Abstract
Background
Insulin injection pens are the predominant devices for insulin delivery in Europe and Japan because of their ease of use and convenience. This study compared clinically relevant technical attributes of durable insulin pens that are important to people with diabetes, specifically functions relating to cartridge-fitting, dose-setting, and dose-delivery on NovoPen® 4, ClikStar®, HumaPen Luxura®, Itango®, and Biosulin® Pen.
Methods
Frequency components and duration of audible clicks on dose setting and injection were measured using audio equipment when setting and delivering 20 IU of insulin. To assess cartridge-fitting torque, each pen was attached to a torque gauge via the attached needle, and torque was measured with each 180° turn as the cartridge was screwed into the body of the pen. Rotary torque of the dose-setting dial was measured when setting the dose to the maximum dose unit of the pen. Injection force was measured when delivering 20 IU at 5 mm/s in a vertical position and at a tilt of 14.7° from vertical.
Results
Audible clicks on dose-setting and dose-delivery were most distinguishable on NovoPen 4, while NovoPen 4 and ClikStar had generally lower cartridge-fitting torque and injection (both vertical and angled) force values.
Conclusion
Overall, the results showed that durable insulin pens such as NovoPen 4 have useful features related to assembly, dose-setting, and injection, which may facilitate ease of use for diabetes patients, particularly elderly patients and those with visual and/or manual dexterity impairments.
Keywords: audible clicks, cartridge-fitting torque, diabetes management, dose-setting dial torque, injection force, insulin pen
Introduction
Insulin injection pens are the predominant devices used for insulin delivery, especially in Europe and Japan, because of their ease of use and convenience. They overcome many problems associated with using the traditional vial and syringe, including fear of injection, social stigma, low confidence in dose-delivery, and inconvenience.1–8
Insulin injection pens are available as either prefilled or durable (with interchangeable cartridges filled with insulin) pens, and these are constantly being updated with improved features to increase user-friendliness. A number of durable insulin pens are available in Japan, each with various patient-centric features and insulin formulations. A notable introduction includes the durable insulin pen NovoPen® 4 (Novo Nordisk A/S, Bagsvaerd, Denmark), which offers access to a range of modern insulin formulations, including basal (insulin detemir), bolus (insulin aspart), and various premixes (biphasic insulin aspart).
Accuracy, injection force, durability, and other features are important components of newer insulin pens. This study was conducted to compare the many clinically relevant technical attributes of durable insulin pens that are important to people with diabetes, many of whom have visual or manual dexterity impairments. Indeed, the Centers for Disease Control and Prevention estimate that, among the 17.6 million adults with diabetes in the United States, 3.6 million, or approximately 20%, have visual impairment.9 Moreover, polyneuropathy eventually affects approximately 40% of patients with diabetes.10–12
The attributes of interest in this study included cartridge-fitting torque, injection force, dose-setting dial torque, and the frequency components and duration of audible clicks on dose-setting and injection. These were compared between NovoPen 4, HumaPen Luxura® (Eli Lilly & Co., Indianapolis, IN), ClikStar® and Itango® (both Sanofi, Paris, France), and Biosulin® Pen (Bioton S.A., Warsaw, Poland). Some additional attributes of these five insulin pens were measured as part of the current study and are summarized in Table 1.
Table 1.
Specifications of the Five Durable Insulin Pens
| Measurement | NovoPen 4 | HumaPen Luxura | Itango | ClikStar | Biosulin Pen | |
|---|---|---|---|---|---|---|
| Max unit (IU) | 60 | 60 | 60 | 80 | 21 | |
| Dose increment (IU) | 1 | 1 | 1 | 1 | 1 | |
| Dose display unit size (mm) | 3.7 × 2.5 | 3.0 × 2.0 | 3.6 × 2.0 | 3.2 × 2.2 | 3.0 × 1.7 | |
| Size (mm) | Body thickness | 15.5 | 17.5 | 17.0 | 19.4 | 15.0 |
| Cartridge holder thickness | 13.8 | 14.5 | 14.5 | 13.7-15.0b | 13.8 | |
| Dose-setting dial at thinnest point | 15.5 | 17.5 | 16.0 | 17.9 | 12.0 | |
| Dose-setting dial at thickest point | 16.7 | 17.5 | 17.0 | 19.6 | 15.0 | |
| Weight (g)a | With cap | 47.4 | 56.3 | 33.4 | 37.9 | 24.3 |
| Without cap | 31.8 | 37.0 | 18.7 | 29.4 | 18.3 | |
| Color of unit number/base | Gray/white | Gray/white | White/black | Gray/white | Silver/dark green | |
| Matching point of cap and body | Free | Free | One point | One point | Free | |
Without insulin cartridge.
Thinnest and thickest points on cartridge holder
Methods
Materials
Pens of each type were selected at random from two different lots: NovoPen 4 (lot numbers VUG0519 and VUG0564), ClikStar (lot numbers C005 and C006), HumaPen Luxura (lot numbers 0501B02A and 0501B03A), Itango (lot numbers U022 and C086), and Biosulin Pen (sample pens without lot numbers). Lots for ClikStar and Biosulin Pen were supplied by Novo Nordisk A/S. Lots for the other pen types were drawn randomly from wholesaler stock.
A single type of needle was used for all injection force tests with all pens; this was a BD Micro-Fine® 31 G thin-wall 5 mm needle (Nippon Becton Dickinson, Tokyo, Japan; lot number 7187214). Insulin cartridges used were Levemir® cartridges (Novo Nordisk A/S; lot number XS60801; used with NovoPen 4), Humalog® cartridges (Eli Lilly & Co.; lot number A438184; used with HumaPen Luxura), and Lantus® cartridges (Sanofi; lot number 9H003A; used with ClikStar, Itango, and Biosulin Pen).
Measurement of Cartridge-Fitting Torque
Five pens of each type were used to assess torque while screwing the insulin cartridge into the body of the pen. Each pen was attached to a torque gauge (6BTG-S, Tohnichi Mfg. Co., Ltd., Tokyo, Japan) via the attached needle, and torque was measured with each 180° turn as the cartridge was screwed into the body of the pen.
Measurement of Injection Force
This test was not conducted with Biosulin Pen, as this device has an automatic injection mechanism. Five pens of each of the other pen types were tested.
For each test, the needle was attached to the pen, and air shots were made until a drop of insulin was visible at the needle tip (and the pen was therefore ready to perform an injection). The dial was set to deliver 20 IU, and the pen was attached to the measuring instrument (UTC-500: A&D, Tokyo, Japan). Injection force was then measured when delivering the dose at 5 mm/s both when the pen was fixed to the instrument vertically and when the pen was fixed with a tilt of 14.7° from vertical.
The angled injection force measurement utilized an L-shaped metal device attached to the push-button of the pen so that the device could be attached to the measuring equipment at the appropriate angle. Mean [± standard deviation (SD)] injection force was calculated from the plateau of the resulting injection force versus the push-button extension curve (a section of the plateau corresponding to displacement of the push-button by 6 mm was used for the calculation).
Measurement of Dose-Setting Dial Torque
Five pens of each type were used to assess the torque of the dose-setting dial when setting the dose to the maximum unit for each pen type (21 IU for Biosulin Pen; 60 IU for NovoPen 4, HumaPen Luxura, and Itango; and 80 IU for ClikStar). Each pen was attached to the torque gauge measuring equipment (HTG-500NC, Imada Co. Ltd., Aichi-ken, Japan), and the maximum torque was measured when the dose-setting dial was rotated at a speed of 1 IU/s or less to the maximum dose.
Measurement of Audible Clicks
Click sounds made on dose-setting and on injection were measured for five and four pen types, respectively (Biosulin Pen does not make click sounds on injection and so was excluded from that measurement). Audible clicks were tested on one pen of each pen type. A condenser microphone (C3000, AKG, Struder Japan, Ltd., Tokyo, Japan) was placed 10 cm from the pen to record the click sound, and the sound was quantized with 16 bit/48 kHz using a digital-to-analog converter (Fireface 800 Sound I/O, RME, Germany). A WAV format file was generated on an analytical computer (Dimension 8250, Dell computer; Microsoft Windows XP SP2) using the quantized data.
To measure the sound on dose-setting, each pen was set to 20 IU (the dose-setting dial was rotated from 0 to 20). To measure the sound on injection, the needle was attached to the pen, air shots were delivered until a drop of insulin was visible at the needle tip, and the pen was set to deliver 20 IU. Click sounds were then measured when insulin was delivered at 10 IU/s. For both measurements, the distribution of frequency components per click and the mean time per click were measured and fast Fourier transform analysis was performed.
If any problems or failures were observed during any of the tests, these were recorded.
Statistics
Two-sided Mann-Whitney U nonparametric statistical tests were carried out for the total area under the torque versus rotation curve (t-AUC) for cartridge-fitting (°•N) and dose-setting (kgf•cm), without adjusting for multiple comparisons. In addition, two-sided Mann–Whitney U nonparametric statistical tests were performed on the difference in the mean injection force (N) between vertical and oblique injections for each pen type, without adjusting for multiple comparisons.
Results
Cartridge-Fitting Torque
Mechanisms for fitting the cartridge to the pens differed between pens: NovoPen 4 and ClikStar allow the cartridge to be fitted in a single movement with a 70° and 180° turn, respectively, and HumaPen Luxura, Biosulin Pen, and Itango require the cartridge to be screwed in through several revolutions with a 900°, 1080°, and 990° total turn, respectively.
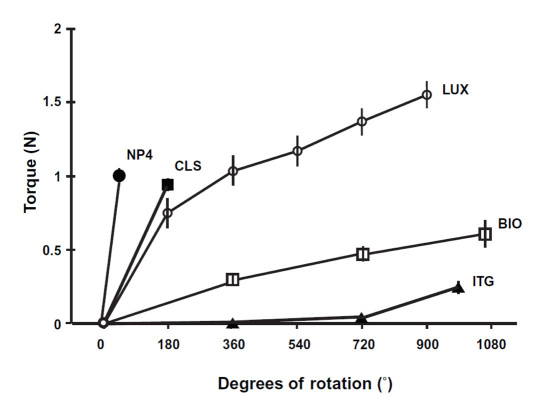
The t-AUC (Figure 1) for the cartridge-fitting torque was calculated for each pen type (mean ± SD). NovoPen 4 and Itango had the lowest t-AUC at 35.1 ± 1.3°•N and 69.4 ± 10.9°•N, respectively; the t-AUC values for HumaPen Luxura, Biosulin Pen, and ClikStar were 928.6 ± 52.4°•N, 415.0 ± 22.0°•N, and 86.0 ± 4.0°•N, respectively. The t-AUC values of the cartridge-fitting torques were significantly different for all comparisons between pen types (p < .001). HumaPen Luxura had the highest maximum cartridge-fitting torque value at 1.56 ± 0.09 N, followed by NovoPen 4 at 1.00 ± 0.04 N, ClikStar at 0.96 ± 0.04 N, Biosulin Pen at 0.61 ± 0.09 N, and Itango at 0.25 ± 0.04 N.
Figure 1.
Cartridge-fitting torque (mean ± SD) for NovoPen 4, HumaPen Luxura, ClikStar, Itango, and Biosulin Pen. NP4, NovoPen 4; LUX, HumaPen Luxura; CLS, ClikStar; ITG, Itango; BIO, Biosulin Pen.
Injection Force
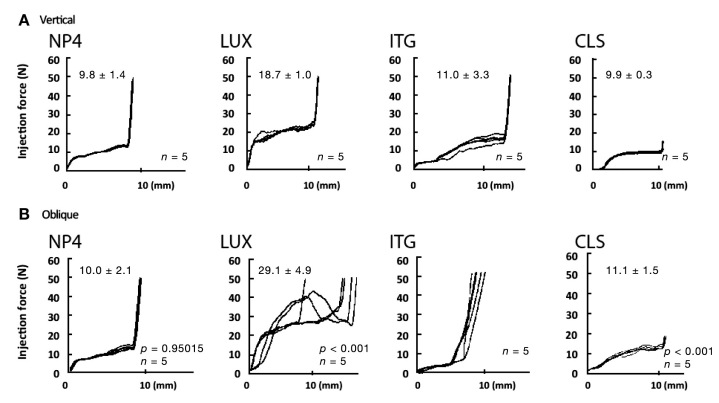
During vertical compression of the push-button, NovoPen 4, ClikStar, and Itango had similarly low injection forces (9.8 ± 1.4 N, 9.9 ± 0.3 N, and 11.0 ± 3.3 N, respectively; Figure 2). HumaPen Luxura had an injection force approximately twice that of other pens (18.7 ± 1.0 N). When the push-button was compressed at an oblique angle, the injection force was low and similar to the force during vertical compression for NovoPen 4 (10.0 ± 2.1 N, p = .95 compared with injection during vertical compression) and ClikStar (11.1 ± 1.5 N, p < .001 compared with injection during vertical compression) but was considerably higher for HumaPen Luxura (29.1 ± 4.9 N, p < .001).
Figure 2.
Injection force profiles for NovoPen 4, HumaPen Luxura, ClikStar, and Itango when push-button was depressed (A) vertically and (B) at a 14.7° angle. P-values relate to the difference between upright and oblique injection force (within pen-type). Numbers shown on each figure are mean ± SD for the five injection force profiles. P-values relate to the difference between upright and oblique injection force (within pen type). NP4, NovoPen 4; LUX, HumaPen Luxura; CLS, ClikStar; ITG, Itango.
In three of the initial five HumaPen Luxura pens and one of the initial five Itango pens, the injection force at the oblique angle failed to produce any measurement.
Therefore, in order to obtain five readings of injection force at an angle for each pen type, eight HumaPen Luxura and six Itango pens were tested in total. Injection force values could still not be obtained for Itango when compressing the push-button at an oblique angle, because the injection force spiked before the push-button had been displaced by 5 mm.
Dose-Setting Dial Torque
Lowest dial torque (mean ± SD) was measured with HumaPen Luxura (0.02 ± 0.01 kgf•cm), and highest dial torque was measured with Biosulin Pen (0.64 ± 0.03 kgf•cm). NovoPen 4, ClikStar, and Itango had dial torque values of 0.37 ± 0.02, 0.26 ± 0.03, and 0.22 ± 0.02 kgf•cm, respectively. Dial torque values were significantly different between all pen types (p < .001).
Measurements of cartridge-fitting torque, dose-setting dial torque, and injection force (both vertical and angled) of the pens were ranked according to the numerical differences between the pens (Table 2).
Table 2.
Ranking of Pen Performance for Cartridge-Fitting Torque, Injection Force, and Dose-Setting Dial Torque
| Measurement | Cartridge-fitting torque (t-AUC;°•N) | Injection force (N) | |||
|---|---|---|---|---|---|
| Verticala | Obliqueb | ||||
| Rankc | 1 | NovoPen 4 | NovoPen 4 | NovoPen 4 | HumaPen Luxura |
| 2 | Itango | ClikStar | ClikStar | Itango | |
| 3 | ClikStar | Itango | HumaPen Luxura | ClikStar | |
| 4 | Biosulin Pen | HumaPen Luxura | NovoPen 4 | ||
| 5 | HumaPen Luxura | Biosulin Pen | |||
Measurement not taken for Biosulin Pen due to automatic injection mechanism
Measurement not taken for Biosulin Pen due to automatic injection mechanism nor Itango because injection force spiked before push-button was displaced by 5 mm
Rankings based on numerical differences; 1 = highest ranking, 5 = lowest ranking
Audible Clicks
During dose-setting, the mean click duration (calculated as the mean of 20 clicks, with each click corresponding to 1 IU on the dose-setting dial) was 0.0099 s in NovoPen 4, 0.0081 s in HumaPen Luxura, 0.0149 s in Itango, 0.0087 s in ClikStar, and 0.0064 s in Biosulin Pen.
The relationship between the duration of click sound for setting the dose to 20 IU, the sound frequency (Hz), and the sound pressure level (dB) showed that NovoPen 4, HumaPen Luxura, and Biosulin Pen had clearer click sounds, with less variability in the distribution of the frequencies, whereas the click sound in Itango had large differences in the sound pressure level and a scattered distribution of the strong frequency component. ClikStar also showed larger variations in frequency of the click sounds on dose-setting.
During delivery of insulin from each pen, mean time per click (with each click signifying the delivery of 1 IU of insulin) was 0.0045 s for NovoPen 4, 0.0088 s for HumaPen Luxura, 0.0073 s for Itango, 0.0075 s for ClikStar, and 0.0091 s for Biosulin Pen.
With NovoPen 4, a sound with a strong frequency component at 8000–22,000 Hz or higher during the first click and the last two clicks was heard. With HumaPen Luxura, sound pressure levels increased with the middle 4 of the 20 clicks and then subsequently decreased.
With Itango, a strong frequency component was distributed at around 2000 Hz and around 5000–24,000 Hz, but the last click produced a sound without any unique features, making it indistinguishable from other clicks. With ClikStar, there was less variability in the distribution of frequency components, and its distribution was stable from the start of insulin injection to the end.
Malfunctions
In one Biosulin Pen, the rubber stopper expanded under the pressure of fitting the insulin cartridge to the body of the pen. In one ClikStar pen, the dose-setting dial stopped rotating spontaneously when setting the dose. These pens were replaced with new pens from the same lot.
Discussion
This technical study assessed some features of durable insulin pens that may affect their ease of use by diabetes patients, particularly those with manual dexterity or visual impairments. Audible clicks on dose-setting were most distinguishable on NovoPen 4, HumaPen Luxura, and Biosulin Pen, whereas NovoPen 4 and ClikStar had generally lower cartridge-fitting torque and injection (both upright and angled) force values.
Confidence in dose-setting is important to insulin pen users. Visual appearance of the dose-setting dial is one aspect, but so are the audible clicks as the dial turns.13,14 NovoPen 4, HumaPen Luxura, and Biosulin Pen had the clearest click sounds on dose-setting. Another important feedback mechanism for confident delivery of insulin are the audible clicks heard when injecting. This end-of-dose click confirmation is particularly important for users with visual impairments in providing confirmation that the complete dose has been delivered, thereby enhancing trust in the device. NovoPen 4 had the clearest end-of-dose confirmatory click of all the pens tested.
The ease with which the insulin cartridge can be fitted to the pen can also affect usability. Maximum cartridge-fitting torque may not be the best measure to assess this, because some pens require a single movement to perform this task and others require repeated turning. Therefore, while maximum torque values were recorded, the most important measure of how much effort is needed by the patient is the t-AUC during cartridge-fitting. NovoPen 4 clearly required the least overall effort to fit the cartridge to the pen body.
NovoPen 4, ClikStar, and Itango all had low injection force when the push-button was depressed vertically. However, in everyday use, and particularly among users with impaired manual dexterity, it is unreasonable to assume that every injection is made with the thumb applying pressure on the push-button at an exactly vertical angle. Therefore, testing injection force at a relatively small oblique angle may represent the daily injection technique for many patients, and results from this test suggest that HumaPen Luxura and Itango may be difficult to use in this situation. NovoPen 4 and ClikStar had the optimum injection force, and one advantage that NovoPen 4 may have over ClikStar is that the push-button extends less (at a 38 IU setting, the extension is 21.5 mm and 31.0 mm, respectively, and at a 59 IU setting, the extension is 27.5 mm and 41.0 mm, respectively). The shorter push-button extension on NovoPen 4 is likely to make injecting easier than with ClikStar even though these two pens have equivalent injection forces. This is because short push-buttons are easier to reach, which may make the insulin pen easier to grip firmly while injecting and thus more stable on injection.15
In Japan, there is a particularly large elderly population, and many elderly people with diabetes have visual and/or manual dexterity impairments that can have a major impact on effective self-injection. Pens with features that aid self-injection in these patients, such as those described in this study, are very important to ensure that patients receive optimum treatment and maintain independence. Another such feature is the readability of dose units, which may affect ease of use, particularly for patients with impaired visual acuity—the dose units were found to be larger on NovoPen 4 than any of the other pens (Table 1).
As this was a technical evaluation, further study of whether the features highlighted in this evaluation are preferred by patients or a particular subgroup of patients in every day clinical practice would be of interest.
Malfunction of one Biosulin Pen may have been due to incomplete storing of the piston rod into the pen body, causing it to push abnormally on the rubber stopper, which could cause the user problems, as it may adversely affect dosing accuracy. Malfunction of one ClikStar pen may have been caused by protrusion of the rubber stopper of the cartridge jamming if the push-button was inadvertently pressed when setting the dose, preventing the dial from turning. This appeared to be resolved by reattaching the cartridge holder.
Conclusion
The technical study showed that durable insulin injection pens such as NovoPen 4 have useful attributes that may facilitate the performance of a series of operations related to injection, including pen assembly, dose-setting, and injecting, and may be particularly useful for elderly diabetes patients with visual and/or manual dexterity impairments.
Acknowledgments
Editorial assistance was provided by Nicole Meinel at ESP Bioscience (Crowthorne, United Kingdom).
Glossary
Abbreviations
- (SD)
standard deviation
- (t-AUC)
total area under the curve
Funding
This work was funded by Novo Nordisk A/S (Bagsvaerd, Denmark).
Disclosure
Toshinari Asakura received funding from Novo Nordisk to perform this study.
References
- 1.Bohannon NJ. Insulin delivery using pen devices. Simple-to-use tools may help young and old alike. Postgrad Med. 1999;106(5):57–58. doi: 10.3810/pgm.1999.10.15.751. 61–4, 68. [DOI] [PubMed] [Google Scholar]
- 2.Chantelau E, Schiffers T, Schütze J, Hansen B. Effect of patient-selected intensive insulin therapy on quality of life. Patient Educ Couns. 1997;30(2):167–173. doi: 10.1016/s0738-3991(96)00964-0. [DOI] [PubMed] [Google Scholar]
- 3.Kadiri A, Chraibi A, Marouan F, Ababou MR, el Guermai N, Wadjinny A, Kerfati A, Douiri M, Bensouda JD, Belkhadir J, Arvanitis Y. Comparison of NovoPen 3 and syringes/vials in the acceptance of insulin therapy in NIDDM patients with secondary failure to oral hypoglycaemic agents. Diabetes Res Clin Pract. 1998;41(1):15–23. doi: 10.1016/s0168-8227(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 4.Albano S ORBITER Study Group. Assessment of quality of treatment in insulin-treated patients with diabetes using a pre-filled insulin pen. Acta Biomed. 2004;75(1):34–39. [PubMed] [Google Scholar]
- 5.Lombardo F, Salzano G, Messina MF, De Luca F. Compliance and administration methods in management of type 1 diabetes. Acta Biomed. 2005;76(Suppl 3):66–69. [PubMed] [Google Scholar]
- 6.Summers KH, Szeinbach SL, Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26(9):1498–1505. doi: 10.1016/j.clinthera.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Rubin RR, Peyrot M. Quality of life, treatment satisfaction, and treatment preference associated with use of a pen device delivering a premixed 70/30 insulin aspart suspension (aspart protamine suspension/soluble aspart) versus alternative treatment strategies. Diabetes Care. 2004;27(10):2495–2497. doi: 10.2337/diacare.27.10.2495. [DOI] [PubMed] [Google Scholar]
- 8.Keith K, Nicholson D, Rogers D. Accuracy and precision of low-dose insulin administration using syringes, pen injectors, and a pump. Clin Pediatr (Phila) 2004;43(1):69–74. doi: 10.1177/000992280404300109. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2010 Diabetes complications. http://www.cdc.gov/diabetes/statistics/complications_national.htm. Accessed December 6. [Google Scholar]
- 10.Løseth S, Mellgren SI, Jorde R, Lindal S, Stålberg E. Polyneuropathy in type 1 and type 2 diabetes: comparison of nerve conduction studies, thermal perception thresholds and intraepidermal nerve fibre densities. Diabetes Metab Res Rev. 2010;26(2):100–106. doi: 10.1002/dmrr.1049. [DOI] [PubMed] [Google Scholar]
- 11.Miralles-García JM, de Pablos-Velasco P, Cabrerizo L, Pérez M, López-Gómez V Sociedad Española de Endocrinología y Nutrición. Prevalence of distal diabetic polyneuropathy using quantitative sensory methods in a population with diabetes of more than 10 years' disease duration. Endocrinol Nutr. 2010;57(9):414–420. doi: 10.1016/j.endonu.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 13.Asakura T, Seino H, Yohkoh N. Comparative study of intuitive impression from use of two types of insulin device (Levemir FlexPen and OptiClik) and usability/reliability after instruction. J Jap Diab Soc. 2008;51(Suppl 1):S283. [Google Scholar]
- 14.Asakura T, Seino H, Kageyama M, Yohkoh N. Comparative study on click sounds produced by three types of prefilled pen devices (FlexPen, SoloSTAR, MirioPen) on dose setting. Jpn J Pharm Health Care Sci. 2008;34:1023–1027. [Google Scholar]
- 15.Asakura T, Seino H, Soeta K, Matsui Y, Kageyama M, Yasue N. Push-button displacement on insulin pens and usability. Practice. 2004;21(6):740–744. [Google Scholar]