Abstract
Background
A concept for a tear glucose sensor based on amperometric measurement of enzymatic oxidation of glucose was previously presented, using glucose dehydrogenase flavin adenine dinucleotide (GDH-FAD) as the enzyme. Glucose dehydrogenase flavin adenine dinucleotide is further characterized in this article and evaluated for suitability in glucose-sensing applications in purified tear-like saline, with specific attention to the effect of interfering substances only. These interferents are specifically saccharides that could interact with the enzymatic activity seen in the sensor's performance.
Methods
Bench top amperometric glucose assays were performed using an assay solution of GDH-FAD and ferricyanide redox mediator with samples of glucose, mannose, lactose, maltose, galactose, fructose, sucrose, and xylose at varying concentrations to evaluate specificity, linear dynamic range, signal size, and signal-to-noise ratio. A comparison study was done by substituting an equivalent activity unit concentration of glucose oxidase (GOx) for GDH-FAD.
Results
Glucose dehydrogenase flavin adenine dinucleotide was found to be more sensitive than GOx, producing larger oxidation currents than GOx on an identical glucose concentration gradient, and GDH-FAD exhibited larger slope response (-5.65 × 10-7 versus -3.11 × 10-7 A/mM), signal-to-noise ratio (18.04 versus 2.62), and linear dynamic range (0–30 versus 0–10 mM), and lower background signal (-7.12 versus -261.63 nA) than GOx under the same assay conditions. GDH-FAD responds equally to glucose and xylose but is otherwise specific for glucose.
Conclusion
Glucose dehydrogenase flavin adenine dinucleotide compares favorably with GOx in many sensor-relevant attributes and may enable measurement of glucose concentrations both higher and lower than those measurable by GOx. GDH-FAD is a viable enzyme to use in the proposed amperometric tear glucose sensor system and perhaps also in detecting extreme hypoglycemia or hyperglycemia in blood.
Keywords: biosensor, diabetes mellitus, glucose dehydrogenase flavin adenine dinucleotide, tear glucose monitoring
Introduction
Diabetes mellitus (DM) is fast becoming one of the largest constituents of health care costs worldwide, accounting for an estimated 12% of all health care expenditures as of 2010.1 Self-monitoring of blood glucose (SMBG) is an integral component of DM management, allowing both the health care practitioner and the patient to assess glucose control and make changes in therapy. However, commercially available methods for SMBG require invasive blood sampling, with its associated pain and inconvenience. A SMBG method that could avoid the need for blood sampling could potentially increase patient compliance with recommended monitoring and thus prevent many complications and costs of DM in the near future through improved management of hyperglycemia and avoidance of hypoglycemia.2 One such method might be to use tear fluid, rather than blood, as the analyte since a correlation has been documented between tear and blood glucose levels, including now in rabbits with a “wearable” (albeit with external wires) device.3–5 The overall objective of this research effort is to develop such a bloodless tear-based SMBG device, as has previously been proposed.2
The most popular SMBG method currently on the market is the amperometric electrochemical test strip that uses an enzyme such as glucose oxidase (GOx) to oxidize glucose and feed electrons into measurement electrodes to produce a measured current proportional to the glucose concentration. These sensors suffer from several known limitations.6 Glucose measurements in systems using GOx, the single most commonly used glucose biosensing enzyme, are affected by the amount of oxygen in the sample, as well as by uric acid, ascorbic acid, acetaminophen, L-dopa, and tolazamide, while measurements using the major available alternative enzyme, glucose dehydrogenase pyrroloquinoline quinone (GDH-PQQ), are affected by galactose, maltose, and xylose.5 These substances are often referred to as “interferents” as they interfere with accurate glucose concentration measurements by altering the measured current for a given glucose concentration and are an obstacle for all SMBG systems.
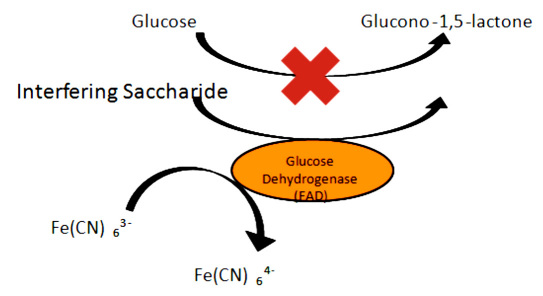
There are two major mechanisms of interference with GOx systems used in current SMBG technology. Some substances act as interferents because they are electro-active at the voltages used in SMBG amperometry so that they oxidize under the applied potential and generate a current that is indistinguishable from the current produced by enzymatic oxidation of glucose. This class of interferents includes uric acid, acetaminophen, and ascorbic acid. There are also some interferents that are not oxidizable on their own but are oxidized nonspecifically by enzymes intended for glucose or otherwise interact with the enzyme. This is speculated to be the mechanism of certain saccharides' (particularly maltose's) interference with GDH-PQQ amperometry.7 Using glucose dehydrogenase flavin adenine dinucleotide (GDH-FAD) as an example, Figure 1 diagrams the effect of enzymatic oxidation of nonglucose saccharides on an amperometric application. Partly due to noise caused by interference, all systems currently on the market are also generally unable to measure extremely low glucose concentrations of the level found in tear fluid or in extreme hypoglycemic states in blood. Instead, state-of-the-art SMBG systems often simply indicate that the glucose concentration is too low to measure.8–11
Figure 1.
Schematic diagram of saccharide interference that may compete with glucose as a substrate for enzyme activity, thereby falsely increasing the signal strength and masking the true glucose value. Fe(CN), ferricyanide.
In 2006, researchers in Japan reported a newly discovered enzyme, GDH-FAD, with potential SMBG applications.12 GDH-FAD was initially reported to give negligible responses to oxygen, lactose, maltose, and mannose, in contrast to the oxygen-dependent response of GOx. Other work has suggested greater sensitivity and dynamic range, potentially enabling differentiation of very low glucose levels found in extreme hypoglycemia or even in bloodless tear glucose sensing.13 As the Food and Drug Administration begins to tighten accuracy requirements for SMBG devices from 20% error to 15% error, enzyme attributes may increasingly constrain the operating parameters of SMBG devices.14
Previous work has suggested a plausible application of GDH-FAD to tear glucose sensing in which interference might come from both oxygen and nonglucose saccharides.2,13 This article presents a comprehensive study of the performance of GDH-FAD in a glucose assay application with saccharide interference in idealized, saline-like matrix as compared with GOx with the same interference conditions and will contribute to an eventual evaluation of GDH-FAD as a potentially superior replacement for GOx in tear glucose sensing applications. GDH-FAD-based assays might offer increased dynamic range and finer sensitivity to severe hypoglycemia, which would enable more precise control of treatment for patients and thus bring medical science one step closer to the ideal of tight glycemic control.
Materials and Methods
Chemicals
All reagents were obtained from Sigma or Sigma-Aldrich unless otherwise specified. GDH-FAD with an activity of 183 U/mg was donated generously by Amano, Inc. (Japan). The GOx used had an activity of 155.6 U/mg. All saccharide solutions were prepared in phosphate-buffered saline (PBS) unless otherwise specified.
Electrochemical Detection
Commercial “Zensor” three-screen-printed carbon ink (working and counter) and silver/silver chloride reference electrodes were obtained from CH Instruments (Austin, TX). Electrochemical measurements were taken using a CHI 1230A (CH Instruments, Austin, TX) and desktop computer. A redox mediator solution was prepared using 100 mM potassium ferricyanide in PBS. The assay solution was produced by dissolving the enzyme (either GDH-FAD or GOx) in 100 mM potassium ferricyanide solution to reach a final enzyme concentration of 183 U/ml. For each assay, 90 μl of enzyme/mediator solution was pipetted onto the electrode area of a Zensor screen-printed electrode and manipulated with a pipette tip so as to entirely cover the working, reference, and counter electrodes. A total of 10 μl of sample was pipetted onto the top of the globule of enzyme/mediator solution already on the electrode, either in a single 10 μl injection of sample or in two separate 5 μl injections. After sample addition was completed, the globule was permitted to sit on the Zensor for 30 s, after which a voltage of +0.35 V was applied and current measurement was simultaneously begun, a similar procedure as has been used previously.2,13 Current was measured every 0.1 s for a total duration of 30 s. The current measurement taken 10 s after beginning measurement was taken as the representative current for the sample, and this measurement was the one used in all data analysis unless otherwise specified.
A glucose assay was made using 10 μl of the sample (glucose) being added to 90 μl of the assay solution in a single injection for a final assay volume of 100 μl and a final assay concentration of 1/10 the glucose concentration of the original sample. Samples for the activity with nonglucose saccharides with GDH-FAD and GOx were made at 10 mM. Concentrations of maltose, galactose, fructose, sucrose, and xylose were compared with assays of glucose and control assays using PBS only. Amperometric current-over-time (i-t) assays using GDH-FAD in the described protocol were performed on a concentration gradient of glucose solutions in PBS with the actual concentration of glucose in the assay solution on the Zensor ranging from 0 to 1000 μM. A 10 μl of the sample (at 10× concentration) was added to 90 μl of the assay solution in a single injection for a final assay volume of 100 μl. Data points were taken at glucose concentrations of 0, 125, 250, 500, 750, and 1000 μM (n = 4). The order in which concentrations were sampled was randomized to prevent run-order effects.
Saccharide Sample and Interferent Assays
Amperometric i-t assays using GDH-FAD in the stated protocol were performed at 1000 μM concentrations of maltose, galactose, fructose, sucrose, and xylose and compared with assays of 1000 μM glucose (n = 3). Control assays using PBS-only samples (n = 3) were also performed. Assays were performed in randomized order alternating among the samples. Mannose and lactose were excluded from the initial run because of reagent unavailability but were used in later experiments performed using GDH-FAD and GOx with 300 μM concentrations of fructose, galactose, maltose, mannose, sucrose, and xylose, along with glucose and a PBS-only blank sample (n = 4). A subsequent set of amperometric i-t assays was performed using both enzymes and all the listed sugars, plus lactose, at a 2 mM concentration (n = 3) to obtain a clearer signal.
A final set of amperometric i-t assays was performed, once using GDH-FAD and once using GOx (n = 1 each enzyme), on an extended pure-glucose-in-PBS concentration gradient with glucose concentrations of 0, 0.25, 0.5, 0.75, 1, 2.5, 5, 10, 20, 30, and 50 mM, in order to compare the dynamic range and sensitivity of each enzyme.
Results and Discussion
Electrochemical Detection
The 10 s current measured by amperometry was found to correlate with the glucose concentration in the assay solution on the Zensors over the range of 0 to 1000 μM, confirming results previously published.2,13 The best-fit regression line was calculated to be
Saccharide Sample and Interferent Assays
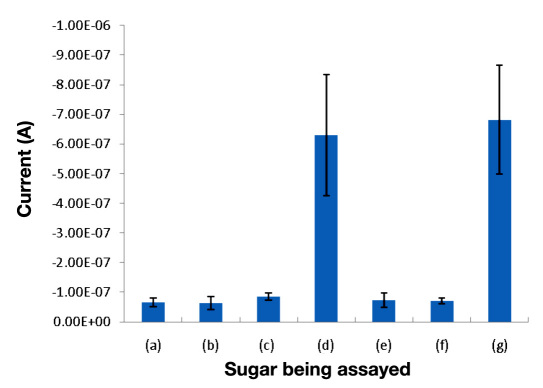
The assay did not show interference from any of the tested saccharides except xylose, which gives a signal comparable to that of glucose at the same concentration (Figure 2). Assay measurements of 1 mM solutions of the other saccharides were no higher than the background signal from PBS alone. These results show that GDH-FAD does not share the susceptibility to maltose interference, which has been documented in older GDH-PQQ-based sensors.5,6,15 GDH-FAD does, however, respond equally to xylose and glucose, most likely because xylose has a near-identical chemical structure to glucose and may act as an alternate substrate for GDH-FAD.
Figure 2.
Amperometric 10 s current responses of a GDH-FAD assay to (a) a blank solution of pure PBS, (b) 1 mM fructose, (c) 1 mM galactose, (d) 1 mM glucose, (e) 1 mM maltose, (f) 1 mM sucrose, and (g) 1 mM xylose (n = 3). All sample solutions were made in standard PBS at pH 7.4. Error bars represent one standard deviation from the mean.
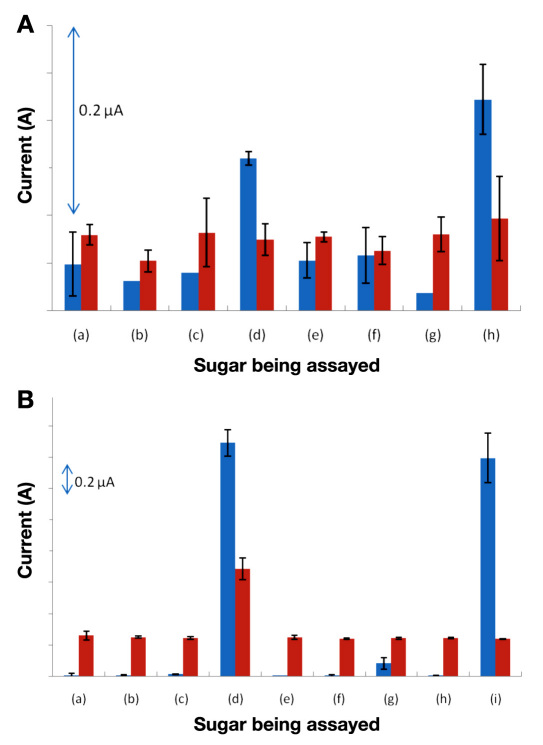
However, the proposed tear glucose sensing application (or any other application with ultra-low glucose concentration) would require enzyme specificity to be maintained substantially below 1 mM. A typical tear glucose concentration of 500 μM or less is below the capabilities of even the best GOx amperometric devices on the market.8 Figure 3A shows the advantage of GDH-FAD over GOx in applications requiring an ability to produce signals with very low concentrations of glucose. The amperometric assay using GDH-FAD was able to distinguish 300 μM glucose (and xylose) from PBS and other saccharides (n = 3), while the amperometric assay using GOx showed no significant difference between the current with 300 μM glucose and the background current with PBS only, indicating a complete absence of signal with 300 μM glucose (n = 3). Thus, in this study, GOx was not able to detect the sub-500 μM glucose concentration range found in normal tear fluid. While GOx detection of sub-500 μM glucose concentrations has been achieved in other studies (though not in any presently available commercial sensor), these have used enzyme activities many times greater than was used in this study.16 Thus these results suggest that, compared with GOx, GDH-FAD generates a greater signal per unit of enzyme per concentration of substrate (glucose), a favorable characteristic for sensor manufacturing, as it would allow far less enzyme and reagent material to be used for each sensor strip, decreasing the average cost of each sensor.
Figure 3.
(A) Comparison of GDH-FAD (blue) and GOx (red) amperometric assay signals for (a) a blank solution of pure PBS, (b) 300 μM fructose, (c) 300 μM galactose, (d) 300 μM glucose, (e) 300 μM maltose, (f) 300 μM mannose, (g) 300 μM sucrose, and (h) 300 μM xylose (n = 3). (B) Comparison of GDH-FAD and GOx amperometric assay signals for (a) a blank solution of pure PBS, (b) 2 mM fructose, (c) 2 mM galactose, (d) 2 mM glucose, (e) 2 mM lactose, (f) 2 mM maltose, (g) 2 mM mannose, (h) 2 mM sucrose, and (i) 2 mM xylose (n = 3). All sample solutions were made in PBS at pH 7.4. Error bars represent one standard deviation from the mean.
Despite the limited signal strength (current flow) provided by GOx amperometry as compared with GDH-FAD amperometry, GOx is an established market standard (used in Yellow Springs Instrument reference systems) because of its excellent specificity, which this study further confirms. Figure 3B shows that, when used at sufficient concentration, GOx does exhibit good specificity, easily distinguishing glucose from all other sugars. This is the one point in which GOx is clearly superior to GDH-FAD as a glucose amperometry enzyme, as GDH-FAD is again unable to distinguish glucose from xylose. However, GDH-FAD can distinguish either sugar from all others with a signal-to-noise ratio far greater than GOx (18.04 for GDH-FAD versus 2.62 for GOx), suggesting a tradeoff between absolute substrate specificity and greater ability to distinguish signals from the background. Interestingly, GDH-FAD exhibits a slight but noticeable response to mannose above the background level, although the mannose signal remains far below the glucose signal. Were it not for the extra noise (interference) from mannose, the GDH-FAD signal-to-noise ratio would be an astonishing 209.28 times the PBS blank background signal.
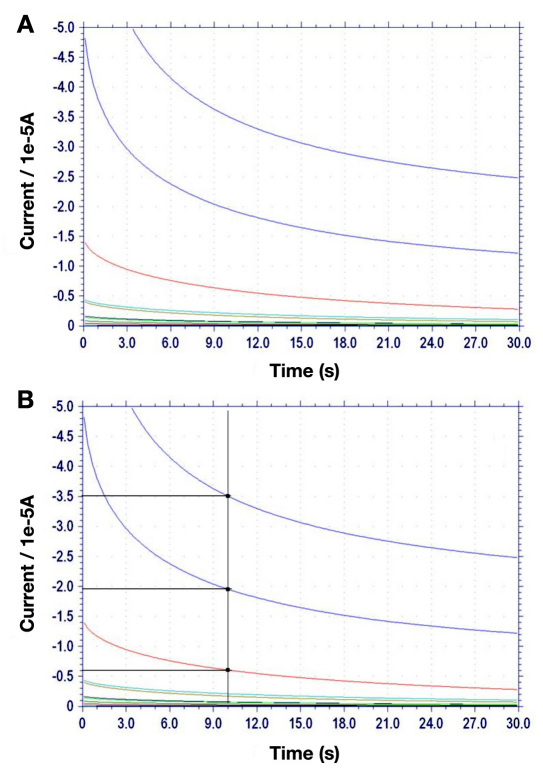
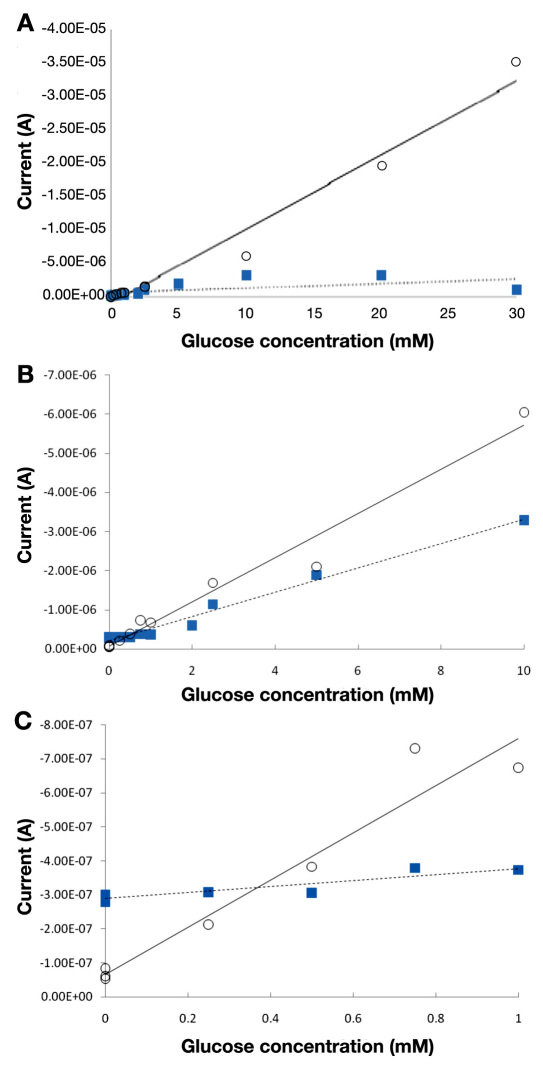
The potential tradeoff is made even more favorable by the wider dynamic range and larger slope of an amperometric glucose assay using GDH-FAD, compared with an identical assay using GOx (Figures 4 and 5). Figure 4 shows the raw amperometry data for GDH-FAD with glucose concentrations from 0 to 30 mM, from which the current values at 10 s are shown in Figure 5. Glucose oxidase data were acquired in an identical manner (raw data not shown). The best-fit regression line for glucose dehydrogenase (solid line) has an R2 value of 0.970, as compared with 0.346 for GOx (dashed line), indicating that GOx is unable to maintain linearity over as wide a range. While GOx produces a linear correlation between current and glucose concentration only up to 10 mM glucose, GDH-FAD can produce a correlation all the way up to 30 mM (Figure 5A). In addition, within the 0–10 mM range in which both enzyme responses are linear and in the 0–1 mM range most relevant for tear glucose applications (Figures 5B and 5C), it can be clearly seen that the slope of the response curve using GDH-FAD is approximately twice as large in magnitude as the slope obtained with GOx at its optimal positive voltage of 0.6 V. At its optimal negative voltage of -0.45 V, GOx produces a nonlinear current–glucose concentration relationship with much lower slope (data not shown). These data suggest that GDH-FAD might have potential applications in blood glucose sensing, as it covers all the concentration range measured by state-of-the-art SMBG sensors and may potentially provide greater resolution at the low end of the concentration range, enabling better management of hypoglycemia.
Figure 4.
(A) Amperometry curves for GDH-FAD. Comparable data were taken for GOx (not shown). (B) Data showing the 10 s mark at which data were taken, highlighting three representative data points and how current is extrapolated from the curves.
Figure 5.
(A) Comparison of GOx (squares) and GDH-FAD (circles) across the linear range of glucose dehydrogenase (0–30 mM). (B) A closer look at GDH-FAD and GOx across the 0–10 mM range, within which both GOx and glucose dehydrogenase display an acceptably linear current–glucose concentration curve. The best-fit regression equation for glucose dehydrogenase (solid line) has a slope of -5.65 × 10-7 A/mM, which is almost double the GOx (dashed line) best-fit slope of -3.11 × 10-7 A/mM. (C) An even closer look at both enzymes in the 0–1 mM range, which would be relevant for tear glucose sensing.
However, it should also be recognized that SMBG sensors generally do not operate with the simplified model used in this study. Although pure sample solutions were used in this study for the purpose of evaluating intrinsic enzyme performance in the absence of confounding variables, actual tear or blood samples would include mixtures of interferents, including nonsaccharide interferents, which are not considered in this study because their interference mechanisms are independent of the enzyme. In addition, currently marketed SMBG sensors frequently use measures such as polymer films or membranes to improve assay performance and extend their dynamic ranges beyond what can be achieved with simple solution-on-electrode assays. These measures, demonstrated in literature as early as 1984,17–19 can currently extend the dynamic range of GOx to as wide as 0.6 to 33.3 mM in devices on the market, which, although not quite low enough for tear glucose sensing in the 0.5 mM range, is an impressive achievement in blood sensing and far exceeds the 1–10 mM range shown in Figure 5A.8
Conclusion
The data presented in this study show that in our glucose assay system GDH-FAD generates a sufficient current to detect glucose in the concentration range found in nondiabetic tear fluid, while GOx does not. GDH-FAD is also shown to be specific to glucose (with the exception of xylose) and, in particular, does not respond to maltose, thus eliminating the danger of maltose-inflated measurements known to occur with GDH-PQQ sensors on the market.15 Finally, GDH-FAD generates almost twice as much signal as GOx under identical conditions, and that signal remains linear over a much wider concentration range. Thus this study shows that GDH-FAD holds three major advantages over GOx in potential glucose sensing applications: (1) a lower limit of detection, (2) a wider linear dynamic range, and (3) a much larger signal size. These characteristics make GDH-FAD better suited than GOx for detecting extremely low levels of glucose, such as those found in tears or in severely hypoglycemic patients. A tear glucose sensor using GDH-FAD would be viable. GDH-FAD might also potentially be used in blood glucose sensors to measure extreme hypoglycemia or even hyper-glycemia, which is beyond the range of GOx sensors.
The single drawback of GDH-FAD is its inability to distinguish between glucose and xylose; thus, for applications requiring specificity between glucose and xylose, GDH-FAD can only be used if measures are taken to remove xylose or otherwise counteract its interference. However, if xylose is not present, or can be prevented from coming into contact with the enzyme in a given sensing application, the given favorable attributes of GDH-FAD highly recommend its use in glucose sensing. It should also be noted that, although GOx exhibits superior specificity among saccharides, GOx also suffers from interference by oxygen, which was not considered in this study.2,6,12 Thus, for a tear glucose application in which samples may vary wildly in oxygen content, GDH-FAD offers the advantage of oxygen insensitivity in addition to all the others listed here. Because free xylose is not created or utilized in normal human metabolism and is absorbed through digestion and excreted intact in urine, xylose sensitivity is not likely to be a particularly severe problem for SMBG applications, as high levels of xylose in the body should typically occur only for a short time following food intake before the xylose is excreted. Thus the only advantage of a xylose-insensitive SMBG system would be the ability to test just after eating a meal without waiting, although this may be a significant issue for certain patients. For other applications, glucose biosensor manufacturers may wish to consider whether xylose or oxygen interference is more important in their particular application when choosing an enzyme to use.
Further work for the development of the proposed GDH-FAD glucose sensor will include many technical refinements to move the electrochemical assay from bench-top equipment to a smaller hand-held system. These include first testing the sensor in a tear matrix in an animal model. This will provide initial prototype efficacy and safety prior to further refinements. These other refinements include: a more ergonomic design for easier self-use, possible materials development for easier manufacturing, investigation into sterilization methods for all materials used, reduction of the sample volume to approximately 3 μl to conform to the available volume of tears on the eye, and development of countermeasures for nonsaccharide electroactive interferents, which affect all state-of-the-art electrochemical glucose sensors regardless of the enzyme used.
Acknowledgments
Enzyme samples were generously donated by Amano, Inc.
Glossary
Abbreviations
- (DM)
diabetes mellitus
- (GDH-FAD)
glucose dehydrogenase flavin adenine dinucleotide
- (GDH-PQQ)
glucose dehydrogenase pyrroloquinoline quinone
- (GOx)
glucose oxidase
- (PBS)
phosphate-buffered saline
- (SMBG)
self-monitoring of blood glucose
Funding
This work was partially supported by ASU Fulton Undergraduate Research Initiatives program, Barrett, the Honors College, and seed funding from the Biodesign Institute at Arizona State University, Tempe, AZ.
Disclosure
Jeffrey T. La Belle has filed a U.S. patent relating to this research.
References
- 1.Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, Nichols G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(3):293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Bishop DK, La Belle JT, Vossler SR, Patel DR, Cook CB. A disposable tear glucose biosensor-part 1: design and concept testing. J Diabetes Sci Technol. 2010;4(2):299–306. doi: 10.1177/193229681000400209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baca JT, Taormina CR, Feingold E, Finegold DN, Grabowski JJ, Asher SA. Mass spectral determination of fasting tear glucose concentrations in nondiabetic volunteers. Clin Chem. 2007;53(7):1370–1372. doi: 10.1373/clinchem.2006.078543. [DOI] [PubMed] [Google Scholar]
- 4.Baca JT, Finegold DN, Asher SA. Tear glucose analysis for the noninvasive detection and monitoring of diabetes mellitus. Ocul Surf. 2007;5(4):280–293. doi: 10.1016/s1542-0124(12)70094-0. [DOI] [PubMed] [Google Scholar]
- 5.Chu MX, Miyajima K, Takahashi D, Arakawa T, Sano K, Sawada S, Kudo H, Iwasaki Y, Akiyoshi K, Mochizuki M, Mitsubayashi K. Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta. 2011;83(3):960–965. doi: 10.1016/j.talanta.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3(4):903–913. doi: 10.1177/193229680900300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirrane BM, Duthie EA, Nelson LS. Unrecognized hypoglycemia due to maltodextrin interference with bedside glucometry. J Med Toxicol. 2009;5(1):20–23. doi: 10.1007/BF03160976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nova Biomedical. StatStrip® glucose. http://www.novabiomedical.com/products/strip_based_clinical_analyzers/statstrip.php.
- 9.Abbott. FreeStyle Freedom Lite: owner's booklet. http://www.abbottdiabetescare.com/static/cms_workspace/document/ART12214_Rev-B_web.pdf.
- 10.LifeScan. OneTouch Ultra 2: owner's booklet. http://www.lifescan.com/pdf/AW_06344502A.pdf.
- 11.Target. EasyPro Plus: user instruction manual. 2007. Target Brands, Inc.
- 12.Tsujimura S, Kojima S, Kano K, Ikeda T, Sato M, Sanada H, Omura H. Novel FAD-dependent glucose dehydrogenase for a dioxygen-insensitive glucose biosensor. Biosci Biotechnol Biochem. 2006;70(3):654–659. doi: 10.1271/bbb.70.654. [DOI] [PubMed] [Google Scholar]
- 13.La Belle JT, Bishop DK, Vossler SR, Patel DR, Cook CB. A disposable tear glucose biosensor-part 2: system integration and model validation. J Diabetes Sci Technol. 2010;4(2):307–311. doi: 10.1177/193229681000400210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malone B. Blood glucose meters: is FDA ready to tighten up accuracy standards? Clin Lab News. 2010;36(5):1–4. [Google Scholar]
- 15.U.S. Food and Drug Administration. FDA public health notification: potentially fatal errors with GDH-PQQ glucose monitoring technology. http://www.fda.gov/medicaldevices/safety/alertsandnotices/publichealthnotifications/ucm176992.htm.
- 16.Kagie A, Bishop DK, Burdick J, La Belle JT, Dymond R, Felder R, Wang J. Flexible rolled thick-film miniaturized flow-cell for minimally invasive amperometric sensing. Electroanalysis. 2008;20(14):1610–1614. [Google Scholar]
- 17.Cass AE, Davis G, Francis GD, Hill HA, Aston WJ, Higgins IJ, Plotkin EV, Scott LD, Turner AP. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal Chem. 1984;56(4):667–671. doi: 10.1021/ac00268a018. [DOI] [PubMed] [Google Scholar]
- 18.Gregg BA, Heller A. Cross-linked redox gels containing glucose oxidase for amperometric biosensor applications. Anal Chem. 1990;62(3):258–263. doi: 10.1021/ac00202a007. [DOI] [PubMed] [Google Scholar]
- 19.Csöregi E, Schmidtke DW, Heller A. Design and optimization of a selective subcutaneously implantable glucose electrode based on “wired” glucose oxidase. Anal Chem. 1995;67(7):1240–1244. doi: 10.1021/ac00103a015. [DOI] [PubMed] [Google Scholar]