Abstract
Background
Continuous glucose monitoring for patients with diabetes is of paramount importance to avoid severe health conditions resulting from hypoglycemia or hyperglycemia. Most available methods require an invasive setup and a health care professional. Handheld devices available on the market also require finger pricking for every measurement and do not provide continuous monitoring. Hence, continuous glucose monitoring from human tears using a glucose sensor embedded in a contact lens has been considered as a suitable option. However, the glucose concentration in human tears is very low in comparison with the blood glucose level (1/10–1/40 concentration). We propose a sensor that solves the sensitivity problem in a new way, is flexible, and is constructed onto the oxygen permeable contact lens material.
Methods
To achieve such sensitivity while maintaining a small sensor footprint suitable for placement in a contact lens, we increased the active electrode area by using three-dimensional (3-D) electrode micropatterning. Fully flexible 3-D electrodes were realized utilizing ordered arrays of pillars with different shapes and heights.
Results
We successfully fabricated square and cylindrical pillars with different height (50, 100, and 200 μm) and uniform metal coverage to realize sensor electrodes. The increased surface area produces high amperometric current that increases sensor sensitivity up to 300% using 200 μm tall square pillars. The sensitivity improvement closely follows the improvement in the surface area of the electrode.
Conclusions
The proposed flexible glucose sensors with 3-D microstructure electrodes are more sensitive to lower glucose concentrations and generate higher current signal than conventional glucose sensors.
Keywords: 3-dimensional electrodes, flexible electrodes, glucose sensor, pillar electrodes
Introduction
Different types of glucose sensors have been developed and can be classified by different aspects of the detection principle.1–6 However, the oldest electro-enzymatic detection principle, developed by Clark in the 1960s, is still of interest to many researchers because of its high selectivity to glucose. The stage of proof of concept for such a device has passed long ago, and currently, efforts are aimed at improving the performance of such electroenzymatic sensors.
Many efforts have been aimed at developing small and reliable sensors that would allow continuous glucose monitoring in patients with diabetes. Noninvasive or minimally invasive sensing techniques have been especially developed that offer an alternative to invasive blood sampling by a needle draw or a pinprick.7 Some alternative biofluids (such as urine, saliva, and tears) for noninvasive and continuous glucose monitoring have become popular.
The validity of using tear glucose measurements as an indicator of blood glucose levels is controversial. Some studies conclude that tear and blood glucose levels are poorly correlated,8 whereas other studies have found that there is a correlation.9,10 When considering reasons for the discrepancy, it has been suggested that the act of tear fluid sampling could introduce a false bias in the results and thus obscure possible correlation between blood and tear glucose concentrations. Indeed, there is evidence that the collection methods that irritate the conjunctiva can increase tear glucose significantly.10
To eliminate possible biasing of samples via collection methods, a contact lens has been suggested as an ideal vehicle for continuous tear glucose monitoring. Although a novel platform for sensing purposes, a contact lens is a long-proven concept that has been engineered to the point where irritation to the eye is minimal and insertion/ removal is a simple process. Despite the promising scenario of contact-lens-borne glucose sensing, practical applications have been slow to materialize.
Previously, a glucose-sensitive contact lens has been prepared by immobilizing two types of fluorescent indicators in the lens material as it is polymerized.11 Tear glucose level is read using an illumination/recording unit held in front of the eye. A similar system is based on embedding photonic crystals in a hydrogel patch, creating a holographic hydrogel.12 When the hydrogel is illuminated, the wavelength of the refracted light varies with the glucose concentration. It has been proposed that such a hydrogel material could be incorporated in a contact lens and the wearer could read out the glucose level by matching the color of the patch to a precalibrated scale. However, a drawback of this technique is that it is only qualitative and relies on the user to match the colors, and thus it is not very accurate.
Alternatively, preparing an electrochemical sensor on a flat plastic support, either by microfabrication methods13 or by screen-printing techniques,14 has been developed. Because such sensors are flexible, they can either be attached onto the eye directly13 or rolled up and inserted into the tear canal.14 This approach shows promise since both devices were demonstrated to have sufficient sensitivity for glucose detection by using tear fluids. The current response from the tear glucose level is, however, significantly lower than the blood glucose levels, and many times, the resulting low current was not easily discernible from the noise response of the sensor. Additionally, interfering species that are present in the tear fluid (such as ascorbic acid) also generate false signals in the same order of magnitude as tear glucose levels.
Sensitivity enhancement is especially important for a successful glucose measurement in tears. This is because typical tear glucose levels are of the order of 1.8–10.8 mg/dl, which is considerably lower than glucose concentration in serum (72–108 mg/dl).15 This article describes a novel method of increasing glucose sensor sensitivity employing three-dimensional (3-D) electrodes that have been directly integrated onto a flexible and gas-permeable contact lens material. The proposed polymer microelectromechanical-system-based fabrication process is practical because all electrodes are prepared using biocompatible materials. The sensor structure is highly flexible and moldable with a very high degree of repeatability and gas permeability. By immobilizing glucose oxidase (GOx) onto the 3-D electrodes, sensitivity can be greatly enhanced in comparison with immobilization of GOx on flat electrodes. This increased sensitivity could satisfy the low-level detection require-ment for glucose determination in human tear fluid.
Methods
Materials and Supplies
The gas-permeable contact lens material, poly dimethyl-siloxane (PDMS), is prepared using a SYLGARD® 184 elastomer kit (Dow Corning Corp., MI). Different formulations of SU-8 are purchased from Microchem Corp., MA. SU-8 is mainly used for preparing molds for the PDMS-based 3-D electrodes. Additionally, SU-8 is also used as a mechanical metal lift-off layer.16 Gold (Au) on chrome (Cr) is used as a working electrode material, and silver/silver chloride (Ag/AgCl) is used as a reference electrode material.
Glucose oxidase from Aspergillus niger (type VII), bovine serum albumin, D-glucose, and clear phosphate buffer solution (pH = 7.4) are purchased from Sigma-Aldrich, Ontario, Canada.
Sensor Design and Fabrication
Sensor Design
Electroenzymatic glucose sensors are the most popular glucose sensors because of their high selectivity to glucose and ease of operation. Glucose oxidase is the most popular enzyme for fabricating glucose sensors because it is easily available at a lower cost than other enzymes. The main reaction for such a glucose sensors is
| (1) |
| (2) |
| (3) |
The two electrons generated from Reaction (3) are collected by the working electrode and measured to evaluate the glucose concentration of the analyte solution.
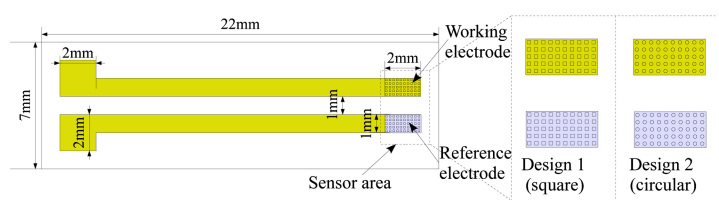
The proposed electroenzymatic glucose sensor with 3-D electrodes is completely designed on a flexible elastomer (PDMS; Figure 1), which is used as a contact lens material. For our experimental purpose, we designed the glucose sensors using a two-electrode system instead of the classical three-electrode system, because developments17 indicate that the two-electrode system performs similarly to the three-electrode system. In our design, the first electrode fabricated using Cr/Au is utilized as a working electrode, as well as an active electrode. The other electrode is fabricated using Ag/AgCl, which is a well-known reference electrode material. In our designs, we fabricate both electrodes with pillars to realize a 3-D topology for the electrode surfaces. Hence, improvement in the surface area is achieved for the same footprint of the sensor electrode. This improvement in the sensing area increases the sensitivity of the proposed glucose sensor. For our experiments, different shapes and geometrical height of the pillars were investigated. Circular and square cross sections of the pillars along with different heights (50, 100, and 200 μm) were selected for our experiments to evaluate the effect of the surface area.
Figure 1.
Experimental sensor design with metal connections. The glucose sensor (2 × 3 mm2) realized with Cr/Au working electrode (2 × 1 mm2) and Ag/AgCl reference electrode (2 × 1 mm2). Both electrodes are designed with an ordered array of 3-D pillars with a uniform metal coating.
As shown in Figure 1, the working electrode footprint in our design is 2 × 1 mm2. Hence, for flat electrodes (without 3-D pillar features), the working electrode surface area is 2 mm2. By incorporating 3-D pillar features, the surface area of the working electrode was increased according to the physical geometry and number of pillars. Different designs utilizing the pillar shape and height are listed in Table 1. For each square pillar, 100 μm length and 100 μm width was chosen. Similarly, for each cylindrical pillar, a 100 μm diameter was selected (see Figure 1 and Table 1). For 2 × 1 mm2 footprint, we fit 10 pillars lengthwise and 5 pillars widthwise. Based on the different heights (50, 100, and 200 μm) investigated here, different surface areas for the flexible glucose sensor were achieved (see Table 1). Exact surface area calculations for different pillar electrode options are shown in Table 1.
Table 1.
Design Details of Different Electrode Configurationsa
| Pillar array | Pillar geometry | |||||||
|---|---|---|---|---|---|---|---|---|
| # per length x # per width | Length (μm) | Width (μm) | Height (μm) | Increase in area per pillar (mm2) | Increase in area per electrode (mm2) | Final effective area (mm2) | Surface area with respect to flat electrode (%) | |
| Flat | 0 × 0 | 0 | 0 | 0 | 0.0000 | 0.00 | 2.00 | 100 |
| Square pillars | ||||||||
| Design 1S | 10 × 5 | 100 | 100 | 50 | 0.0200 | 1.00 | 3.00 | 150 |
| Design 2S | 10 × 5 | 100 | 100 | 100 | 0.0400 | 2.00 | 4.00 | 200 |
| Design 3S | 10 × 5 | 100 | 100 | 200 | 0.0800 | 4.00 | 6.00 | 300 |
| Cylindrical pillars | Diameter (μm) | |||||||
| Design 1C | 10 × 5 | 100 | 50 | 0.0157 | 0.79 | 2.78 | 139 | |
| Design 2C | 10 × 5 | 100 | 100 | 0.0314 | 1.57 | 3.57 | 179 | |
| Design 3C | 10 × 5 | 100 | 200 | 0.0628 | 3.14 | 5.14 | 257 | |
Different geometric variations tested during our study are listed in this table.
During our experiments, up to 300% increase in surface area for the same footprint was achieved using the pillar electrodes. The increased surface area of the working electrode is expected to increase the sensitivity of the glucose sensor. Hence, low concentrations of glucose from human tears can be measured more accurately by mounting such a sensor onto a contact lens. Additionally, the flexible 3-D glucose sensor is fabricated on a biocompatible PDMS material using an easy fabrication process flow (see Figure 2). Simple rectangular electrodes are used to prove the concept. However, in the future, it would not be difficult to shape them into a form compatible with a contact lens and mold them to match the curvature of the human eye.
Figure 2.
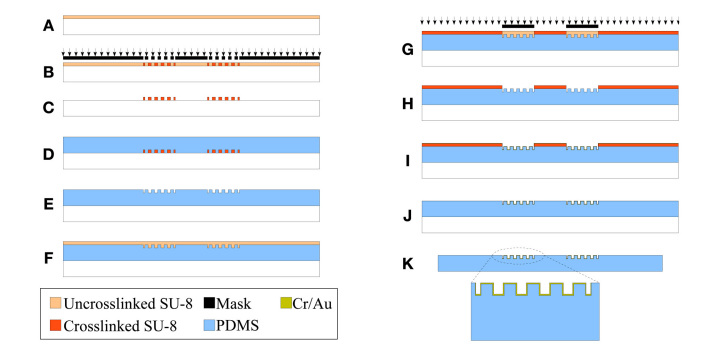
Complete fabrication process flow for flexible 3-D electrodes. (A) Spin the SU-8 layer onto a cleaned glass substrate for use as the soft lithography mold; (B) bake and expose the SU-8 layer with the desired mask pattern for the pillar array; (C) bake and develop the uncross-linked SU-8 to obtain the desired mold pattern; (D) after hard baking the mold, pour the PDMS mixture onto the mold and cure at an elevated temperature; (E) transfer the molded PDMS layer (with the pillar structures) onto another glass substrate; (F) spin the SU-8 layer for the metallization shadow mask; (G) bake and expose the SU-8 layer to pattern openings for the metal deposition; (H) bake and develop away the uncross-linked SU-8 to realize the SU-8 shadow mask; (I) deposit Au after Cr adhesion layer; (J) mechanically peel off the SU-8 shadow mask to realize the desired metal pattern; and (K) remove the PDMS-based glucose sensor from the glass substrate and electroplate the reference electrode with Ag followed by chloridation to realize the Ag/AgCl reference electrode.
Fabrication
To fabricate the flexible, 3-D glucose sensor electrode, we started with a clean glass substrate. To prepare a mold for the 3-D electrodes using PDMS, the SU-8 layer of the desired thickness (50, 100, or 200 μm thick) was spun (Figure 2A). The SU-8 layer was baked and exposed with the i-line ultraviolet source using the mask for the pillar electrode (Figure 2B). Subsequently, the exposed SU-8 layer is baked again and developed using the SU-8 developer to obtain the mold pattern for the 3-D pillar electrodes (Figure 2C). The SYLGARD 184 (PDMS) mixture is prepared and degassed before being poured onto the prepared mold (Figure 2D). After complete curing, the PDMS-based 3-D pillars were removed from the mold and directly transferred onto another cleaned glass substrate with the pillars on the top (Figure 2E). An approximately 100 μm thick SU-8 layer was spun onto the PDMS layer with pillars and baked using the low-temperature baking profile18 (Figure 2F). This SU-8 layer was exposed with the mask to open areas for the following metal deposition step (Figure 2G). By using this technique, we were able to obtain reliable metal coverage onto the pillars unlike conventional metal patterning processes. The metal coverage on the pillar sidewalls was verified visually under a high-magnification microscope (discussed later). Additionally, continuity of the metal from the top of each pillar to the contact pad was also verified using digital Multimeter. The SU-8 layer was baked again at low temperature,18 and the unexposed SU-8 is removed using the SU-8 developer (Figure 2H). Next, 5 nm Cr followed by 300 nm Au was sputtered to obtain uniform metal coverage all over the electrode features (Figure 2I). The SU-8 layer was then mechanically peeled off from the flexible PDMS-based 3-D sensors to obtain the desired metal patterns16 (Figure 2J). The PDMS-based fully flexible 3-D sensor electrodes were then peeled from the glass substrate for subsequent processing. Finally, one of the electrodes was electroplated with Ag followed by the chloridation of the Ag electrode to obtain the Ag/AgCl electrode before enzyme immobilization and testing (Figure 2K).
Immobilizing Glucose Oxidase
To prepare the enzyme solution, GOx powder was mixed with a 7.4 pH phosphate buffer. After the addition of glutaraldehyde and bovine serum albumin, the solution was stored in a 4 °C fridge until needed. Meanwhile, the electrodes were cleaned with oxygen plasma, washed with high-purity deionized water, and dried under a filtered stream of nitrogen gas. The gold electrodes were flooded with chilled enzyme solution and left at room temperature for 30 min to immobilize the enzyme onto the electrodes. Subsequently, the excessive enzyme solution was rinsed using the phosphate buffer. The same concentration of enzyme solution is used for flat and pillar electrodes. Hence the effective amount of enzyme onto the electrode depends on the surface area of the electrode. The sensors with immobilized enzyme were again stored at 4 °C until used for further processing or analysis.
Experimental Methods for Evaluation of the Sensor Characteristics
For electrochemical measurement of the flexible 3-D sensors, current response at 0.5 V with different glucose concentrations was measured using a potentiostat (AFCBP1 Biopotentiostat, Pine Instrument Company, PA). For amperometric measurements, glucose concentrations from 0.75 to 15 mg/dl were prepared, and the test solution was continuously stirred during the entire period of the measurement.
Results and Discussions
Effect of the Surface Area
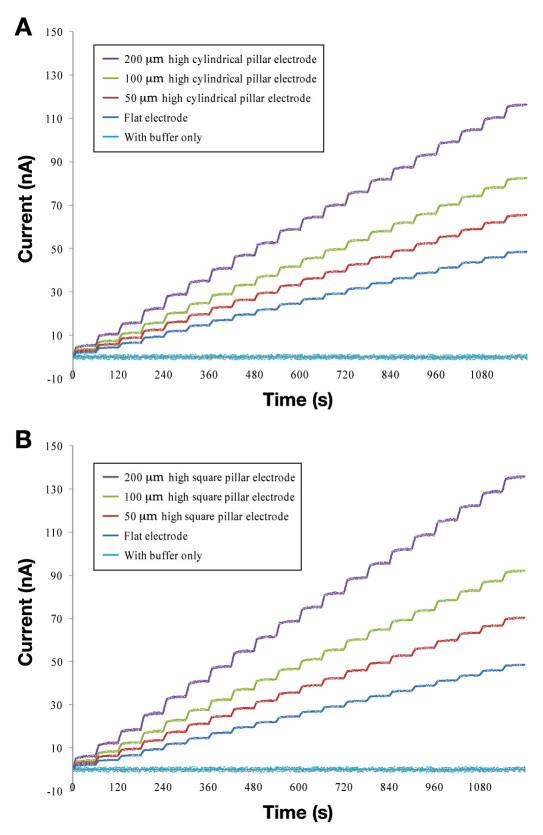
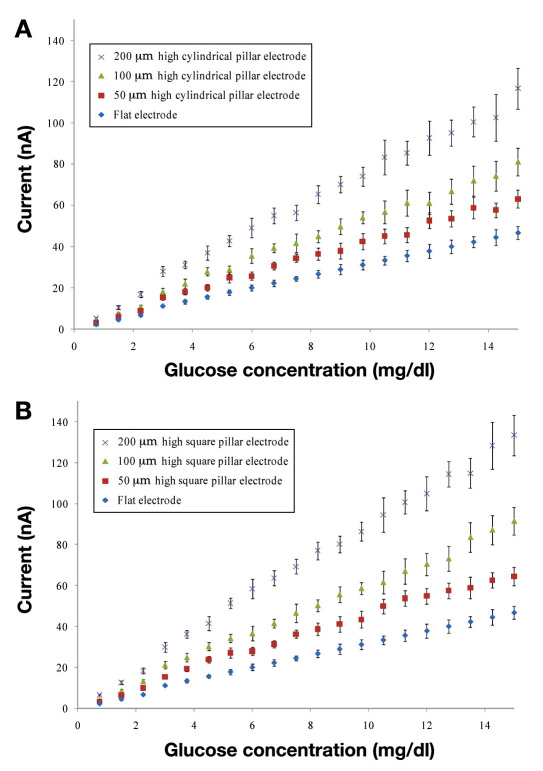
In order to systematically study the effect of the surface area of the sensor, we prepared the sensors with different pillar geometries (as described earlier). Figures 3A and 3B show the measured performance versus different glucose concentrations for the different electrode designs. Different designs of the 3-D electrode system are described in Table 1.
Figure 3.
Measured amperometric response for different 3-D electrode geometries. The response is measured at 0.5 V with respect to the Ag/AgCl (reference) electrode. (A) Amperometric response for different designs with the cylindrical pillars along with the flat electrode response. The amperometric response in phosphate buffer is also shown for comparison of the noise. The amperometric current increases with improvement in the electrode surface area. (B) Amperometric response for different designs with the square pillars along with the flat electrode response. Similar to the cylindrical pillars, the amperometric current increases with improvement in the surface area of the electrode.
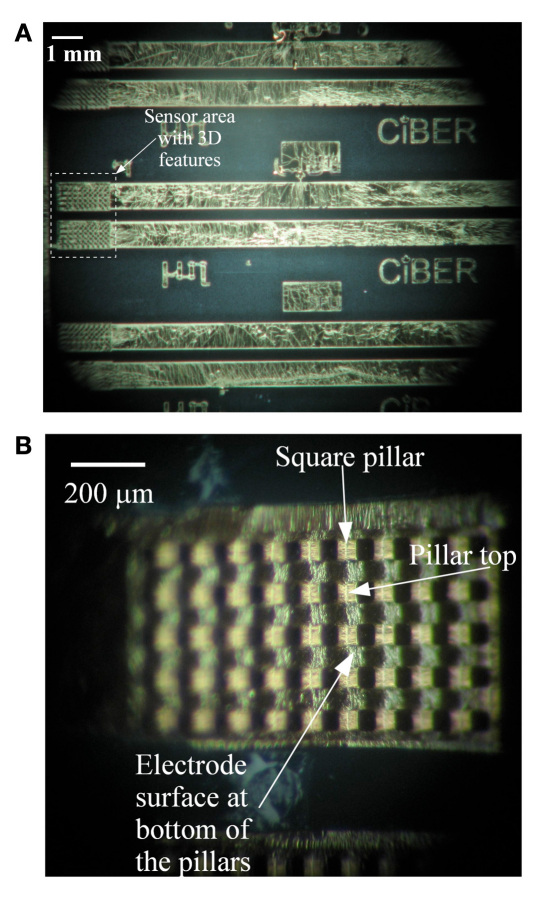
As evident from comparing Figures 3A and 3B, sensors with 3-D features (pillars) greatly increase the glucose signals (according to the increased surface area). The glucose signals approached a 300% increase in signal using 200 μm high square pillar patterns rather than flat electrodes. Additionally, the percentage of sensitivity rises in the sensor signal closely follow the percentage increase in the surface area of the electrode. This linearly increasing trend was observed for both the square and the cylindrical pillar geometry. Hence, very high sensitivity is achieved using the pillar-electrode-based glucose sensors rather than the flat-electrode-based sensors. Additionally, 3-D electrodes have a different diffusion profile and could also be a reason for the observed sensitivity increase. The microscopic image of the glucose sensor electrode with 100 μm high square pillars is shown in Figures 4A and 4B.
Figure 4.
Microscopic images of electrodes immediately after PDMS-based sensors are removed from the glass backing plate. (A) Three consecutive sensors with pillar electrodes are shown along with connecting conductors for testing. The actual sensor area is highlighted in the image. (B) Magnified view of the pillar electrodes with square pillars. Ordered array of square pillars on working electrode are uniformly covered with Cr/Au metal layer. The square pillar feature along with top and bottom of the metal covered pillar is indicated in the image.
Previously, 3-D electrodes were fabricated for retinal implants in order to improve their performance.19 However, fully flexible 3-D electrodes using the ordered array of pillars have never been fabricated for electrochemical glucose sensors. Additionally, our system offers fully flexible and ordered arrays of pillar electrodes directly in a contact lens material (PDMS) that offers desired oxygen permeability and biocompatibility.17 Besides patterning electrodes onto a contact lens for tear glucose monitoring, a number of applications that utilize completely conformable 3-D electrodes can be easily realized using the proposed technology.
Electrochemical Response and Calibration Curve
Figures 3A and 3B show a typically measured ampero-metric response acquired during successive addition of 0.75 mg/dl glucose every 60 s. The response is very quick, reaching 90% of the maximum value in less than 20 s, which signifies the possibility of its practical application on a contact lens. Figures 3A and 3B also show the control measurement curve acquired by successive addition of the same amount of buffer (no glucose), verifying the specificity of the sensor response. The calibration curve in Figures 5A and 5B was generated by averaging the current values between 20 and 55 s following each addition of the glucose solutions. A linear relationship between the current and the glucose concentration is observed between 0.75 and 15.0 mg/dl. The R2 values for different types of the electrodes are shown in Table 2. The values indicate a very good linear response from all the sensor types.
Figure 5.
Calibration currents for different concentrations of glucose based on five different sensors. Response is measured at 0.5 V with respect to the Ag/AgCl (reference) electrode. The current signal is generated by averaging current values from 20 to 55 s after each successive addition of 0.75 mg/dl glucose. (A) Calibration currents for pillar electrodes with circular cross section. (B) Calibration currents for pillar electrodes with square cross section.
Table 2.
Sensitivity Measurement of Different Sensor Designs along with Percentage Improvement and Worst Case Standard Deviation from Three Different Measurements of Five Sensorsa
| Measured sensitivity (nA(mg/dl)-1(mm)-2) | % improvement in sensitivity | % increase in surface area from Table 1 | Worst case standard deviation (%) | R2 values | |
|---|---|---|---|---|---|
| Flat electrode | 1.56 | 100% | 100% | 8% | 0.9978 |
| Square pillars | |||||
| Design 1S | 2.31 | 148% | 150% | 8% | 0.9923 |
| Design 2S | 2.90 | 186% | 200% | 10% | 0.9958 |
| Design 3S | 4.45 | 285% | 300% | 8% | 0.9963 |
| Cylindrical pillars | |||||
| Design 1C | 2.21 | 142% | 139% | 9% | 0.9936 |
| Design 2C | 2.73 | 175% | 179% | 8% | 0.9946 |
| Design 3C | 3.59 | 230% | 257% | 9% | 0.9945 |
Response is measured at 0.5 V with respect to the Ag/AgCl (reference) electrode.
Considering the area of the working electrode (2 mm2), the sensitivity of different designs are shown in Table 2. As we can clearly see from Table 2, there is improvement in the sensitivity of the working electrode with pillars. Additionally, it is also clear that the desired sensitivity of sensor electrodes can be easily achieved by changing the physical geometry of pillars as well as pillar array configuration. The sensitivity increase is correlated with the increase in surface area of pillar electrodes (from Tables 1 and 2). The improvement in sensitivity is again due to the increased surface area of the 3-D pillar electrode.
Furthermore, it is estimated that electrodes with pillar structures would be capable of measuring tear glucose levels up to 0.75 mg/dl, which is 1/5 of the minimum tear glucose level.15,20 The noise current for different types of electrode is consistently ∼0.2 nA. However, the average current generated at a 0.75 mg/dl concentration using the flat electrode is ∼2.25 nA. The lowest detection limit for the pillar electrodes could be below 0.75 mg/dl. However, we have only tested the electrodes up to a 0.75 mg/dl concentration and used this value as the lowest detection limit for our experiments.
Repeatability and Stability of 3-D Flexible Sensors
A typical amperometric test arrangement is used to validate sensor operation. The measurement protocol consisted of successive additions of 0.75 mg/dl glucose solution in buffer. Figure 5 shows average value obtained from three independent measurement results of five different sensors. The current signals for each sensor are generated by averaging current values between 20 and 55 s following each addition.
Our results indicate that the sensor has good repeatable and stable characteristics. For the proposed flexible 3-D sensors, the worst case standard deviation is ±9.63% (among all different options). Additionally, as we can see from Figures 5A and 5B, all the different designs with pillar electrodes repeatedly gave a stable response.
Conclusions
A noninvasive glucose sensor for continuous monitoring on a contact lens could make personalized medicine cheaper, simpler, and more reliable. Here we have successfully fabricated a microsized, fully flexible, 3-D glucose sensor with improved sensitivity for an oxygen-permeable polymer contact lens. Fabricated with 3-D flexible electrodes, the sensors exhibit a fast response, high sensitivity, and good repeatability in the range of low concentrations of glucose found in the human tear. Although further testing for long-term stability and improvements for the same is required, the results lay the initial foundation for building a multifunctional contact lens capable of chemical analysis in the future.
Future improvements for sensor stability will likely include long-term stability improvement as well as protecting and stabilizing the active area with a hydrogel film without losing the performance of the 3-D sensor. Interference studies should also be performed along with improvements to overcome the interference issue.
Acknowledgments
This work made use of 4D LABS shared facilities supported by the Canada Foundation for Innovation, British Columbia Knowledge Development Fund, Western Economic Diversification Canada, and Simon Fraser University.
Glossary
Abbreviations
- (Ag/AgCl)
silver/silver chloride
- (3-D)
three-dimensional
- (GOx)
glucose oxidase
- (PDMS)
poly dimethylsiloxane
Funding
This work was funded by the Natural Sciences and Engineering Research Council of Canada. Fabrication and testing equipment funding was also provided by the Canadian Foundation for Innovation and CMC Microsystems, respectively. This research was also supported in part by the Natural Sciences and Engineering Research Council of Canada and the Canada Research Chairs Program.
References
- 1.Badugu R, Lakowicz JR, Geddes CD. Ophthalmic glucose monitoring using disposable contact lenses—-a review. J Fluoresc. 2004;14(5):617–633. doi: 10.1023/b:jofl.0000039349.89929.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ersöz A, Denizli A, Ozcan A, Say R. Molecularly imprinted ligand-exchange recognition assay of glucose by quartz crystal microbalance. Biosens Bioelectron. 2005;20(11):2197–2202. doi: 10.1016/j.bios.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Godber B, Thompson KS, Rehak M, Uludag Y, Kelling S, Sleptsov A, Frogley M, Wiehler K, Whalen C, Cooper MA. Direct quantification of analyte concentration by resonant acoustic profiling. Clin Chem. 2005;51(10):1962–1972. doi: 10.1373/clinchem.2005.053249. [DOI] [PubMed] [Google Scholar]
- 4.Martin WB, Mirov S, Venugopalan R. Using two discrete frequencies within the middle infrared to quantitatively determine glucose in serum. J Biomed Opt. 2002;7(4):613–617. doi: 10.1117/1.1501893. [DOI] [PubMed] [Google Scholar]
- 5.Rolinski OJ, Birch DJ, McCartney L, Pickup JC. Molecular distribution sensing in a fluorescence resonance energy transfer based affinity assay for glucose. Spectrochim Acta A Mol Biomol Spectrosc. 2001;57(11):2245–2254. doi: 10.1016/s1386-1425(01)00475-9. [DOI] [PubMed] [Google Scholar]
- 6.Springsteen G, Wang B. A detailed examination of boronic acid-diol complexation. Tetrahedron. 2002;58(26):5291–5300. [Google Scholar]
- 7.Ferrante do Amaral CE, Wolf B. Current development in non-invasive glucose monitoring. Med Eng Phys. 2008;30(5):541–549. doi: 10.1016/j.medengphy.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 8.LeBlanc JM, Haas CE, Vicente G, Colon LA. Evaluation of lacrimal fluid as an alternative for monitoring glucose in critically ill patients. Intensive Care Med. 2005;31(10):1442–1445. doi: 10.1007/s00134-005-2747-5. [DOI] [PubMed] [Google Scholar]
- 9.Baca JT, Taormina CR, Feingold E, Finegold DN, Grabowski JJ, Asher SA. Mass spectral determination of fasting tear glucose concentrations in nondiabetic volunteers. Clin Chem. 2007;53(7):1370–1372. doi: 10.1373/clinchem.2006.078543. [DOI] [PubMed] [Google Scholar]
- 10.Baca JT, Finegold DN, Asher SA. Tear glucose analysis for the noninvasive detection and monitoring of diabetes mellitus. Ocul Surf. 2007;5(4):280–293. doi: 10.1016/s1542-0124(12)70094-0. [DOI] [PubMed] [Google Scholar]
- 11.March WF, Mueller A, Herbrechtsmeier P. Clinical trial of a noninvasive contact lens glucose sensor. Diabetes Technol Ther. 2004;6(6):782–789. doi: 10.1089/dia.2004.6.782. [DOI] [PubMed] [Google Scholar]
- 12.Alexeev VL, Das S, Finegold DN, Asher SA. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin Chem. 2004;50(12):2353–2360. doi: 10.1373/clinchem.2004.039701. [DOI] [PubMed] [Google Scholar]
- 13.Iguchi S, Kudo H, Saito T, Ogawa M, Saito H, Otsuka K, Funakubo A, Mitsubayashi K. A flexible and wearable biosensor for tear glucose measurement. Biomed Microdevices. 2007;9(4):603–609. doi: 10.1007/s10544-007-9073-3. [DOI] [PubMed] [Google Scholar]
- 14.Kagie A, Bishop DK, La Belle JT, Dymond R, Felder R, Wang J. Flexible rolled thick-film miniaturized flow-cell for minimally invasive amperometric sensing. Electroanalysis. 2008;20:1610–1614. [Google Scholar]
- 15.Berman ER. New York: Plenum; 1991. Biochemistry of the eye. [Google Scholar]
- 16.Patel JN, Kaminska B, Gray BL, Gates BD. A sacrificial SU-8 mask for direct metallization on PDMS. J Micromech Microeng. 2009;19:1–10. 115014. [Google Scholar]
- 17.Mitsubayashi K, Wakabayashi Y, Tnimoto S, Murotomi D, Endo T. Optical-transparent and flexible glucose sensor with ITO electrode. Biosens Bioelectron. 2003;19(1):67–71. doi: 10.1016/s0956-5663(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 18.Patel JN, Kaminska B, Gray BL, Gates BD. PDMS as a sacrificial substrate for SU-8-based biomedical and microfluidic applications. J Micromech Microeng. 2008;18(9):1–11. 095028. [Google Scholar]
- 19.Kim ET, Seo JM, Woo SJ, Zhou JA, Chung H, Kim SJ. Fabrication of pillar shaped electrode arrays for artificial retinal implants. Sensors. 2008;8(9):5845–5856. doi: 10.3390/s8095845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whikehart DR. 2nd ed. Philadelphia: Butterworth-Heinemann; 2003. Biochemistry of the eye. [Google Scholar]