effects of dynamic bilevel positive airway pressure support on central sleep apnea in men with heart failure. Chest. 2008;134:61–6. doi: 10.1378/chest.07-1620. [DOI] [PubMed] [Google Scholar]
- 21.Randerath WJ, Galetke W, Stieglitz S, et al. Adaptive servo-ventilation in patients with coexisting obstructive sleep apnoea/hypopnoea and Cheyne-Stokes respiration. Sleep Med. 2008;9:823–30. doi: 10.1016/j.sleep.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Banno K, Okamura K, Kryger MH, et al. Adaptive servo-ventilation in patients with idiopathic Cheyne-Stokes breathing. J Clin Sleep Med. 2006;2:181–6. [PubMed] [Google Scholar]
- 23.Javaheri S, Goodwin J, Wylie P, et al. Complex central sleep apnea: Treatment with auto servo ventilation-abstract. Chest. 2009;136:43. [Google Scholar]
- 24.Iber C Ancoli-Israel S, Chesson AL, Quan SF, et al. Westchester, IL: The American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events rules, terminology and technical specifications. [Google Scholar]