Electronic nicotine delivery devices (E-cigarettes) are marketed to deliver nicotine without tobacco toxicants and are sold in shopping malls and over the internet despite no published safety or efficacy data.1,2 These unregulated products consist of a battery, heater and cartridge containing a solution of nicotine, propylene glycol and other chemicals.3 Puffing activates the heater and the solution is vaporised and inhaled. Cartridges can be refilled using drops of solution sold in bottles labelled as containing over 500 mg nicotine, approximately 10 times the lethal dose. Some smokers emphasise the products’ potential as a cessation aid,1 while some public health advocates highlight possible health risks and uncertain effects.1,4 Because there are no published studies examining the products’ nicotine delivery or subjective and cardiovascular effect profile, this study examined how two brands of electronic nicotine delivery devices (E-cigarettes) influence plasma nicotine levels, heart rate and cigarette craving in cigarette smokers, and compared these effects to those produced by smokers’ usual brand of cigarettes.
METHODS
Using previously described methods,5 smokers in this institutional review board-approved clinical laboratory study (n = 16; all naïve to electronic nicotine delivery devices (E-cigarettes): 5 women; 8 non-white; mean age=29.8 years, SD=10.7; mean cigarettes/day=18.5, SD=2.2) each provided informed consent and participated in 4 Latin-square ordered conditions (each separated by 48 h) that differed by product: own brand cigarettes, sham smoking (puffing an unlit cigarette), ‘NPRO’ (NJOY, Scottsdale, Arizona, USA) with a 16 mg nicotine cartridge, or ‘Hydro’ (Crown Seven, Scottsdale, Arizona, USA) with a 16 mg nicotine cartridge. Cartridge flavour (menthol or regular) was chosen to match participant's preferred cigarette flavour. A new cartridge (within its expiration date) and a fully charged battery were used for each session. Conditions were preceded by >12 h tobacco/nicotine abstinence (verified with expired air carbon monoxide <10 ppm) and began with forearm vein catheter insertion and continuous heart rate recording followed by subjective measures and blood sampling. Participants were instructed to puff normally and then puffed ad libitum 10 times (30-s interpuff interval) from the product of the day (bout 1). At 5, 15, 30 and 45 min after the first puff, subjective measures were completed and blood sampled. At time +60 min assessments were repeated, product was administered (bout 2), and identical subsequent assessments completed. Plasma nicotine levels were assayed and heart rate data were averaged as reported previously.5 Data were analysed using a condition×bout×time within-subject analysis of variance; The Tukey honestly significant difference test was used to explore mean differences (p<0.05).
RESULTS
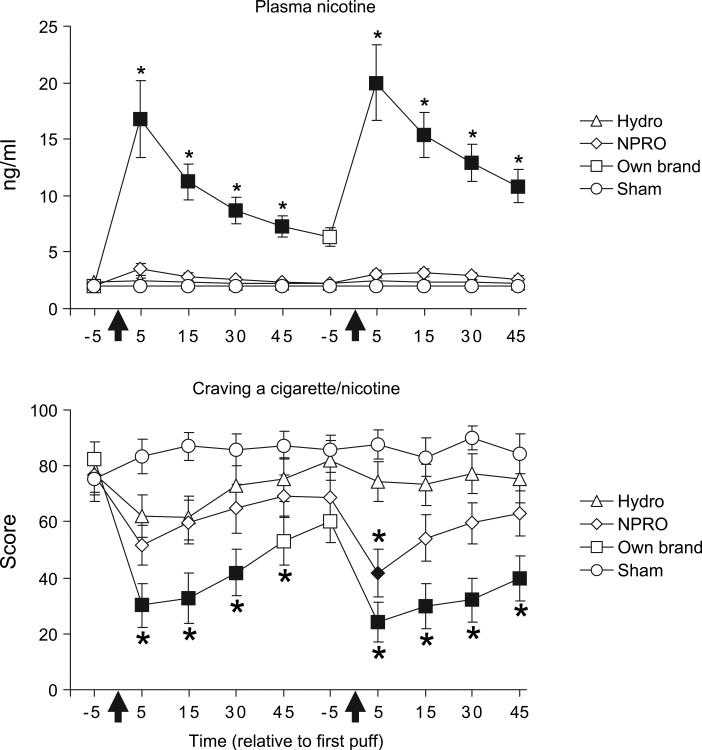
For plasma nicotine and ‘craving for a cigarette/nicotine’, significant condition×bout (Fs (3, 45)>12.4; p<0.001) and condition×time (Fs(12,180)>11.8; p<0.001) interactions were observed (figure 1). Relative to before bout 1, own brand cigarettes increased plasma nicotine and decreased craving significantly at most post-administration timepoints (ps<0.05). Hydro and NPRO failed to increase nicotine levels significantly and NPRO decreased craving significantly 5 min after bout 2 only (p<0.05). Mean plasma nicotine levels in the sham condition never were greater than 2.0. After bout 1, Own brand plasma nicotine level was significantly greater than either Hydro or NPRO at 5 (own mean=16.8 ng/ml, SEM=3.4; Hydro mean=2.5 ng/ml, SEM=0.2; NPRO mean=3.5 ng/ml, SEM=0.5), 15 (own mean=11.2 ng/ml, SEM=1.6; Hydro mean=2.3 ng/ml, SEM=0.2; NPRO mean=2.8 ng/ml, SEM=0.3) and 30 min (own mean=8.7 ng/ml, SEM=1.2; Hydro mean=2.2 ng/ml, SEM=0.1; NPRO mean=2.6 ng/ml, SEM=0.2), and also for bout 2 at 5 (own mean=20.0 ng/ml, SEM=3.3; Hydro mean=2.5 ng/ml, SEM=0.3; NPRO mean=3.0 ng/ml, SEM=0.3), 15 (own mean=15.4 ng/ml, SEM=2.0; Hydro mean=2.3 ng/ml, 0.2; NPRO mean=3.1 ng/ml, SEM=0.4) and 30 min (own mean=12.9 ng/ml, SEM=1.7; Hydro mean=2.3 ng/ml, SEM=0.1; NPRO mean=2.9 ng/ml, SEM=0.3; all ps<0.05). For heart rate, a significant condition×bout×time interaction was observed (F(12,180)=2.3; p<0.05). Relative to before bout 1, significant increases in heart rate were observed 5 and 15 min after bouts 1 and 2 for own brand only (ps<0.05).
Figure 1.
Mean (±1 SEM) plasma nicotine (top panel; assay's limit of quantitation=2 ng/ml) and response to a visual analogue scale item assessing ‘craving for a cigarette/nicotine’ (bottom panel; 0–100 scale) from 16 cigarette smoking participants who each abstained from tobacco/nicotine for at least 12 h before completing each of the study's 4 conditions. Arrows indicate timing of product administration (each administration consisted of 10 puffs with a 30-s interpuff interval). Filled symbols indicate a significant difference from the first assessment timepoint; asterisks (*) indicate a significant difference from sham smoking at each timepoint (ps<0.05; Tukey honestly significant difference test).
DISCUSSION
Relative to a tobacco cigarette, 10 puffs from either of these electronic nicotine delivery devices (E-cigarettes) with a 16 mg nicotine cartridge delivered little to no nicotine and suppressed craving less effectively (see Bullen et al).6 Importantly, these results were from two specific products tested under acute conditions in which puff number was controlled. Variability in product design may influence vapour content7 and chronic use and/or more intensive puffing (ie, more puffs, greater puff volume) may influence nicotine delivery. Given these and other factors, there is an ongoing need to evaluate electronic nicotine delivery devices (E-cigarettes). These evaluations should be conducted in a manner that takes into account variability in design (including cartridge nicotine content), examines the effects of user behaviour over time and compares these products to existing methods of delivering therapeutic nicotine safely and effectively. Taken together, the well known lethality of nicotine, variability in cartridge/vapour content,7 and the results reported here all support the notion that electronic nicotine delivery devices (E-cigarettes) and their nicotine-containing solution should be evaluated, regulated, labelled and packaged in a manner consistent with cartridge content and product effect. At the least, consumers should be aware that, unlike several regulated nicotine products (eg, gum,8 patch9), these putative drug delivery systems do not delivery nicotine effectively after acute administration.
Acknowledgements
The author acknowledges with gratitude the professional and swift work of the staff and students of VCU's Behavioral Pharmacology Laboratory (Ms Barbara Kilgalen, Ms Janet Austin and Ms Caroline Cobb) as well as the staff of VCU's Bioanalytical Core Laboratory Service Center. This work was supported by USPHS grants R01CA103827 and R01CA120142. The sponsor had no role in the design and conduct of the study; the collection, analysis and interpretation of the study; or in the preparation, review, or approval of the manuscript. The author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding US National Cancer Institute, US NIH.
Footnotes
Competing interests None.
Ethics approval This study was conducted with the approval of the Virginia Commonwealth University.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Zezima K. Cigarettes without smoke, or regulation. [23 June 2009];NY Times. 2009 http://www.nytimes.com/2009/06/02/us/02cigarette.html?_r=1.
- 2.World Health Organization . World Health Organization, Geneva. World Health Organization; Geneva: 2009. Technical report series: the scientific basis of tobacco product regulation: report of a WHO Study Group. [Google Scholar]
- 3.Laugesen M. Ruyan e-cigarette bench-top tests.. Poster presented to the joint conference of the Society for Research on Nicotine and Tobacco and Society for Research on Nicotine and Tobacco-Europe; April 30 2009. [Google Scholar]
- 4.World Health Organization Marketers of electronic cigarettes should halt unproved therapy claims. [23 June 2009];2008 http://www.who.int/mediacentre/news/releases/2008/pr34/en/index.html.
- 5.Cobb C, Weaver MF, Eissenberg T. Evaluating the acute effects of oral, non-combustible potential reduced exposure products marketed to smokers. Tob Control. 2009 doi: 10.1136/tc.2008.028993. Published Online first. doi:10.1136/tc.2008.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullen C, Glover M, Laugesen M, et al. Effect of an e-cigarette on craving and withdrawal, acceptability and nicotine delivery: randomized crossover trial.. Poster presented to the joint conference of the Society for Research on Nicotine and Tobacco and Society for Research on Nicotine and Tobacco-Europe; April 30 2009. [Google Scholar]
- 7.US Food and Drug Administration ‘Evaluation of e-cigarettes’. [15 Oct 2009];2009 http://www.fda.gov/downloads/Drugs/ScienceResearch/UCM173250.pdf.
- 8.Heishman SJ, Henningfield JE. Tolerance to repeated nicotine administration on performance, subjective, and physiological responses in nonsmokers. Psychopharmacology. 2000;152:321–33. doi: 10.1007/s002130000541. [DOI] [PubMed] [Google Scholar]
- 9.Kleykamp BA, Jennings JM, Sams C, et al. The influence of transdermal nicotine on tobacco/nicotine abstinence and the effects of a concurrently administered cigarette in women and men. Exp Clin Psychopharmacol. 2008;16:99–112. doi: 10.1037/1064-1297.16.2.99. [DOI] [PubMed] [Google Scholar]