Abstract
Background
Sensor-augmented insulin pumps may be programmed to suspend insulin delivery in response to hypoglycemia. The Medtronic Paradigm® Veo™ pump with automatic low glucose suspend (LGS) was released in 2009. Data from 7 months of real-world use of the system were analyzed to assess usage patterns and effectiveness of LGS.
Method
Data from 935 patients totaling 49,867 patient days were collected; the LGS feature was on for 82% of these days. A subset of 278 subjects who used the pump for ≥3 months was analyzed separately; these subjects provided 28,401 patient days of data, with LGS used for 92% of the time.
Results
The LGS threshold was most commonly set between 50 and 60 mg/dl. A total of 27,216 LGS events occurred, and 60% began in the afternoon or evening. The median duration of LGS events was 9.87 min, 45% lasted for <5 min, and 11% lasted for >115 min (equivalent to the full extent of the LGS event between 115 and 120 min). Among the episodes lasting for >115 min, the mean sensor glucose (SG) was 58.8 ± 12.4 mg/dl at LGS activation (time 0), rose to 102.2 ± 52.8 mg/dl by the end of the LGS episode (when insulin delivery was automatically resumed), and was 150.1 ± 68.6 mg/dl at 240 min. In the 278-subject subgroup, LGS usage significantly reduced the number of SG readings <50 mg/dl (p = 0.001) and >300 mg/dl (p = 0.001).
Conclusions
The LGS feature was on for most of the patient days in the study. Most LGS episodes lasted for <10 min. Use of the LGS feature significantly reduced exposure to hypoglycemia. Profound hyperglycemia resulting from LGS episodes lasting >115 min was not observed.
Keywords: hypoglycemia avoidance, low glucose suspend, pump suspension, semi-closed loop, Veo insulin pump
Introduction
Severe hypoglycemia remains the main impediment to the implementation of intensive diabetes management for many children and adults with type 1 diabetes.1 Severe hypoglycemic events leading to seizures, coma, and death still occur at unacceptable rates. Therefore, strategies to decrease the occurrence of severe hypoglycemia are warranted.
The STAR 3 study showed the benefit of combining insulin pump therapy with continuous glucose monitoring (CGM) technology in a regimen known as sensor-augmented pump (SAP) therapy. Adult and pediatric STAR 3 subjects randomized to SAP therapy realized lower glycated hemoglobin levels than those randomized to multiple daily injections, with no increased risk of hypoglycemia.2 Importantly, glucose values from the CGM system did not directly control the insulin pump in STAR 3. The Medtronic Paradigm® Veo™ System (Medtronic Minimed, Inc., Northridge, CA) improves on SAP therapy by addition of the low glucose suspend (LGS) feature, which allows for automatic pump suspension for up to 120 min when a preprogrammed CGM threshold value is reached. If the threshold value is reached, and in the absence of intervention, the pump displays an informative message, sounds a continuous alarm, and begins a 6 h cycle of no insulin delivery for 120 min followed by insulin delivery at the previously set basal rate for 240 min. The 6 h LGS cycle continues until it is manually interrupted, at which time normal insulin delivery is resumed. The LGS feature may be turned on or off independently of other pump functions, but it does not work in the absence of incoming sensor glucose (SG) data.
The Veo System became available in 2009, and users had access to CareLink™ Therapy Management Software for Diabetes (CareLink), which simplifies data analysis and facilitates therapy management. Here we report on the use and efficacy of the Veo System through analysis of CareLink data uploaded from January to July 2010.
Methods
During the 7-month data-collection period, 935 subjects who had activated the LGS feature of the Veo System at least once were found in the CareLink database. They had uploaded data representing 49,867 patient days, during which the LGS feature was turned on for 82% of the time. Data from this cohort were analyzed to determine the distribution of LGS events by time of day and duration. This cohort experienced 2986 long-duration (>115 min) LGS episodes; SG values collected at the beginning of, during, and after these episodes were summarized as mean ± standard deviation (SD). A subset of 278 subjects who used the Veo System with CGM sensors for ≥3 months provided 28,401 patient days of data; LGS was turned on for 92% of these patient days. These data were analyzed to determine the effect of LGS on the frequency of SG levels in the hypoglycemic and hyperglycemic ranges; significance was calculated with the Kruskal–Wallis test.
Results
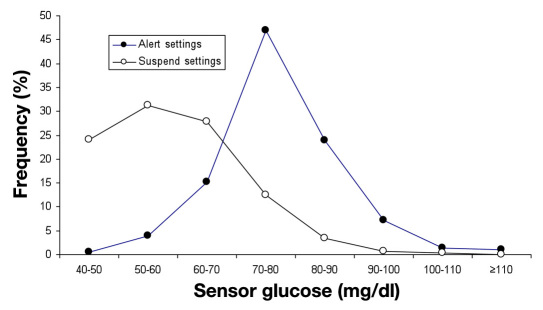
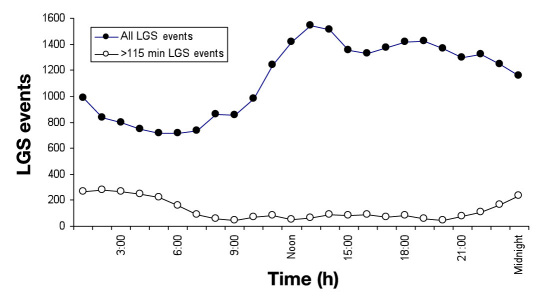
The distributions of SG threshold setting for the Veo System's hypoglycemic alert and its LGS feature among 935 users are given in Figure 1. Hypoglycemic alerts were typically set for higher SG values than were pump suspensions. During the evaluation time period, a total of 27,216 LGS events were recorded throughout the diurnal cycle; the hour-by-hour number of occurrences is given in Figure 2. More LGS events occurred in the early afternoon hours than at other times. The duration of LGS events was bimodal, with most events lasting either <5 or >115 min, as follows: 45% (12,309) lasted for 0–5 min, 21% (5606) lasted for 5–30 min, 13% (3598) lasted for 30–60 min, 7% (1788) lasted for 60–90 min, 3% (929) lasted for 90–115 min, and 11% (2986) lasted for >115 min. The distribution for the 2986 maximal-duration LGS events (Figure 2) shows that many occur during late-night and early morning hours. A total of 1974 maximal-duration LGS events occurred in the 10 h between 2200 and 0800, and only 1012 occurred in the 14 h from 0800–2200. The mean SG value at the beginning of the 2986 maximal-duration LGS episodes is given in Table 1, along with mean SG values at hours 1–4 after the beginning of pump suspension. Sensor glucose values were in the hypoglycemic range at the beginning of pump suspension and rose gradually to ∼150 mg/dl after 4 h (comprising 2 h of pump suspension and 2 h of basal delivery).
Figure 1.
Distributions of time spent at a SG threshold setting for hypoglycemic alerts (filled circles) and pump suspensions (open circles) among 935 Veo System users.
Figure 2.
Diurnal distribution of all LGS episodes (filled circles, n = 27,216) and LGS episodes lasting >115 min (open circles, n = 2986) among 935 Veo System users.
Table 1.
Sensor Glucose Values during and after 2986 Low Glucose Suspend Episodes of >115 Minutes
| Event | Time (min) | Mean (SD) SG, mg/dl |
|---|---|---|
| LGS Start | 0 | 58.8 (12.4) |
| 1 h after LGS Start | 60 | 77.0 (36.6) |
| End of LGS | 120 | 102.2 (52.8) |
| 1 h after LGS End | 180 | 136.8 (64.9) |
| 2 h after LGS End | 240 | 150.1 (68.6) |
Sensor glucose data reflecting hypoglycemia and hyperglycemia in the 278-subject subgroup are given in Table 2. The percentage of abnormal SG readings reflects the duration of hypoglycemia and hyperglycemia, and the area under the glucose concentration time curve [area under the curve (AUC)] reflects both the duration and severity of glycemic excursions. There was a significant reduction in the percentage of SG values <50, >240, and >300 mg/dl when LGS was turned on compared with when the feature was turned off. There was a trend for the percentage of SG values >180 mg/dl to be lower during the LGS-off period, but the difference was not statistically significant. There was a significant difference in favor of LGS-on periods for AUC <50 and AUC <60 values. The AUC values for hyperglycemia (>180, >240, and >300 mg/dl) were all significantly lower during periods when the LGS feature was turned on.
Table 2.
Sensor-Recorded Hypoglycemia and Hyperglycemia in 278 Subjects with ≥3 Months' Use of Veo System
| SG (mg/dl) | % of SG values, LGS off | % of SG values, LGS on | P valuea | AUC, LGS off | AUC, LGS on | P valuea |
|---|---|---|---|---|---|---|
| <50 | 1.33 | 0.92 | <0.01 | 0.09 | 0.07 | <0.001 |
| <60 | 3.58 | 2.63 | 0.14 | 0.32 | 0.22 | 0.016 |
| <70 | 6.73 | 5.48 | 0.43 | 0.80 | 0.59 | 0.145 |
| >180 | 28.02 | 31.34 | 0.05 | 18.13 | 17.50 | 0.029 |
| >240 | 11.65 | 11.28 | 0.02 | 6.98 | 5.58 | 0.006 |
| >300 | 4.64 | 3.41 | <0.01 | 2.48 | 1.65 | <0.001 |
P values compare LGS-on and LGS-off intervals.
Table 3 shows the accuracy of the SG values responsible for triggering the LGS feature, which was assessed by comparing contemporaneous SG and capillary blood glucose (BG) values at the beginning of LGS episodes. Of the 1499 LGS events for which there was a BG value recorded within 15 min of a triggering SG value of ≤70 mg/dl, 902 (60%) BG values were <70 mg/dl and only 63 (4%) corresponding BG values were ≥180 mg/dl.
Table 3.
Distribution of Contemporaneous Blood Glucose Values at the Start of Low Glucose Suspend Episodes Triggered by Sensor Glucose Values ≤70 mg/dl
| Blood glucose (mg/dl) | N (%) |
|---|---|
| <70 | 902 (60) |
| 70–100 | 333 (22) |
| 100–180 | 201 (13) |
| ≥180 | 63(4) |
| Total | 1499 (100) |
Discussion
This is the first report on the real-world usage and effectiveness of an insulin pump that responds to low glucose levels by suspending insulin delivery. It represents an important milestone in the drive toward eventual closure of the “loop” of fully automated insulin delivery.
By analyzing data in the CareLink Therapy Management System, we were able to find a large number of users in Europe within the first year of commercialization of the Veo System to assess effectiveness of the LGS feature.
Data representing approximately 49,000 patient days of use of the LGS feature show that the pumps were suspended over 27,000 times, or approximately 0.55 times per patient day. Most pump suspensions occurred during daytime hours, and most were of short duration, perhaps representing users who took corrective action after dismissing the alarm and resuming basal delivery. Only 11% of pump suspensions lasted for >115 min. Most of the maximal-duration pump suspensions occurred during normal sleeping hours, and many may reflect a scenario in which a patient was awakened by the audible alarm and chose to dismiss it without restarting basal insulin delivery. This LGS scenario is preferable to continued insulin delivery in the face of hypoglycemia. A case report by Tanenberg and colleagues3 presents data from a pump-wearing subject who succumbed to nocturnal hypoglycemia, which was reflected in blinded CGM sensor data and postmortem vitreous humor examination.
To attenuate the risk of ketoacidosis resulting from pump suspension, the LGS feature resumes basal insulin delivery for 4 h after a 2 h period of pump suspension. The automatic restart of basal insulin was effective in preventing extreme hyperglycemia, as evidenced by SG values recorded during and after maximal-duration pump suspensions. Previous studies confirm the safety of pump suspensions lasting between 90 and 165 min; these occur frequently and are effective in preventing hypoglycemia.4 Buckingham and associates5 and Cengiz and coworkers6 have also demonstrated the effectiveness and safety of insulin suspension up to 2 h to prevent impending hypoglycemia.
The LGS feature increased the number of in-target SG readings by reducing the number of hypoglycemic readings and, notably, by also reducing the number of hyperglycemic readings. Reduction of hyperglycemia may reflect fewer episodes of excessive carbohydrate intake in response to symptomatic hypoglycemia.
Any system that can reduce severity and duration of nocturnal hypoglycemia and, almost as importantly, reduce fear and anxiety around nocturnal hypoglycemia may be valuable to patients with type 1 diabetes. It is clear that the function of this system still depends on accurate data from the sensor and that patients need to confirm SG readings with traditional BG meter readings before taking corrective action. The ∼4% rate at which LGS events were triggered near the time of BG readings ≥180 mg/dl is likely acceptable, given the reduction in nocturnal hypoglycemia or values <50 mg/dl. During a Medtronic-sponsored user evaluation of the Veo System, there was a similar rate of SG/BG discordancy and no reported episodes of diabetic ketoacidosis.7 Furthermore, the SG/BG discordancies shown in Table 3 may reflect BG values that are rapidly rising due to recently ingested carbohydrates. Algorithms designed to reduce the false positive rate of the LGS feature are under development.
This study's conclusions are limited and may not be generalizable; the study was a retrospective data-mining effort to gain insight into real-world utilization of the commercialized LGS feature. In particular, patients turned the LGS feature on or off at times of their own choosing, which may have influenced our results. Studies of the LGS feature in a controlled protocol-driven setting and its long-term effects are in progress.
Conclusions
Commercial availability of an insulin pump system that suspends insulin delivery in response to CGM-sensor-detected hypoglycemia represents an important milestone in our progress toward full automation of insulin delivery. The Medtronic Paradigm Veo pump and its algorithm for pump suspension appear to safely and effectively reduce exposure to both hypoglycemia and hyperglycemia.
Glossary
Abbreviations
- (AUC)
area under the curve
- (BG)
blood glucose
- (CGM)
continuous glucose monitoring
- (LGS)
low glucose suspend
- (SAP)
sensor-augmented pump
- (SD)
standard deviation
- (SG)
sensor glucose
Funding
This work was funded by Medtronic, Inc.
Disclosures
All authors are employees of Medtronic, Inc.
References
- 1.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care. 2010;33(5):1004–1008. doi: 10.2337/dc09-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Peoples T, Perkins BA, Welsh JB, Willi SM, Wood MA STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 3.Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract. 2010;16(2):244–248. doi: 10.4158/EP09260.CR. [DOI] [PubMed] [Google Scholar]
- 4.Elleri D, Acerini CL, Allen JM, Hayes J, Pesterfield C, Wilinska ME, Dunger DB, Hovorka R. Parental attitudes towards overnight closed-loop glucose control in children with type 1 diabetes. Diabetes Technol Ther. 2010;12(1):35–39. doi: 10.1089/dia.2009.0084. [DOI] [PubMed] [Google Scholar]
- 5.Buckingham B, Cobry E, Clinton P, Gage V, Caswell K, Kunselman E, Cameron F, Chase HP. Preventing hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Technol Ther. 2009;11(2):93–97. doi: 10.1089/dia.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cengiz E, Swan KL, Tamborlane WV, Steil GM, Steffen AT, Weinzimer SA. Is an automatic pump suspension feature safe for children with type 1 diabetes? An exploratory analysis with a closed-loop system. Diabetes Technol Ther. 2009;11(4):207–210. doi: 10.1089/dia.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhary P, Shin J, Wang Y, Evans ML, Hammond PJ, Kerr D, Shaw JA, Pickup JC, Amiel SA. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in exposure to hypoglycemia. Diabetes Care. doi: 10.2337/dc10-2411. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]