Abstract
Background
A high dosing accuracy is needed to maintain normal glycemia in patients with diabetes. This study investigated the dose accuracy of the commonly used reusable insulin pens ClikSTAR®, NovoPen® 4, and Luxura®.
Methods
Pens were tested in a laboratory setting by one trained technician who delivered four doses of 30 U from each of 15 pens per pen model (a total of 60 doses from each pen model). Pens were also tested in a simulated clinical setting by 48 people with diabetes. Each participant delivered 27 doses: three doses of 30 U from each of three pens per pen model. Overall, the technician delivered 180 doses and the participants 1296 doses.
Results
All pens met the tolerance limits defined by the German edition of the International Standardization Organization (ISO) 11608-1:2000 standard [30 ± 1.5 U (28.5–31.5 U)]. All doses were delivered within the limits proposed by the ISO, except for two doses with Luxura in the clinical setting. In laboratory testing, the mean dose delivered by ClikSTAR (29.69 U) or Luxura (29.89 U) was less than the expected 30 U and significantly less than the mean dose delivered by NovoPen 4 (30.04 U; p < .001 for both comparisons). Similar results were observed in the simulated clinical setting. NovoPen 4 had the greatest variance in laboratory testing but the least in the simulated clinical setting.
Conclusions
This study demonstrates comparable dose accuracy and variability of the ClikSTAR, Luxura, and NovoPen 4 insulin pens. The slight differences in mean doses between pens are unlikely to be clinically significant.
Keywords: Berlipen, ClikSTAR, dose accuracy, insulin pens, Luxura, NovoPen 4
Management of type 1 or type 2 diabetes mellitus is based on controlling hyperglycemia with exogenous insulin only (for patients with type 1 diabetes) or oral antihyperglycemic drugs with the eventual addition of exogenous insulin (for patients with type 2 diabetes).1 For patients whose diabetes regimen includes self-injected insulin, dosing accuracy is critically important maintaining optimal glycemic control.
The two predominant means of injecting insulin are vial and syringe and insulin pens. Insulin pens are now used worldwide by patients with diabetes, but there are marked differences in their use between countries.2 Compared with vial and syringe, insulin pens offer improvements in compliance and flexibility.3,4 Studies have demonstrated lower annual treatment costs4 and user preference for pens over vial and syringe.5 Insulin pens that are manufactured for use by the general public are subject to medical device standards set by the International Standardization Organization (ISO).6 In addition, independent verification of the data is desirable in order to provide patients and their physicians with the confidence that their chosen pen device provides accurate dosing.
ClikSTAR® is a pen manufactured by sanofi-aventis Deutschland GmbH, Frankfurt am Main, Germany, for the administration of insulins. It was designed to provide the state-of-the-art performance of the disposable SoloSTAR® pen in a reusable format. After only a brief training of health care professionals, ClikSTAR has been shown to be easy to use and to provide accurate dosing for people with diabetes.7 A user study showed that the ClikSTAR pen offers significant advantages over other reusable pens tested in terms of ease of use, ease of cartridge replacement, and feeling of the clicks.8 Overall, the ClikSTAR pen was significantly easier to use than all other pens. Significant advantages were also seen with the ClikSTAR pen compared with at least one other pen in terms of audibility of the clicks, overall rating of ease in completing tasks, as well as ability to perform any step without assistance (including the safety step). A separate study showed that ClikSTAR, in comparison with other reusable pens, had the lowest injection force, irrespective of injection speeds (constant button speed or constant flow rate).9 This is an important benefit for patients with diabetes, especially for those with limited finger joint mobility and low hand strength.
The aim of this study was to investigate the accuracy of ClikSTAR according to ISO 11608-1:2000 limits6 at a single intermediate dose of 30 U in both a laboratory and a simulated clinical setting (field data). Furthermore, the study aimed to compare the accuracy of ClikSTAR with that of other widely available reusable insulin pens.
Methods
Study Design
This study was conducted in two phases using ClikSTAR, Luxura® (Eli Lilly, Indianapolis, IN), and NovoPen® 4 (Novo Nordisk, Bagsvaerd, Denmark) pens (Table 1). Pens were tested in a laboratory setting in the first phase, with doses delivered by a trained technician. In the second phase, the pens were tested in a simulated clinical setting by people with diabetes. All pens in both phases were equipped with the same needle type [BD Micro-Fine + 0.25 mm (31 G) × 8 mm; phase 1: lot 9125266; phase 2: lot 9034692].
Table 1.
Insulin Pens
| Insulin pen | Manufacturer (batch) | Insulin (batch) |
|---|---|---|
| Laboratory testing | ||
| ClikSTAR | sanofi-aventis (C006) | Lantus (40C610) |
| Luxura | Eli Lilly (not available) | Huminsulin Basal (A490065C) |
| NovoPen 4 | Novo Nordisk (not available) | Levemir (VT60651) |
| Simulated clinical setting | ||
| ClikSTAR | sanofi-aventis (C002 0123) | Lantus (40C610) |
| Luxura | Eli Lilly (A604286, A591581) | Huminsulin Basal (A591132H) |
| NovoPen 4 | Novo Nordisk (XSG0423) | Levemir (XT60348) |
In the laboratory setting, one trained technician delivered four doses of 30 U from each of the 15 pens per pen model (a total of 60 doses). The pens for each pen model were labeled from 1–15. Before starting a new measurement, one pen was chosen from the pool of unused pens of that model by a random software routine and used for the test. After all 15 pens for one model had been finished, another pen model was chosen.
For each dose, a cup with paraffin was weighed on a balance. After a priming dose, 30 U were injected into the paraffin while the cup and paraffin were still on the balance. The needle remained in the paraffin for the amount of time specified in the instruction manual for each pen model. After removing the pen and waiting an additional 4 s, the new weight was recorded. A new 30 U dose was dialed and the procedure repeated for a total of four doses. The mass of each dose was obtained on an OHaus Discovery DV 215 CD precision and analytical balance (capacity 210 g, repeatability 0.1 mg, linearity ±0.2 mg), which was calibrated before use. The actual dose (U) was calculated from gravimetric determination of the delivered mass, taking into consideration the density and nominal insulin concentration.
For the simulated clinical setting, 24 men and 24 women with diabetes (12 of each gender had prior pen experience) were selected from a single private practice in Leipzig, Germany. The physician was responsible for recruitment and any medical assistance but was not actively involved in the study design. Participants were given a small fee whether they finished the study or dropped out, which they were free to do at any time. There were 144 different pens per pen model used by the participants, with each pen used three times for a total of 432 trials. Each participant delivered three doses of 30 U from each of three pens per pen model. With three pen models, each participant delivered 27 doses. Overall, the 48 participants delivered 1296 doses.
Participants were given 5–10 min of instruction by the study monitor that covered the study description and pen usage based on the manual, i.e., handling, dosing, priming, injecting insulin, leaving the needle in the sponge, and safety instructions. The monitor demonstrated pen usage and injection into a sponge once and then trained each participant once. An assistant fitted the cartridge to the pen while the monitor fitted the needle to the pen. The participant was then asked to prime the pen. The pen was then weighed on a balance by the study monitor. The participant injected a 30 U dose into a sponge, with the needle remaining in the sponge for the amount of time specified in the instruction manual. The pen was weighed again, with the difference in weight being equal to the mass of the dose. The procedure was repeated until a total of three doses were delivered per pen. The entire procedure was repeated with a new pen chosen randomly as described earlier until the mass of all 27 doses for each pen model was recorded. The actual dose (U) was calculated as described earlier.
Statistical Analyses
All analyses were performed as described in the German edition of ISO 11608-1:2000.6 The tolerance interval was calculated, where is the mean of the actual dose for each pen at each dosage level, s is the standard deviation (SD), and k is the tolerance limit factor. The latter was 2.335 for n = 60 in the laboratory test and was 2.38 for n = 432 in the simulated clinic setting. The tolerance interval should lay within the upper and lower acceptance limits for the dosage level. For 30 U, the acceptance limits are 30 ± 1.5 U (28.5–31.5 U). Arithmetic mean of the actual dose, SD, variation coefficient, and difference from the expected dose were also calculated. The Kolmogorov–Smirnov test was performed to confirm normality of distribution and a t test was applied to determine whether the mean dose delivered by each pen model was significantly different from the expected mean value (30 U). Differences between pen models were assessed by F test to compare sample variance and then by t test to compare sample means.
Results
Comparison of Pens in Laboratory Testing and a Simulated Clinical Setting
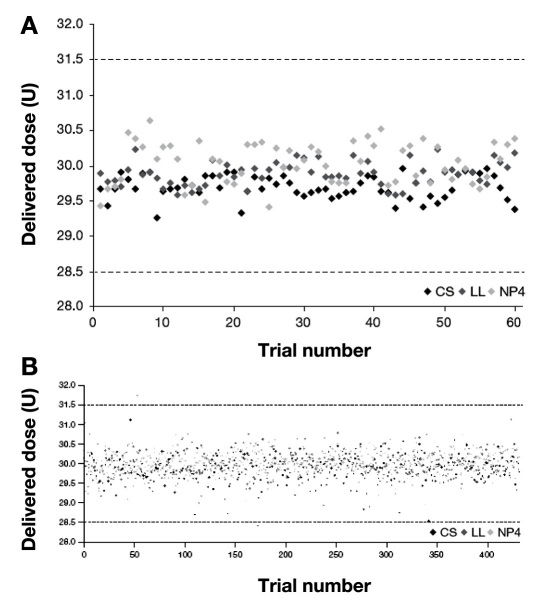
No single dose exceeded the German edition of ISO 11608-1:2000 limits6 for a 30 U dose during laboratory testing (Figure 1A), and only two individual doses delivered using Luxura were outside the limits in the simulated clinical setting (Figure 1B). Results for each pen model are summarized in Table 2. A total of 180 singles doses were recorded in the laboratory setting, 60 from each pen model. In the simulated clinical setting, 1296 doses were recorded, 432 from each pen model.
Figure 1.
Distribution of individual doses by pen in (A) a laboratory setting or (B) a simulated clinical setting. CS, ClikSTAR; LL, Luxura; NP4, NovoPen 4.
Table 2.
Comparison of Pens in Laboratory Testing and a Simulated Clinical Setting
| Laboratory testing | Simulated clinical setting | |||||
|---|---|---|---|---|---|---|
| ClikSTAR | Luxura | NovoPen 4 | ClikSTAR | Luxura | NovoPen 4 | |
| Number of trials | 60 | 60 | 60 | 432 | 432 | 432 |
| Number of pens | 15 | 15 | 15 | 144 | 144 | 144 |
| Trials per pen | 4 | 4 | 4 | 3 | 3 | 3 |
| Lowest dose, units | 29.26 | 29.58 | 29.42 | 28.52 | 28.40 | 28.92 |
| Highest dose, units | 29.96 | 30.23 | 30.64 | 31.10 | 31.72 | 31.00 |
| Mean dose, units | 29.69 | 29.89 | 30.04 | 29.85 | 29.96 | 30.02 |
| SD, units | 0.17 | 0.17 | 0.29 | 0.32 | 0.31 | 0.25 |
| Variance, units | 0.03 | 0.03 | 0.09 | 0.10 | 0.10 | 0.06 |
| Difference from expected dose, units | -0.31 | -0.11 | 0.04 | -0.15 | -0.04 | 0.02 |
| Difference from expected dose, % | -1.02 | -0.38 | 0.13 | -0.49 | -0.12 | 0.08 |
| SD of difference from expected dose, % | 0.57 | 0.57 | 0.98 | 1.07 | 1.04 | 0.84 |
In laboratory testing (Table 2), the mean dose delivered by ClikSTAR (29.69 U) was significantly less than the expected 30 U [95% lower and upper confidence interval (CI): 29.65, 29.74; p < .001], as was the mean dose delivered by Luxura (29.89 U; 95% CI: 29.84, 29.93; p < .001). Mean dose delivered by NovoPen 4 (30.04 U) was not significantly different from the expected mean (95% CI: 29.96, 30.11; p = .31). Mean doses delivered by ClikSTAR and Luxura were significantly lower than that delivered by NovoPen 4 (p < .001 for both comparisons). Mean dose delivered by ClikSTAR was also significantly lower than that delivered by Luxura (p = .002). Sample variance was significantly greater for NovoPen 4 (0.09 U) than for ClikSTAR (0.03 U) or Luxura (0.03 U; p < .001 for both comparisons), while there was no difference between ClikSTAR and Luxura (p = 0.97).
In the simulated clinical setting, mean dose delivered by ClikSTAR (29.85 U) was significantly less than the expected 30 U (95% CI: 29.82, 29.88; p < .001), as was the mean dose delivered by Luxura (29.96 U; 95% CI: 29.93, 29.99; p = .01). Mean dose delivered by NovoPen 4 (30.02 U) was significantly greater than the expected mean (95% CI: 30.00, 30.04; p = .04). Mean doses delivered by ClikSTAR and Luxura were significantly lower than that delivered by NovoPen 4 (p < .001 for both comparisons), while there was no difference between the mean dose delivered by ClikSTAR and that delivered by Luxura (p = 1.00). Sample variance was significantly less for NovoPen 4 (0.06 U) than for ClikSTAR (0.10 U) or Luxura (0.10 U; p < .001 for both comparisons), while there was no difference in variance between ClikSTAR and Luxura (p = .68).
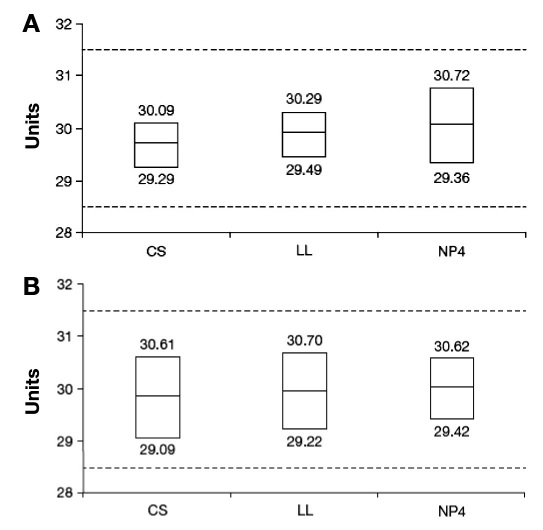
Tolerance Intervals
All three pens met the tolerance limits defined by the ISO standards in both laboratory testing (Figure 2A) and the simulated clinical setting (Figure 2B). Calculated ISO tolerance intervals were within the acceptance range for each pen at the tested dosage level (30 ± 1.5 U).
Figure 2.
Tolerance intervals for each pen model in (A) a laboratory setting or (B) a simulated clinical setting. CS, ClikSTAR; LL, Luxura; NP4, NovoPen 4.
Influence of Test Participants
Demographic characteristics of the 48 people who participated in the simulated clinical setting are shown in Table 3. Gender had no influence on the delivered dose using any pen model. Prior experience of the participants with pens had no effect on the delivered dose using ClikSTAR or Luxura, but participants with pen experience delivered significantly lower doses (30.00 ± 0.25 U) using NovoPen 4 than did those without experience (30.05 ± 0.25 U; p = .048).
Table 3.
Demographic Characteristics of Study Participants
| Male (n = 24) | Female (n = 24) | Total (n = 48) | |
|---|---|---|---|
| Age, years (SD) | 60.3 (11.8) | 64.7 (11.0) | 62.5 (11.5) |
| Height, cm (SD) | 172.1 (5.1) | 162.8 (6.0) | 167.5 (7.2) |
| Weight, kg (SD) | 97.1 (22.2) | 90.5 (25.7) | 93.8 (24.0) |
| Handedness, n | |||
| Right | 21 | 17 | 38 |
| Left | 3 | 5 | 8 |
| Both | 0 | 2 | 2 |
Discussion
Accurate insulin dosing is required for patients with diabetes to maintain normal glycemic levels and to minimize the risk of hypoglycemia. For these patients, insulin pens offer substantial improvements compared with vial and syringe.3–5 Accuracy of an insulin pen must be demonstrated by the manufacturer before it can be introduced to the marketplace. However, independent assessment of dose accuracy in both laboratory and clinical settings can provide people with diabetes and their health care practitioners with added reassurance that the device will perform correctly and with good accuracy in clinical practice.
The current study examined the accuracy of the ClikSTAR pen to deliver a 30 U dose of insulin in both a laboratory and simulated clinical setting and compared the accuracy with that of the reusable insulin pens Luxura and NovoPen 4. In both settings, all three insulin pens demonstrated excellent dosing accuracy that was within tolerance limits defined by ISO standards. In the laboratory setting, no single dose from any pen model was detected outside of specified ISO limits. In the simulated clinical setting, no single dose from the ClikSTAR or NovoPen 4 pens was outside the ISO limits, while only two doses using the Luxura pen were outside the limits. The results with the ClikSTAR pen in the simulated clinical setting are in good agreement with an earlier design validation study.7
There was a trend for both the ClikSTAR and Luxura pens to deliver mean doses that were less than the target dose of 30 U, while, with NovoPen 4, the mean dose was not different from the target dose in the laboratory setting and slightly greater than the target dose in the simulated clinical setting. Likewise, the variance of the ClikSTAR and Luxura pens was comparable in both the laboratory and simulated clinical settings but was less than that of NovoPen 4 in the laboratory and greater in the simulated clinical setting. Despite these differences, the mean dose and variance obtained by the different insulin pens were similar to each other, indicating a comparable reliability of all pens in this study. All pens are thus capable of delivering a 30 U insulin dose accurately. A limitation to this study was the fact that only the single intermediate insulin dose was tested, rather than a range of doses.
Conclusions
This study demonstrates comparable dose accuracy and variability of the ClikSTAR pen compared with Luxura and NovoPen 4 pens when tests were performed by a trained technician or by people with diabetes after appropriate instruction. Although there were slight differences in mean values, these are unlikely to be clinically significant and may reflect measurement precision rather than a potential risk for underdosing/overdosing with any pen. These findings confirm the dose accuracy of each pen. Other features such as injection force9 and ease of use and overall performance, as previously demonstrated with ClikSTAR,8 may also influence selection of insulin pens in everyday clinical practice.
Acknowledgments
Editorial support was provided by Tom Claus of PPSI and funded by sanofi-aventis.
Glossary
Abbreviations
- (CI)
confidence interval
- (ISO)
International Standardization Organization
- (SD)
standard deviation
Funding
This study was funded by sanofi-aventis, Deutschland GmbH.
Disclosure
Arnd Friedrichs and Steffen Adler are employees of LWS Risk Management Consult GmbH, a paid consultant to sanofi-aventis. Nils Basso is an employee of sanofi-aventis.
References
- 1.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perfetti R. Reusable and disposable insulin pens for the treatment of diabetes: understanding the global differences in user preference and an evaluation of inpatient insulin pen use. Diabetes Technol Ther. 2010;12(Suppl 1):S79–85. doi: 10.1089/dia.2009.0179. [DOI] [PubMed] [Google Scholar]
- 3.Korytkowski M, Bell D, Jacobsen C, Suwannasari R, FlexPen Study Team A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25(11):2836–2848. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28(10):1712–1725. doi: 10.1016/j.clinthera.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Summers KH, Szeinbach SL, Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26(9):1498–1505. doi: 10.1016/j.clinthera.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.ISO 11608–1:2000 Pen-injectors for medical use – part 1: pen injectors –requirements and test methods. 2010. http://www.iso.org/iso/catalogue_detail.htm?csnumber=19545. Accessed May 14.
- 7.Schwartz S. Correct use of a new reusable insulin injection pen by patients with diabetes: a design validation study. J Diabetes Sci Technol. 2010;4(5):1229–1235. doi: 10.1177/193229681000400523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penfornis A. Performance of a new reusable insulin pen. Diabetes Technol Ther. 2011;13(3):373–379. doi: 10.1089/dia.2010.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedrichs A, Korger V, Adler S. Injection force of reusable insulin pens: Novopen 4, Lilly Luxura, Berlipen, and ClikSTAR. J Diabetes Sci Technol. 2011;5(5):1185–1190. doi: 10.1177/193229681100500523. [DOI] [PMC free article] [PubMed] [Google Scholar]