Abstract
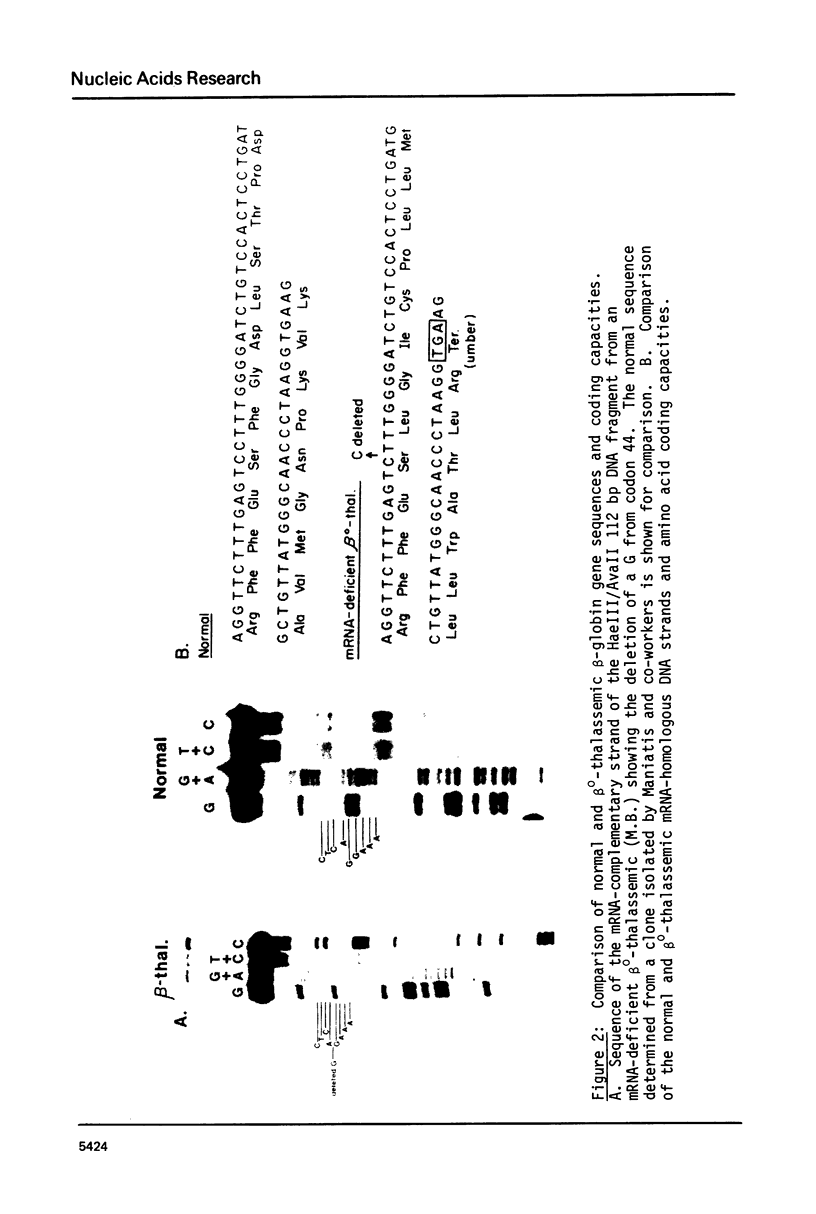
The beta-globin gene of a patient with mRNA-deficient beta o-thalassemia has been sequenced. We find a single nucleotide deletion in amino acid codon 44 that produces a UGA terminator at codon 60. We have previously shown that the beta-globin mRNA of this patient is correctly spliced and polyadenylated, but rapidly turns over with a half-life of less than 30 min. We suggest that the rapid mRNA turnover is influenced by the deletion of this single nucleotide as well as by the nonsense codon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. G., 3rd, Steinberg M. H., Newman M. V., Morrison W. T., Benz E. J., Jr, Iyer R. beta-Thalassemia present in cis to a new beta-chain structural variant, Hb Vicksburg [beta 75 (E19)Leu leads to 0]. Proc Natl Acad Sci U S A. 1981 Jan;78(1):469–473. doi: 10.1073/pnas.78.1.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Baird M., Driscoll C., Schreiner H., Sciarratta G. V., Sansone G., Niazi G., Ramirez F., Bank A. A nucleotide change at a splice junction in the human beta-globin gene is associated with beta 0-thalassemia. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4218–4221. doi: 10.1073/pnas.78.7.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Brimhall B., Jones R. T., Schneider R. G., Hosty T. S., Tomlin G., Atkins R. Two new hemoglobins. Hemoglobin Alabama (beta39(C5)Gln leads to Lys) and hemoglobin Montgomery (alpha 48(CD 6) Leu leads to Arg). Biochim Biophys Acta. 1975 Jan 30;379(1):28–32. [PubMed] [Google Scholar]
- Busslinger M., Moschonas N., Flavell R. A. Beta + thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981 Dec;27(2 Pt 1):289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Kan Y. W. beta 0 thalassemia, a nonsense mutation in man. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2886–2889. doi: 10.1073/pnas.76.6.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Solal M., Blouquit Y., Garel M. C., Thillet J., Gaillard L., Creyssel R., Gibaud A., Rosa J. Haemoglobin Lyon (beta17-18 (A 14-15) Lys-Val leads to O). Determination by sequenator analysis. Biochim Biophys Acta. 1974 Jun 7;351(2):306–316. [PubMed] [Google Scholar]
- Gander E. S., Stewart A. G., Morel C. M., Scherrer K. Isolation and characterization of ribosome-free cytoplasmic messenger-ribonucleoprotein complexes from avian erythroblasts. Eur J Biochem. 1973 Oct 18;38(3):443–452. doi: 10.1111/j.1432-1033.1973.tb03078.x. [DOI] [PubMed] [Google Scholar]
- Gorski J., Fiori M., Mach B. A new nonsense mutation as the molecular basis for beta thalassaemia. J Mol Biol. 1982 Jan 25;154(3):537–540. doi: 10.1016/s0022-2836(82)80012-0. [DOI] [PubMed] [Google Scholar]
- Kantor J. A., Turner P. H., Nienhuis A. W. Beta Thalassemia: mutations which affect processing of the beta-Globin mRNA precursor. Cell. 1980 Aug;21(1):149–157. doi: 10.1016/0092-8674(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Kinniburgh A. J., McMullen M. D., Martin T. E. Distribution of cytoplasmic poly(A+)RNA sequences in free messenger ribonucleoprotein and polysomes of mouse ascites cells. J Mol Biol. 1979 Aug 25;132(4):695–708. doi: 10.1016/0022-2836(79)90383-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Beach L. R., Honig G. R., Lazerson J., Ershler W. B., Ross J. Processing of human beta-globin mRNA precursor to mRNA is defective in three patients with beta+-thalassemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4287–4291. doi: 10.1073/pnas.77.7.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Rachmilewitz E. A., Ross J. Unstable beta-globin mRNA in mRNA-deficient beta o thalassemia. Cell. 1981 Dec;27(3 Pt 2):543–553. doi: 10.1016/0092-8674(81)90396-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen M. D., Shaw P. H., Martin T. E. Characterization of poly(A+)RNA in free messenger ribonucleoprotein and polysomes of mouse Taper ascites cells. J Mol Biol. 1979 Aug 25;132(4):679–694. doi: 10.1016/0022-2836(79)90382-6. [DOI] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Moschonas N., de Boer E., Grosveld F. G., Dahl H. H., Wright S., Shewmaker C. K., Flavell R. A. Structure and expression of a cloned beta o thalassaemic globin gene. Nucleic Acids Res. 1981 Sep 11;9(17):4391–4401. doi: 10.1093/nar/9.17.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Norvell W. A., Frink C. R., Hill D. E. Phosphorus in Connecticut lakes predicted by land use. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5426–5429. doi: 10.1073/pnas.76.11.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C. Nonsense and frameshift mutations in beta 0-thalassemia detected in cloned beta-globin genes. J Biol Chem. 1981 Oct 10;256(19):9782–9784. [PubMed] [Google Scholar]
- Orkin S. H., Old J. M., Weatherall D. J., Nathan D. G. Partial deletion of beta-globin gene DNA in certain patients with beta 0-thalassemia. Proc Natl Acad Sci U S A. 1979 May;76(5):2400–2404. doi: 10.1073/pnas.76.5.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Ramirez F., Starkman D., Bank A., Kerem H., Cividalli G., Rachmilewitz E. A. Absence of beta mRNA in beta0-thalassemia in Kurdish Jews. Blood. 1978 Oct;52(4):735–739. [PubMed] [Google Scholar]
- Spritz R. A., Jagadeeswaran P., Choudary P. V., Biro P. A., Elder J. T., deRiel J. K., Manley J. L., Gefter M. L., Forget B. G., Weissman S. M. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2455–2459. doi: 10.1073/pnas.78.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Gallwitz D. Fate of histone messenger RNA in synchronized HeLa cells in the absence of initiation of protein synthesis. Eur J Biochem. 1977 Jan;72(2):385–392. doi: 10.1111/j.1432-1033.1977.tb11263.x. [DOI] [PubMed] [Google Scholar]
- Traeger J., Wood W. G., Clegg J. B., Weatherall D. J. Defective synthesis of HbE is due to reduced levels of beta E mRNA. Nature. 1980 Dec 4;288(5790):497–499. doi: 10.1038/288497a0. [DOI] [PubMed] [Google Scholar]
- Trecartin R. F., Liebhaber S. A., Chang J. C., Lee K. Y., Kan Y. W., Furbetta M., Angius A., Cao A. beta zero thalassemia in Sardinia is caused by a nonsense mutation. J Clin Invest. 1981 Oct;68(4):1012–1017. doi: 10.1172/JCI110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway D., Williamson R. An intron nucleotide sequence variant in a cloned beta +-thalassaemia globin gene. Nucleic Acids Res. 1981 Apr 24;9(8):1777–1788. doi: 10.1093/nar/9.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]