Abstract
Study Objectives:
Major depressive disorder (MDD) is often associated with disturbances in circadian and/or sleep-wake dependent processes, which both regulate daytime energy and sleepiness levels.
Design:
Analysis of continuous electroencephalographic (EEG) recordings during 40 h of extended wakefulness under constant routine conditions. Artifact-free EEG samples derived from 12 locations were subjected to spectral analysis. Additionally, half-hourly ratings of subjective tension and sleepiness levels and salivary melatonin measurements were collected.
Setting:
Centre for Chronobiology, Psychiatric Hospitals of the University of Basel, Switzerland.
Participants:
Eight young healthy women and 8 young untreated women with MDD.
Interventions:
N/A.
Measurements and Results:
MDD women exhibited higher frontal low-frequency (FLA) EEG activity (0.5-5.0 Hz) during extended wakefulness than controls, particularly during the night. Enhanced FLA was paralleled by higher levels of subjective sleepiness and tension. In MDD women, overall FLA levels correlated positively with depression scores. The timing of melatonin onset did not significantly differ between the two groups, but the nocturnal secretion of salivary melatonin was significantly attenuated in MDD women.
Conclusions:
Our data imply that young women with MDD live on a higher homeostatic sleep pressure level, as indexed by enhanced FLA during wakefulness. Its positive correlation with depression scores indicates a possible functional relationship. High FLA could reflect a use-dependent phenomenon in depression (enhanced cognitive rumination or tension) and/or an attenuated circadian arousal signal.
Citation:
Birchler-Pedross A; Frey S; Chellappa SL; Götz T; Brunner P; Knoblauch V; Wirz-Justice A; Cajochen C. Higher frontal EEG synchronization in young women with major depression: a marker for increased homeostatic sleep pressure? SLEEP 2011;34(12):1699-1706.
Keywords: Major depressive disorder, women, frontal low-frequency EEG activity, subjective sleepiness, extended wakefulness, circadian rhythms, sleep-wake homeostat
INTRODUCTION
Major depressive disorder (MDD) is often associated with a dysregulation in circadian rhythmicity and/or sleep regulation. Abnormal circadian rhythms in many variables have been reported over the years, ranging from core body temperature, neurotransmitters, and hormones to physiology of the sleep-wake cycle itself.1,2 Characteristics of the circadian system (amplitude, phase, and/or endogenous period) can be measured under very stringent laboratory conditions using markers such as core body temperature or melatonin. Although both delayed and advanced phases have been found in patients with MDD, several studies using highly controlled protocols such as the constant routine3,4 or forced desynchrony5,6 could not confirm circadian phase changes in MDD. However, reduced circadian amplitude seems to be generally present.2,4,7,8
The process S deficiency hypothesis postulates a deficiency in the homeostatic build-up of sleep pressure during wakefulness in MDD, leading to a shallower dissipation rate of process S during sleep.4,9–12 Changes in homeostatic sleep regulation can be quantified by spectral EEG correlates in the low-frequency range (1-7 Hz) during sleep and wakefulness.13–19 The intensity of low-frequency EEG activity at the beginning of sleep is proportional to the duration of prior wakefulness, and is considered to reflect the homeostatic aspect of sleep regulation.20–22 During sustained wakefulness, EEG activity in the 1-7 Hz range increases and can predict the subsequent homeostatic increase in slow wave activity (SWA, EEG power density 0.75-4.5 Hz) during sleep,16,23,24 a phenomenon that is particularly pronounced in frontal brain regions.23,24 This increased propensity in frontal low-frequency EEG activity (FLA) during sustained wakefulness suggests that frontal regions are more susceptible to sleep deprivation effects than other cortical regions.23,25
The process S deficiency hypothesis for MDD has rarely been tested in either sleep or waking EEG. An early study found lower delta waves during sleep in depressed patients,10 which was later documented only in males with MDD.9 In untreated middle-aged depressives, there was no difference from controls in SWA during sleep.26 Similarly, EEG studies during wakefulness in depression are contradictory and inconclusive.27
It is surprising that this has not received more attention, since waking EEG-derived indices are a desirable biological measure in psychiatric disorders, given its practicability, low budget, and possibility of a greater number of recording sites.27 Thus, here we aimed at investigating sleep-homeostat and circadian-related differences in the EEG during extended wakefulness in MDD and healthy women under very stringently controlled laboratory conditions.
Our main hypotheses were as follows:
Women with MDD undergo a deregulation of sleep-wake homeostasis in comparison to healthy women, as indexed by an altered time course in EEG power density in the 1-5 Hz range during 40 h of extended wakefulness, particularly in frontal derivations, which are more susceptible to the effects of prolonged wakefulness.
Based on these alterations in sleep-wake homeostasis, which can affect subjective parameters, women with MDD experience higher subjective sleepiness and tension levels during 40 h extended wakefulness.
Women with MDD show attenuated amplitude and/or circadian phase advance or delay in the rhythm of melatonin secretion.
METHODS
Study Participants
All study participants were recruited via advertisements at different Swiss universities and on online job advertisement pages for students. A total of 900 candidates were enrolled as potential participants, and all completed a general questionnaire on their health status, medication, and shift work, as well as a Beck Depression Inventory (BDI), Pittsburgh Sleep Quality Index (PSQI), and Chronotype questionnaires. Of these candidates, 80 young women were interviewed (SCID-I), and 25 volunteers were selected for study participation. Of these 25, 16 young women (mean age 24 ± 4.8y [SD]) participated in the study. Most of the potential participants were excluded since they had evening chronotype or high PSQI (> 8). All women were experiencing an episode of a MDD when undertaking the study protocol and fulfilled the diagnostic criteria of MDD according to the DSM-IV-TR. The main reason to include only women was based on the greater prevalence rate of MDD (without comorbidity), in women than men.
Since all of our depressed women were rather young, they did not have a long history of depression. The depressed participants experienced either the first or the second onset episode; none of them had been given psychiatric (including psychotropic drugs) treatment before the study. The episode duration was ≥ 2 weeks, according to DSM-IV-TR criteria (prior mean duration 11.18 ± 7.6 months). They had no atypical symptoms, did not experience severe sleep problems, as measured by the PSQI (PSQI ≤ 8; mean PSQI 6.5 ± 1.6),28 and did not exhibit any comorbid psychiatric DSM-IV-TR-disorder. Each participant underwent a clinical interview, which was performed by the same clinical psychologist (ABP). This interview comprised the structured clinical interview for DSM-IV Axis I Diagnoses of existing symptoms (SCID-I; mean: 5.15 ± 0.37 SD),29 the Hamilton-17 scale, the structured interview guide for the Hamilton depression rating scale with atypical depression supplement (SIGH-ADS; mean for HAMD-17: 12.29 ± 2.49 SD),30,31 the Montgomery-Åsberg Depression Scale (MADRS; mean: 16.71 ± 2.13 SD),32 and the Beck Depression Inventory (BDI; mean value 21.29 ± 6.84 SD).33 Half of the 16 MDD women were allocated to a high sleep pressure protocol (i.e., 40 h of extended wakefulness under constant routine conditions), while the other half participated in a low sleep pressure protocol (to be reported elsewhere).
The control sample comprised 8 healthy young women (age range: 20-31 years; mean age 25 ± 3.3 years, without any sleep problems [mean PSQI 2 ± 1.63 SD]).34,35 All study volunteers underwent a physical examination as well as an interview about sleep quality, life habits, and health state. They were free of any medication intake or treatment (except oral contraceptives) for ≥ 2 months; they had no neurological or sleep disorders. Sleep efficiency did not differ in the 2 groups (P = 0.54) measured by wrist actigraphy (mean value for MDD 88.67 ± 4.3 SD; for healthy 92.41 ± 4.0 SD). Volunteers were included only if their clinical sleep EEG scoring had no pathological findings (apnea-hypopnea index [AHI] < 10/h; periodic leg movements [PLM] index < 10/h). To exclude chronotype-specific differences in circadian phase preference we selected only moderate chronotypes (morning-evening-type [M/E] questionnaire rating between 14 and 21 points).36 Thus, chronotype was not significantly different between the 2 groups (controls 15.6 ± 3.6 vs. depressive 16.1 ± 1.3), nor was the body mass index (BMI, 21.2 ± 2.5 for the depressive and 20.9 ± 1.4 for the healthy volunteers). All participants were nonsmokers and without any drug abuse, as verified by urinary toxicological analysis sensitive for amphetamines, benzodiazepines, opiates, and tetrahydrocannabinol (Drug-Screen Card Multi-6, von Minden GmbH, Moers, Germany). Participants were also required to abstain from excessive caffeine and alcohol consumption and heavy physical exercise. They indicated ≤ 3 cups of caffeinated beverages per day and ≤ 10 glasses of alcohol per week. Other exclusion criteria were: shift work within 3 months and transmeridian flights within 1 month prior to the study. All women (from both of the depressed and healthy controls) started the study on days 1–5 after menses onset in order to complete the entire study block within the follicular phase. Three women with MDD and 5 control women used oral contraceptives. Thus, our study group included 8 young women with MDD and 8 healthy controls. While this sample seems rather low, the study was carried out under very controlled laboratory conditions of a constant routine (CR). In addition, prior to the study, participants were required to adhere to a regular sleep-wake cycle as verified by actigraphy and sleep logs, and all spent an adaption night in the laboratory. Thus, the procedure significantly reduced variability in the output measures.
All procedures conformed to the Declaration of Helsinki. The local ethics committee approved the study protocol, screening questionnaires and consent form,37,38 and all study participants gave signed informed consent.
Study Design
Each participant was instructed to maintain a regular sleep-wake cycle (bed- and wake-times within 30 min of self-selected target time), verified by wrist activity monitors (Cambridge Neurotechnology, UK) and sleep logs for one week prior to the “in laboratory” part of the study. The entire study design entailed 2 protocols, one for high sleep pressure conditions and one for low sleep pressure conditions, with 8 controls and 8 depressive volunteers in each protocol. Participants were assigned randomly to either the low or high sleep pressure protocol. The treatment order (“sleep deprivation” vs. “nap protocol”) was counterbalanced in order to avoid possible order effects. Here we focus only on the high sleep pressure protocol, which comprised an 8-h full polysomnography night in the laboratory, followed by 3.5 consecutive days in the laboratory. During day 1, participants adjusted to the experimental dim light condition (< 8 lux). After a second 8-h sleep episode, all volunteers participated in a 40-h sleep deprivation protocol under controlled conditions (constant routine),23,24,9,40 followed by a recovery night. The timing of the 8-h sleep episode was calculated with respect to the midpoint of each individual's habitual sleep episode, as assessed by actigraphy and sleep logs during the baseline week. All wake episodes were spent under semi-recumbent constant routine conditions (< 8 lux) during wakefulness, with a minor shift to supine posture during scheduled sleep episodes (0 lux).24
EEG Recording, Subjective Ratings, and Melatonin during Wakefulness
The Karolinska Drowsiness Test (KDT)41,42 was performed every hour during scheduled wakefulness, starting 1 h after habitual wake time. During the KDT, volunteers were instructed to relax, to keep their eyes open, and to avoid movement for 3 min, during which they had to fixate on a 5-cm dot attached to the wall at 1.5 m distance. These instructions were intended to maximize signal quality. Waking EEG activity was recorded continuously during the 40 h of extended wakefulness, using the Vitaport Ambulatory system (Vitaport-3 digital recorder TEMEC Instruments BV, Kerkrade, the Netherlands). Twelve EEG derivations (F3, F4, Fz, C3, C4, Cz, P3, P4, Pz, O1, O2, Oz referenced against linked mastoids), 2 electrooculograms, one submental electromyogram, and one electrocardiogram were recorded. All EEG signals were filtered at 30 Hz (fourth-order Bessel-type antialiasing low-pass filter, total 24 dB/Oct), and a time constant of 1.0 second was used prior to online digitization (range 610 μV, 12 bit AD converter, 0.15 μV/bit; storage sampling rate at 128 Hz). The raw signals were stored online on a Flash RAM Card (Viking, Rancho Santa Margarita, CA, USA) and downloaded offline to a PC hard drive. EEGs were subjected to spectral analysis using a fast Fourier transform (10% cosine 2-sec window), which resulted in a 0.5-Hz resolution. The 3-min EEGs during the KDT were manually and visually scored for artifacts (eye blinks, body movements, and slow eye movements) offline. Approximately 80% of the waking EEG data was used after rejecting for epochs with artifacts. The absolute EEG power densities were then calculated for artifact-free 2-s epochs in the frequency range of 0.5 to 20 Hz. For data reduction, artifact-free 2-s epochs were averaged over 20-s epochs.24
Subjective sleepiness was assessed every 30 min on the Karolinska Sleepiness Scale (KSS).42 Subjective tension was assessed by a 100-mm bipolar VAS at 30-min intervals. The participants were asked to indicate how they felt “at the moment” by placing a vertical mark on the VAS ranging from 0 (“worst ever”) to 100 mm (“best ever”). A similar VAS rating for mood was also made.43
Salivary collections for hormonal assays were scheduled during wakefulness at the same 30-min intervals as the subjective ratings. A direct double-antibody radioimmunoassay was used for the melatonin assay (validated by gas chromatography-mass spectroscopy with an analytical least detectable dose of 0.65 pm/mL; Bühlmann Laboratories, Schonenbuch, Switzerland).44 The functional least-detectable dose using the less than 20% coefficient of interassay variation criterion was < 0.65 pg/mL, and individual serum and saliva melatonin profiles showed excellent parallelism (r = 0.977-0.999; slopes = 0.21-0.63).44
Statistics
For all analyses, the statistical packages SAS (SAS Institute Inc., Cary, NC, USA; Version 6.12) and Statistica (Stat-Soft Inc., 2000-2004, Statistica for Windows, Tulsa, OK, USA) were used. Repeated measure analyses of variance (rANOVAs) were performed with the between factor “group” (depressive vs. control). We also considered the within factor “derivation” (EEG channels: F3, Fz, F4, C3, Cz, C4, P3, Pz, P4, O1, Oz, O2) and the within factor “time-of-day” (11 time points; the 3.75-h interval came about 150 min of wakefulness followed by 75 min of the corresponding scheduled sleep (nap). This duration allows starting the recovery night at the same clock time [circadian phase] as the baseline night, since it replaced the last scheduled nap). These 3 factors (“group,” “derivation,” and “time-of-day”) were performed for each 0.5-Hz frequency bin separately in the range of 1-20 Hz. Since we did not observe consistent left-right changes in MDD women vs. control women, frontal (F3, Fz, F4), central (C3, Cz, C4), parietal (P3, Pz, P4), and occipital derivations (O1, Oz, O2) were collapsed per subject into a single frontal derivation (average [F3, Fz, F4]), a single central (average [C3, Cz, C4]), a single parietal (average [P3, Pz, P4]), and a single occipital derivation (average [O1, Oz, O2]). Frequency bins yielding significance for the interaction “group × derivation” were collapsed into frequency bands, averaged per 3.75-h bin per study volunteer, and subjected to rANOVA with the factors ”group,” “derivation,” and “time-of-day.” Similarly, the 30-min subjective ratings and melatonin values were collapsed into 3.75-h time bins resulting in 11 time points and subjected to rANOVAs with the factors mentioned above. All P-values derived from rANOVAs were based on Huynh-Feldt's (H-F) corrected degrees of freedom (significance level: P < 0.05). Alpha adjustment for multiple comparisons was applied according to Curran-Everett.45 Pearson correlation coefficients were computed to compare individual FLA levels with depressions scores derived from the MADRS and Hamilton 7-Item scale in MDD women.
RESULTS
EEG during Wakefulness
Absolute spectral EEG power density for each frequency bin, for each derivation, and for each derivation averaged over eleven 3.75-h time intervals yielded a significant “group” effect for the frequency bins between 1 and 2.5 Hz, a significant “derivation” effect for a broader frequency range 1-13 Hz, (F3,42 ≥ 3.3) and 14.2-20 Hz (F3,42 ≥ 4.7), and a significant interaction “group” × “derivation” effect between 0.5 and 4 Hz (F3,42 ≥ 2.9; P < 0.05; Figure 1). Similarly, when considering averaged derivations (frontal, central, parietal, occipital), a significant “group” effect was elicited between 0.5 and 2 Hz (F1,14 ≥ 4.6), and a significant (i.e., P < 0.05) “derivation” effect was elicited between 1-8 Hz (F3,42 ≥ 5.8), 9.5-12 Hz (F3,42 ≥ 6.4), and 16.5-20 Hz (F3,42 ≥ 4.0). Furthermore, the interaction term “group” × “derivation” yielded significant differences between 0.5 and 5 Hz (F1,3 ≥ 1.0). Thus, EEG power density in the 0.5- 5 Hz range was collapsed per subject in order to investigate the time course of low-frequency EEG activity in the course of the 40-h episode of extended wakefulness.
Figure 1.
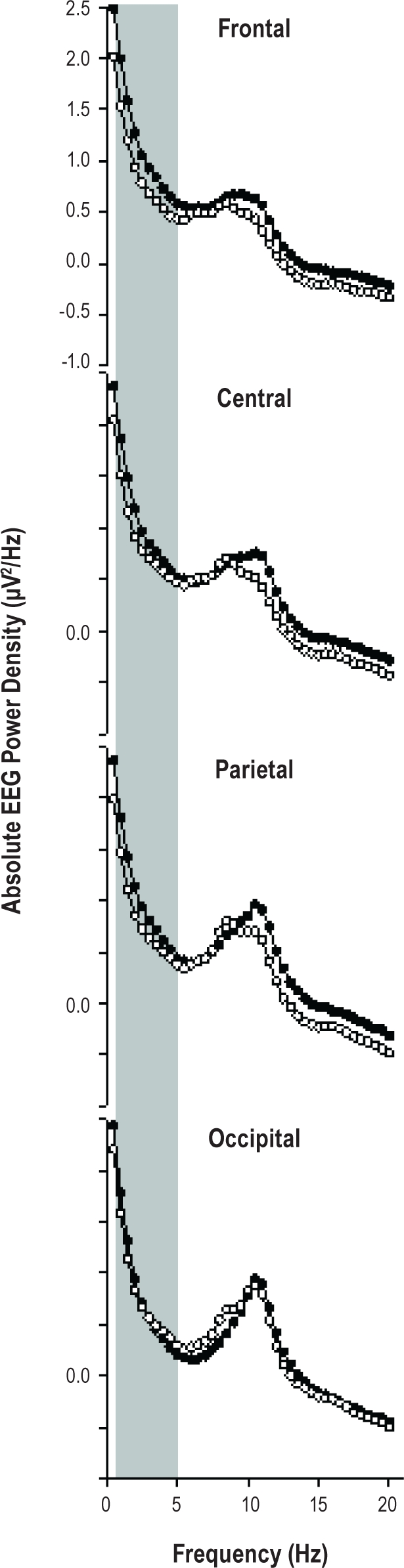
Absolute EEG power spectra during extended wakefulness along the antero-posterior axis (frontal, central, parietal, occipital). Women with MDD are indicated by closed dots and the control group by open symbols. Mean values are shown for each 0.5-Hz frequency bin in the range from 0.5 to 20 Hz. A significant “group” × “derivation” effect was observed between 0.5 and 5 Hz, and a significant group effect in the range of 0.5-2 Hz.
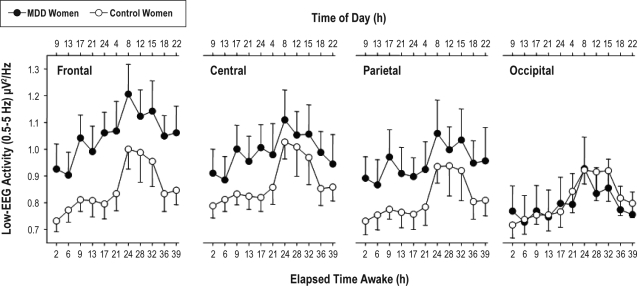
Overall, low-frequency EEG activity (0.5-5 Hz) showed a similar time course in both women with MDD and control women (Figure 2), with no significant differences in the interaction terms “group” × “time” and “group” × “time” × “derivation,” although the interaction “group” × “derivation” yielded significant differences (F3,42 = 2.9; P < 0.05), indicating a frontal predominance of the increase in low-frequency EEG activity in MDD women compared to control women (Figure 2).
Figure 2.
Dynamics of low-frequency EEG activity (EEG power density in the 0.5-5 Hz band) during 40-h of extended wakefulness. Data were binned into 3.75-h time intervals (mean values ± SEM, n = 8) and plotted against relative time of day (h). Relative clock time represents the average clock time at which the time intervals occurred. For statistics see text. Local derivations on waking-EEG are summarized as frontal derivation (mean of F3, F4, Fz) central derivation (mean C3, C4, Cz), parietal derivation (mean of P3, P4, Pz), and occipital derivation (mean of O1, O2, Oz).
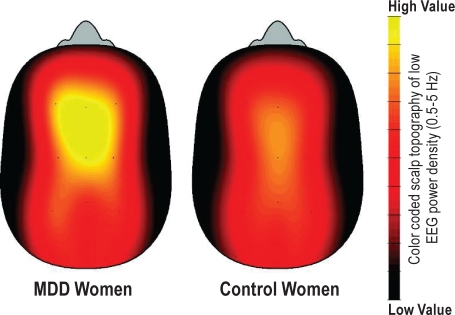
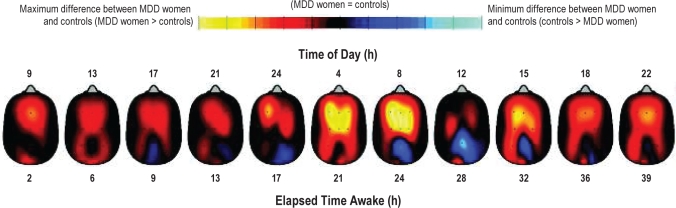
For enhanced visual illustration, a global cortical contour plot with the entire topography is provided in Figure 3 for EEG power density in the 0.5-5 Hz range. Average low-frequency EEG activity indicates higher values in frontal and central derivations in the MDD than control women. Visual inspection of the contour plot over time (Figure 4) shows that MDD women had particularly high low-frequency EEG activity during the subjective night and early morning, as well as at 15:00 on the second day of extended wakefulness.
Figure 3.
Color-coded scalp topography of low-frequency EEG power density in the 0.5-5 Hz range averaged over 40 h of extended wakefulness in women with MDD (left head, n = 8) and control women (right head, n = 8). High temperature (yellow) represents high low-frequency EEG power density values, while low temperature (red) represents low values. Note: higher low-frequency EEG activity in MDD than control women, particularly in frontal and central brain regions.
Figure 4.
Time course of low-frequency EEG activity (in the range of 0.5-5 Hz) during 40 h of extended wakefulness. These contour plots (i.e., heads) describe the difference between low-frequency EEG activity in the MDD and control women across 11 time points in the high sleep pressure protocol. MDD women showed higher low-frequency EEG activity, particularly during the subjective night and early morning, as well as at 15:00 during the second day. Maximum difference between MDD women and controls is shown in yellow (max: MDD women > controls) and the minimum difference between the 2 groups is shown in light blue (max: controls > MDD women).
Subjective Sleepiness
The time course of subjective sleepiness ratings for MDD and control women during the 40-h of extended wakefulness are illustrated in Figure 5 (upper panel). The factor “group” yielded a tendency for higher sleepiness levels in MDD women (F1,15 = 3.6; P = 0.07), and significance for the factor “time-of-day” (F10,150 = 17.1; P < 0.001), the latter showing the expected circadian and wake-dependent modulation of sleepiness in both groups. Certain time points (from 17 h, 21 h, and 24 h elapsed time into protocol) during the biological night yielded significant higher sleepiness levels in the MDD than in the control women (F1,15 = 6.8; P < 0.02).
Figure 5.
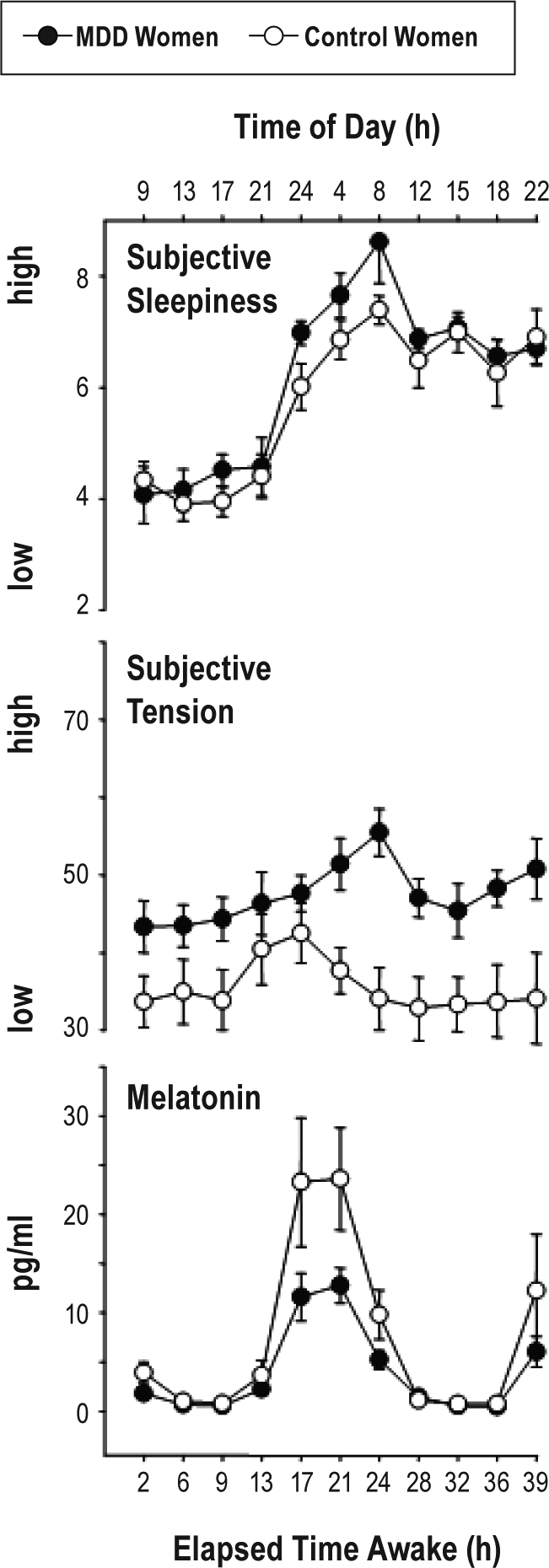
Time course of subjective sleepiness (Karolinska Sleepiness Scale, first panel), subjective tension (VAS, second panel), and salivary melatonin levels (third panel) across the 40 h of extended wakefulness. Women with MDD are plotted in closed dots, the healthy controls with open symbols (mean values ± SEM, n = 8).
Subjective Tension
MDD women indicated significantly higher levels of subjective tension only after 20 h to 40 h of wakefulness compared to the control women (Figure 5, middle panel). Thus, the factor “group” yielded significance (F1,15 = 12.9; P < 0.03), although no differences were observed for the factor “time-of-day” (F10,150 = 0.81; n.s.) and the interaction “group” × “time-of-day.” However, certain time points from 17 h, 21 h, 24 h, and 28 h elapsed time into protocol during the biological night and next morning where significantly higher in the MDD women than in the control women (F1,15 = 18.75; P = 0.0005).
Melatonin
The time course of salivary melatonin levels is illustrated in Figure 5 (lower panel). The factor “group” yielded a tendency for lower melatonin levels in MDD than control women (F1,15 = 3.7; P = 0.06). The factor “time-of-day” yielded significance (F10,150 = 16.2; P < 0.001), and the interaction term “group” × “time-of-day” almost reached significance (F10,150 = 1.9; P = 0.05), indicating an attenuation of nocturnal salivary melatonin secretion in the MDD women.
FLA and Depression Scores
Pearson correlations revealed positive and significant correlations between FLA levels during extended wakefulness and depression baseline severity, derived from both the Hamilton-7 Items (r = 0.75; P < 0.04) and MADRS (Figure 6; r = 0.8; P < 0.02).
Figure 6.
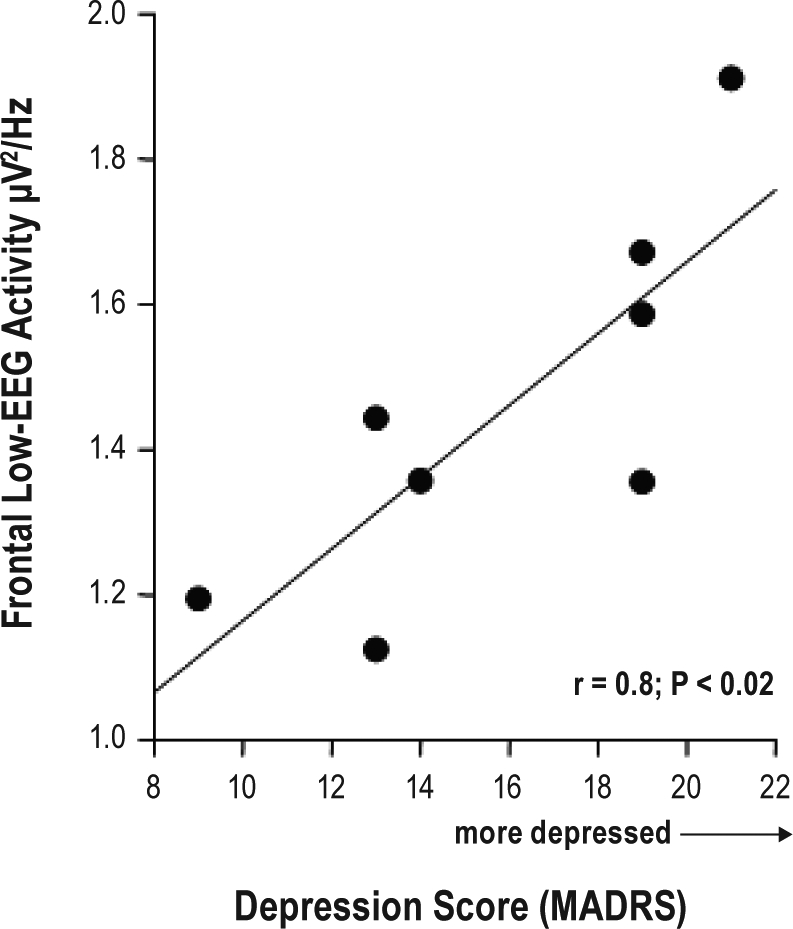
Linear regression between the values of the depression score (MADRS; 8-20) on the x-axis and the value of FLA (y-axis) across the 40 h of extended wakefulness (r = 0.8; P < 0.02).
DISCUSSION
Overall FLA during extended wakefulness was higher in MDD than in healthy control women and correlated positively and significantly with depression severity. The time course of enhanced FLA was paralleled by higher subjective sleepiness and tension levels in MDD than in healthy control women. MDD women did not differ from controls in circadian melatonin phase, but showed a significant attenuation of melatonin secretion during the biological night.
These are the first data in MDD addressing the waking aspect of the S deficiency hypothesis postulated by Borbély and Wirz-Justice.11 Surprisingly, unmedicated young women with MDD, a middle chronotype, and almost no sleep disturbances seem to live on a higher—not the hypothesized lower—homeostatic sleep pressure, but with similar build-up rates during extended wakefulness as found for healthy controls. The sleep aspect of the S-deficiency hypothesis was also studied in this same cohort (to be reported elsewhere),46 and they indeed showed elevated—not diminished—SWA levels during sleep. To our knowledge, there is only one early study that reported elevated SWA during wakefulness in right frontal brain regions in a mixed-gender group of MDD patients with a comparatively brief medication washout period.47 Together with the significant correlation with the MADRS and Hamilton depression scores, elevated FLA levels in our MDD group women most likely reflect certain aspects of the disorder per se, and do not seem to be primarily caused by a sleep disorder or a major circadian misalignment. Thus, we speculate that the elevated FLA levels during wakefulness in an episode of major depression were a state rather than a trait marker in our MDD cohort. Support for this assumption also comes also from elevated SWA levels during sleep in the same MDD women.46 Our study sample comprised women with MDD who are unmedicated and without sleep disturbances. While this may not be a representative sample of patients with major depression, it should be emphasized that MDD itself is a heterogeneous group with symptoms that crucially depend on numerous aspects, such as duration of disorder. Most importantly, we could show that even in patients with MDD without medication and sleep disorders the homeostatic sleep regulation is significantly changed. In this context, one may speculate that changes in sleep homeostasis may anticipate and/or trigger severe MDD episode, especially when considering that our sample included women with mild episodes of MDD. Similarly, FLA could also be seen as a compensatory mechanism and/or reaction to increased depressive levels, as indexed by an “over response” of the homeostatic sleep process when challenged by 40 h of sleep deprivation.
Interestingly, there is more recent work, running in a similar direction, showing that suppressing low-frequency activity in the EEG during sleep in MDD patients leads to mood improvements in those patients.48 On the other hand, enhancing low-frequency activity during sleep marginally decreased positive mood in patients with MDD.49 These two studies lend support to our finding that the amount of FLA is significantly related to depression severity.
The topographic specificity of the increase in low-frequency activity—mainly frontal derivations—are an indication that women with MDD may be more susceptible to homeostatic sleep pressure, in particular the effects of prolonged wakefulness, possibly due to a high “recovery need” of frontal heteromodal association areas of the cortex, which are strongly affected by elevated sleep pressure as shown in PET studies.50 Alternatively, our results of higher FLA could represent a biological correlate of higher cognitive rumination in MDD patients, known as “brooding,” which is a core process in the onset and maintenance of depression.51,52 Depressive rumination could be seen as a state of higher arousal during wakefulness, which could have resulted in elevated subjective tension. If slow wave homeostasis is associated with net synaptic strength, postulated by Tononi et al. to increase during wakefulness and decrease during sleep,53 then higher cognitive rumination should lead to higher FLA during wakefulness, and FLA could be a proxy of more intense upscaling of synaptic strength in MDD. Thus, one could speculate that our subjects with MDD had an altered regulation of synaptic plasticity. Results from a recent study indeed support the hypothesis of decreased synaptic plasticity in patients with MDD.54
Enhanced FLA levels affect the circadian timing system. There is recent evidence for a crosstalk between systems regulating sleep-wake homeostasis and endogenous circadian rhythmicity in the hypothalamus.55 High sleep pressure levels considerably suppressed activity in the anterior hypothalamus, including the suprachiasmatic area, the brain site of the central circadian pacemaker.55 Thus, the observed decrease of circadian melatonin secretion in our MDD cohort could reflect an attenuation of the circadian wake-promoting signal by increased homeostatic sleep pressure. This finding is consistent with previous forced desynchrony studies in seasonal affective disorder (SAD) patients5,6 and non-circadian protocols with depressed patients.48,49,53
It may be that low nocturnal melatonin levels and indeed low amplitude in many other variables5,6,47,56,59 are a reflection of diminished circadian signal in MDD. Lack of an adequate wakefulness signal may permit the expression of increased FLA and sleepiness in these patients. On the other hand, one could also argue that, since our results on the circadian system focus only on melatonin as a circadian marker, that the attenuated profile of melatonin in MDD could also reflect impaired nocturnal melatonin secretion. Taken together, these findings provide a possible explanation as to why bright light exposure, which increases the amplitude of the circadian system, may improve depressive symptoms in MDD.60
Limitations
Measuring behavior under highly controlled laboratory conditions is pivotal to assess the contributions of circadian and homeostatic processes to the EEG during wakefulness. Our sample comprised young, unmedicated, depressed women, and excluded those with sleep disturbances; thus, our findings are not representative for all patients suffering MDD. Likewise, the directionality of the results (MDD prior to changes in sleep homeostasis and vice-versa) cannot be predicted by this study design, since a cross-sectional design and a constant routine protocol cannot provide a causal effect for these results. However, since the only difference between the depressive and control women was the depression per se, we could ideally test whether the observed changes are related to circadian and/or sleep homeostatic alterations.
CONCLUSIONS
The concomitant findings of higher FLA, subjective sleepiness, tension, and attenuated melatonin levels in untreated young women with MDD under stringent laboratory conditions indicate that major depression per se is associated with impaired nighttime melatonin secretion and altered sleep-wake-homoeostatic processes.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Wirz-Justice has participated in speaking engagements for Servier. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Claudia Renz, Marie-France Dattler, Giovanni Balestrieri, and the student workers for their help in data acquisition. The authors also thank all the study volunteers for their participation in our study, Marcel Hofstetter for developing the software for the EEG topographical mapping, and Dr. Antoine Viola for statistical advice.
This research was supported by Swiss National Science Foundation Grants START # 3100-055385.98, and 3130-0544991.98 and 320000-108108 to Dr. Cajochen, the Velux Foundation (Switzerland), the Daimler-Benz Foundation (Germany) and Bühlmann Laboratories, Allschwil (Switzerland).
This study was conducted at Centre for Chronobiology, Psychiatric Hospital of the University of Basel, Wilhelm Kleinstrasse 27, CH-4012 Basel, Switzerland.
REFERENCES
- 1.Boivin DB. Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. J Psychiatry Neurosci. 2000;25:446–58. [PMC free article] [PubMed] [Google Scholar]
- 2.Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23:571–85. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buysse DJ, Monk TH, Kupfer DJ, Frank E, Stapf D. Circadian patterns of unintended sleep episodes during a constant routine in remitted depressed patients. J Psychiatr Res. 1995;29:407–16. doi: 10.1016/0022-3956(95)00021-v. [DOI] [PubMed] [Google Scholar]
- 4.Wirz-Justice A. Biological rhythms in mood disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. New York: Raven Press; 1995. pp. 999–1017. [Google Scholar]
- 5.Koorengevel KM, Beersma DG, den Boer JA, van den Hoofdakker RH. A forced desynchrony study of circadian pacemaker characteristics in seasonal affective disorder. J Biol Rhythms. 2002;17:463–75. doi: 10.1177/074873002237140. [DOI] [PubMed] [Google Scholar]
- 6.Koorengevel KM, Beersma DG, den Boer JA, van den Hoofdakker RH. Mood regulation in seasonal affective disorder patients and healthy controls studied in forced desynchrony. Psychiatry Res. 2003;117:57–74. doi: 10.1016/s0165-1781(02)00305-0. [DOI] [PubMed] [Google Scholar]
- 7.Souêtre E, Salvati E, Belugou JL, et al. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28:263–78. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 8.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl. 2007:104–15. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 9.Armitage R, Hoffmann R, Trivedi M, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000;95:201–13. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 10.Kupfer DJ, Ulrich RF, Coble PA, et al. Application of automated REM and slow wave sleep analysis: II. Testing the assumptions of the two-process model of sleep regulation in normal and depressed subjects. Psychiatry Res. 1984;13:335–43. doi: 10.1016/0165-1781(84)90081-7. [DOI] [PubMed] [Google Scholar]
- 11.Borbély AA, Wirz-Justice A. Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Hum Neurobiol. 1982;1:205–10. [PubMed] [Google Scholar]
- 12.Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21(Suppl 1):S11–5. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- 13.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: evidence for frequency-specific circadian and homeostatic influences. Neurosci Lett. 1997;239:121–4. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- 14.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. Am J Physiol. 1999;277:R1771–9. doi: 10.1152/ajpregu.1999.277.6.R1771. [DOI] [PubMed] [Google Scholar]
- 15.Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience. 2002;114:1047–60. doi: 10.1016/s0306-4522(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 16.Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–9. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 17.Makeig S, Jung TP, Sejnowski TJ. Awareness during drowsiness: dynamics and electrophysiological correlates. Can J Exp Psychol. 2000;54:266–73. doi: 10.1037/h0087346. [DOI] [PubMed] [Google Scholar]
- 18.Torsvall L, Åkerstedt T. Sleepiness on the job: continuously measured EEG changes in train drivers. Electroencephalogr Clin Neurophysiol. 1987;66:502–11. doi: 10.1016/0013-4694(87)90096-4. [DOI] [PubMed] [Google Scholar]
- 19.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–68. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 20.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 21.Daan S, Beersma DG, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–83. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 22.Tobler I, Borbély AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–6. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 23.Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 24.Cajochen C, Knoblauch V, Kräuchi K, Renz C, Wirz-Justice A. Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. Neuroreport. 2001;12:2277–81. doi: 10.1097/00001756-200107200-00046. [DOI] [PubMed] [Google Scholar]
- 25.Horne JA. Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993;162:413–9. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 26.Landolt HP, Gillin JC. Similar sleep EEG topography in middle-aged depressed patients and healthy controls. Sleep. 2005;28:239–47. doi: 10.1093/sleep/28.2.239. [DOI] [PubMed] [Google Scholar]
- 27.Pollock V, Schneider L. Quantitative waking EEG in depression. Biol Psychiatry. 1991;27:757–80. doi: 10.1016/0006-3223(90)90591-o. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M. Strukturiertes Klinisches Interview für DSM-IV (SKID) Göttingen: Beltz-Test; 1996. [Google Scholar]
- 30.Ramos-Brieva J, Cordero-Villafafila A. A new validation of the Hamilton Rating Scale for Depression. J Psychiatr Res. 1988;22:21–8. doi: 10.1016/0022-3956(88)90024-6. [DOI] [PubMed] [Google Scholar]
- 31.Janet BW, Williams DSW, Terman M. Structured interview guide for the Hamilton Depression Rating Scale with atypical depression supplement (SIGH-ADS) 2003 [Google Scholar]
- 32.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 33.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 34.Knoblauch V, Martens WL, Wirz-Justice A, Cajochen C. Human sleep spindle characteristics after sleep deprivation. Clin Neurophysiol. 2003;114:2258–67. doi: 10.1016/s1388-2457(03)00238-4. [DOI] [PubMed] [Google Scholar]
- 35.Birchler-Pedross A, Schröder CM, Munch M, et al. Subjective well-being is modulated by circadian phase, sleep pressure, age, and gender. J Biol Rhythms. 2009;24:232–42. doi: 10.1177/0748730409335546. [DOI] [PubMed] [Google Scholar]
- 36.Torsvall L, Åkerstedt T. A diurnal type scale. Construction, consistency and validation in shift work. Scand J Work Environ Health. 1980;6:283–90. doi: 10.5271/sjweh.2608. [DOI] [PubMed] [Google Scholar]
- 37.Münch M, Knoblauch V, Blatter K, et al. The frontal predominance in human EEG delta activity after sleep loss decreases with age. Eur J Neurosci. 2004;20:1402–10. doi: 10.1111/j.1460-9568.2004.03580.x. [DOI] [PubMed] [Google Scholar]
- 38.Münch M, Knoblauch V, Blatter K, Wirz-Justice A, Cajochen C. Is homeostatic sleep regulation under low sleep pressure modified by age? Sleep. 2007;30:781–92. doi: 10.1093/sleep/30.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knoblauch V, Münch M, Blatter K, et al. Age-related changes in the circadian modulation of sleep-spindle frequency during nap sleep. Sleep. 2005;28:1093–101. doi: 10.1093/sleep/28.9.1093. [DOI] [PubMed] [Google Scholar]
- 40.Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 41.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 42.Gillberg M, Kecklund G, Åkerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17:236–41. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 43.Birchler-Pedross A, Frey S, Knoblauch V, et al. Diurnal variations of mood in drug free unipolar depressed women under high and low sleep pressure conditions: is there a sleep deprivation effect ? Sleep Abstract Supplement. 2010;33:A98. [Google Scholar]
- 44.Weber J, Schwander JC, Unger I, Meier D. A direct ultrasensitive RIA for the determination of melatonin in human saliva: comparison with serum levels. J Sleep Res. 1997:757. [Google Scholar]
- 45.Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 46.Frey S, Birchler-Pedross A, Brunner P, Götz T, Knoblauch V, Cajochen C. Women with major depression live under higher homeostatic sleep pressure. J Sleep Res. 2010 doi: 10.3109/07420528.2012.656163. Abstract P441. [DOI] [PubMed] [Google Scholar]
- 47.Knott VJ, Lapierre YD. Computerized EEG correlates of depression and antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:213–21. doi: 10.1016/0278-5846(87)90063-7. [DOI] [PubMed] [Google Scholar]
- 48.Landsness EC, Goldstein MR, Peterson MJ, Tononi G, Benca RM. Selective slow wave deprivation as a possible acute treament of major depressive disorder. Sleep Abstract Supplement. 2010;33:A 234. [Google Scholar]
- 49.Cheng P, Goldschmied J, Casement M, et al. Slow wave sleep enhancement and positie mood in depressive and controls. Sleep Abstract Supplement. 2010;33:A 234. [Google Scholar]
- 50.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 51.Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100:569–82. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- 52.Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109:504–11. [PubMed] [Google Scholar]
- 53.Tononi G. Slow wave homeostasis and synaptic plasticity. J Clin Sleep Med. 2009;5:S16–9. [PMC free article] [PubMed] [Google Scholar]
- 54.Nissen C, Holz J, Blechert J, et al. Learning as a model for neural plasticity in major depression. Biol Psychiatry. 2010;68:544–52. doi: 10.1016/j.biopsych.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt C, Collette F, Leclercq Y, et al. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–9. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- 56.Wehr TA, Wirz-Justice A. Circadian rhythm mechanisms in affective illness and in antidepressant drug action. Pharmacopsychiatria. 1982;15:31–9. doi: 10.1055/s-2007-1019506. [DOI] [PubMed] [Google Scholar]
- 57.Lavie P. Sleep-wake as a biological rhythm. Annu Rev Psychol. 2001;52:277–303. doi: 10.1146/annurev.psych.52.1.277. [DOI] [PubMed] [Google Scholar]
- 58.Strogatz SH, Kronauer RE, Czeisler CA. Circadian pacemaker interferes with sleep onset at specific times each day: role in insomnia. Am J Physiol. 1987;253:R172–8. doi: 10.1152/ajpregu.1987.253.1.R172. [DOI] [PubMed] [Google Scholar]
- 59.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shirani A, St Louis EK. Illuminating rationale and uses for light therapy. J Clin Sleep Med. 2009;5:155–63. [PMC free article] [PubMed] [Google Scholar]