Abstract
Japanese companies were the first in the world to achieve a colorimetric glucose measurement meter back in 1973. Over the following 40 or so years, they succeeded in achieving a much greater level of user-friendliness and performance and in so doing, have contributed to the spread of self-monitoring of blood glucose. This article aims to unravel the history of blood glucose measurement's technological developments; to look at the direction and features of the development path Japan is taking; as well as to introduce some Japanese products that are on the market.
Keywords: blood glucose, blood glucose meter, colorimetric, electrochemistry, Japan, self-monitoring of blood glucose
Introduction
In 1973, ARKRAY, Inc. (company name changed from Kyoto Daiichi Kagaku Co., Ltd. in 2000) developed a quantitative blood glucose monitoring system based on the technology of colorimetric blood glucose test strips developed in the United States in the 1960s. This system from ARKRAY, Inc., was a portable reflectance spectro-photometer, which became a cornerstone of practical self-monitoring of blood glucose (SMBG) use.
During the 1990s, several Japanese companies succeeded in the development of ultra-easy SMBG systems combining palm size meters and colorimetric or electrochemical-based test strips. Since then, that type of SMBG system with handheld meter/disposable test strip has become the de facto standard in the expanding worldwide market of diabetes care.
Of all SMBG products supplied throughout the world, 20% are Japanese made and are highly regarded for their manufacturing technology and quality control, which provides safety and peace of mind to all patients with diabetes.
SMBG Systems Based on the Colorimetric Principle
The world's first blood glucose test strips that employed the colorimetric method were developed by Dr. Free, from Miles Laboratories Inc., at the start of the 1960s. To make the strip, a filter paper was dipped in an aqueous solution containing glucose oxidase (GOx), peroxidase, and chromogen. The paper was then dried after incubation in the mixture and dipped in a nitrocellulose solution to form a semipermeable membrane to allow serum glucose to pass into the reagent layer while blocking passage of blood cells. In 1963, Dr. Free obtained a U.S. patent on this test strip (USP No. 3092465).1 The following year, he began to market it under its commercial name Dextrostix.2
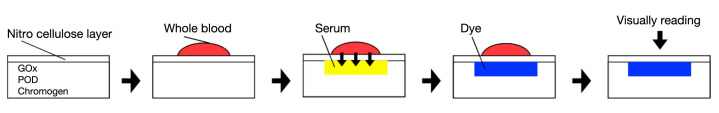
With Dextrostix, the blood glucose level was determined through the application of a large blood drop (approximately 30 μl) taken from a finger needle-stick. After a 2-min reaction time, the user had to rinse the blood cells off the sensor before viewing the color change against a color chart so as to semiquantitatively compare their blood sugar level to a normal (50–100 mg/dl), middle (100–200 mg/dl), or high (200–400 mg/dl) level. The structure of this system is shown in Figure 1 and the principle in Method 1.
Figure 1.
Structure of the Dextrostix test strip.
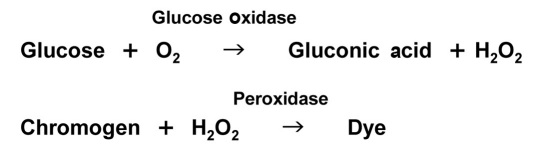
Method 1.
Reaction principle.
In order to achieve quantitative measurement with Dextrostix, Miles Laboratories Inc. decided to develop a spectrophotometer with which to measure light reflectance from the test strip. The company approached a Japanese company, ARKRAY, Inc., and asked them to develop an improved, quantitative system with greater practicality. The new system developed by ARKRAY, Inc. improved precision through the use of integrated sphere measurement to detect all reflectance light from the area of color development. This device initially went on sale in Europe and the United States in 1973, under the commercial name of EYETONE (see Figure 2) and then in Japan under the commercial name DEXTROMETER.3
Figure 2.
EYETONE and the Dextrostix test strips.
Development of this system led companies such as Boehringer Mannheim (now Roche Ltd.), ARKRAY, Inc., LifeScan, Inc. (now a subsidiary of Johnson & Johnson Services, Inc.), and Terumo Co. (of Japan) to develop their own colorimetric test strips and meters, which in turn led to the further spread of SMBG around the world. During this period of development, ARKRAY, Inc. made two major changes to the conventional SMBG test strip method, achieving wide acceptance in the Japanese market. First, they changed the test strip substrate from a paper filter to a plastic film, which enabled a more uniform color development of the reagent as well as changed the way the user removed specimen from the test strip, which was originally achieved by rinsing with water. Removing the specimen from the detection area was important for precise measurement but the measurement performance was dependent on how the user rinsed it. To overcome this imprecision, the company introduced cotton wool to wipe excess blood from the test strip instead of rinsing. This procedure was easier and safer, preventing contamination of the specimen. ARKRAY, Inc. incorporated these technological advances in developing the GLUCOSCOT (Figure 3).4
Figure 3.
GLUCOSCOT system (from ARKRAY, Inc.).
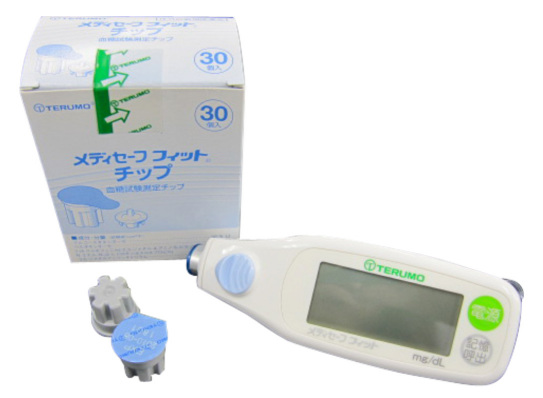
Terumo Co. is a Japanese company with a large share in the medical syringe market not only in Japan but also worldwide. Their lancet device and needle for finger sticking made it possible to obtain a very small volume of whole blood. In addition to this, in the early 1990s, they developed a GOx-based colorimetric system called Mediace. Since then, they have continued to develop and improve colorimetric test strips culminating in their 2009 release of the Medisafe® Fit, a system with performance comparable to those of electrochemical test strips on the market (Figure 4). This company holds a nearly 20% share of the SMBG system market in Japan.
Figure 4.
Medisafe Fit (from Terumo Co.).
A SMBG System Based on the Electrochemical Principle
The next stage of the story came when a group of talented engineers from a venture capital company, Genetics International, Inc., (established in Inverness, United Kingdom) managed to develop a disposable blood glucose test strip using a GOx and electron mediator mixture placed upon an electrode substrate. The test strip was then measured by a handheld, pen-shaped device capable of detecting the small current signal that was generated, which corresponds to the concentration of glucose in the sample. This technology was patented in 1985 (USP No. 4545382).5
The measurement process was very simple: a disposable test strip was placed at the tip of the device to which 10 μl of whole blood from a finger stick was applied. Over the next minute, hydrogen peroxide generated from the catalytic GOx reaction was detected by the electrode and converted into a whole blood glucose concentration and displayed on the screen located on the side of the device.6,7
Efforts were made to market the system under the brand name ExacTech® (Figure 5) but as the meter was just the size of a pen, many people found it too small and difficult to use. In particular, the tiny display screen made it very difficult to read results, which led to disappointing sales.
Figure 5.
ExacTech and its dedicated test strip.
Yet during the same period, Mr. Nankai and his research team from Matsushita Electric Industrial Co. Ltd. (now Panasonic Co.) tried developing a disposable glucose test strip similar to the one from Genetics International, Inc. The principle of its measurement is shown in Figure 6.
Figure 6.
The principle used in the disposable test strip developed by Nankai's group.
In 1987, a joint development agreement was signed between ARKRAY, Inc. and Matsushita Electric Industrial Co. for development of an electrochemical-based SMBG system. By utilizing Nankai's sensor technology, Shigeki Yamada and his colleagues at ARKRAY, Inc. succeeded in developing an epoch-making SMBG system in 1991.
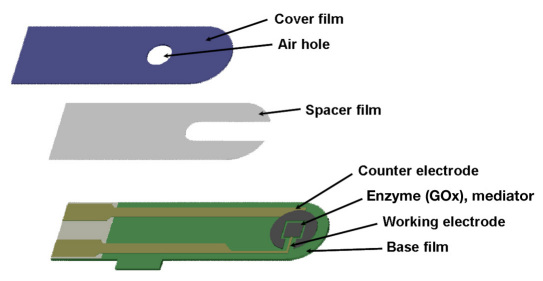
This new system came in the form of a buttonless meter, the same size as a credit card. By just inserting the strip into the meter and applying a small volume of finger-stick whole blood (5 μl), the user could have his or her blood glucose concentration within 60 seconds. These features were a considerable advance from the earlier systems and nothing short of revolutionary in the field of SMBG. The use of this new GLUCOCARD™ product became widespread not only in Japan but elsewhere around the world too. The secret behind its elegant ease of use was the absence of any tricky application of finger-stick blood to the strip, as this had been made redundant by the internal capillary structure. In the Japanese market, GLUCOCARD, with its simplicity of operation and great measurement precision, immediately became popular and soon achieved 80% of share within several years of the launch. As of the middle of 2011, it holds 60% of market share. The structure of this system is shown in Figure 7; its measurement principle in Method 2; and its photograph in Figure 8.
Figure 7.
The structure of the GLUCOCARD sensor.
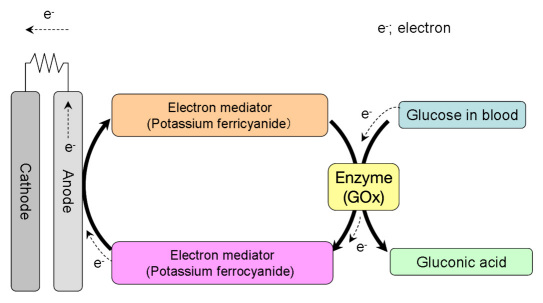
Method 2.
The measurement principle behind the GLUCOCARD sensor.
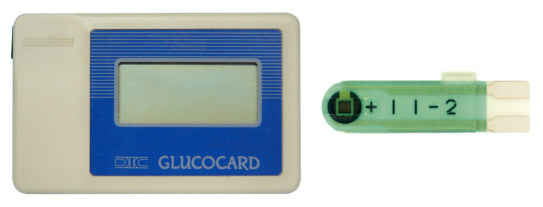
Figure 8.
GLUCOCARD meter and GLUCOCARD sensor.
Yet even with all this simplification, the GLUCOCARD underwent a number of improvements and upgrades. The sensor enzyme was changed from GOx to flavin adenine dinucleotide-dependent glucose dehydrogenase (FAD-GDH) (an enzyme developed by Professor Koji Sode, Tokyo University of Agriculture and Technology8) resulting in the X-SENSOR/X-METER system with an even smaller sample size of 0.3 μl and a measurement time reduced from 60 seconds to just 5 seconds. In a 21st century marked by a mushrooming population of patients with diabetes, this is a high-precision, fast, and innovative product that requires a tiny sample volume and is simple enough for anyone to use. In short, it has contributed significantly to the field of diabetes care. The principle and the detail of the measurement of the X-SENSOR are shown in Method 3, Table 1, and Figure 9.
Method 3.
Measurement principle of the X-SENSOR.
Table 1.
GLUCOCARD X-METER Specifications
| Test | Glucose in whole blood |
|---|---|
| Sample volume | 0.3 μl |
| Test strip | GLUCOCARD X-SENSOR |
| Measuring range | 10–600 mg/dl |
| Measuring time | 5 seconds |
| Power source | A 3-volt Lithium battery (CR2032 or DL2032) |
| Battery life | Approximately 2000 tests |
| Memory | 360 test results |
| Clock accuracy | +75 to -75 s/month |
| Operating environment | Temperature range 10–40 °C (50–104 °F) Humidity 20–80% relative humidity (no dew condensation) |
| Dimensions | 50 × 100 × 12 mm |
| Weight | Approximately 45 g |
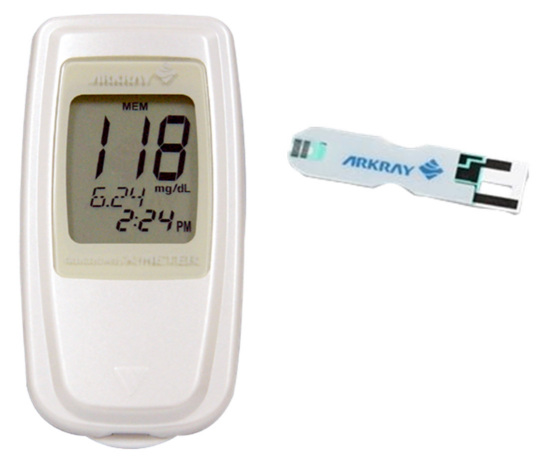
Figure 9.
The GLUCOCARD X-METER and X-SENSOR.
Japanese SMBG Systems Available in the Market
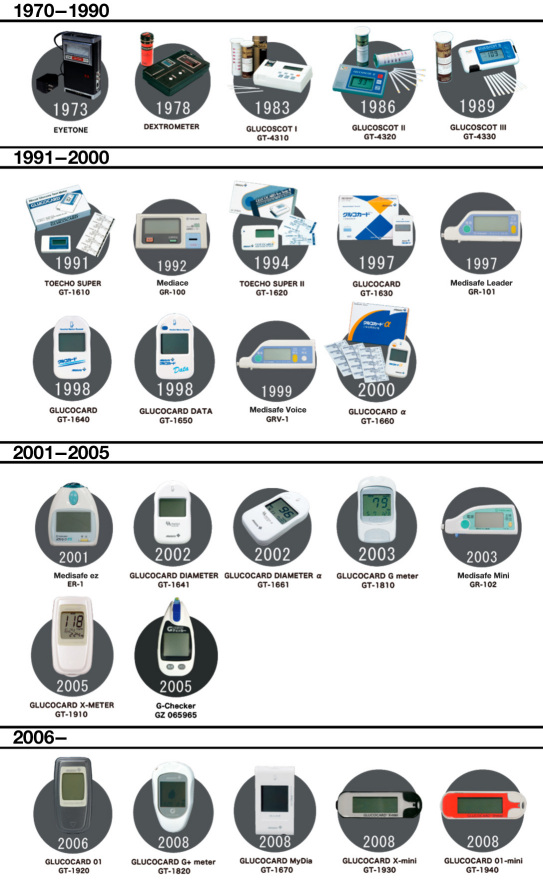
In the 40 years since 1970, ARKRAY, Inc. has developed more than 20 types of blood glucose meters. Together with products from Panasonic Co., Terumo Co., and Gunze Ltd., nine systems developed by Japanese companies are available worldwide as described later in this article. The history of SMBG meters is shown in Figure 10. A comparison of device specifications is shown in Table 2.
Figure 10.
History of SMBG development of Japanese companies
Table 2.
Specification Comparison of Models Developed in Japan
| Name | 1. GT-Series | 2. Contour Series | 3. G-Series | 4. Medisafe Mini | 5. X-Series | 6. G-checker | 7. 01-Series | 8. Medisafe Fit | 9. V1-Series |
|---|---|---|---|---|---|---|---|---|---|
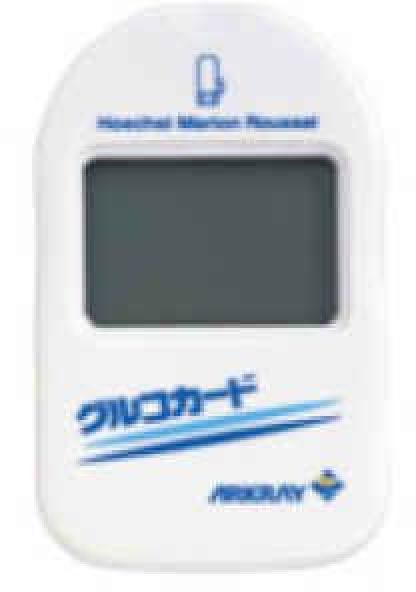
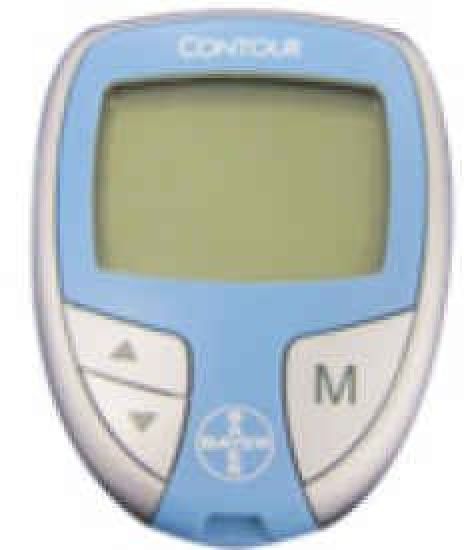
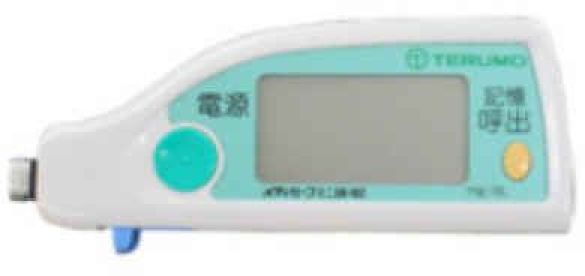
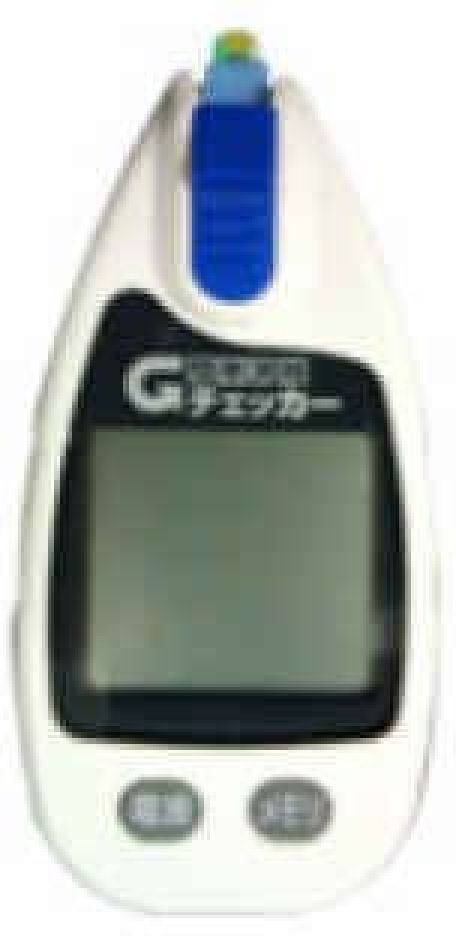
| GLUCOCARD | Contour | GLUCOCARD G+ meter | Medisafe Mini | GLUCOCARD X-METER | G-Checker | GLUCOCARD 01 | Medisafe Fit | GLUCOCARD Vital | |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
| Enzyme | GOx | GDH | GDH | GOx | GDH | GOx | GOx | GOx | GOx |
| Minimum sample volume | 3 μl | 0.6 μl | 0.6 μl | 1.2 μl | 0.3 μl | 2.5 μl | 0.3 μl | 0.8 μl | 0.5 μl |
| Measurement time | 30 s | 5 s | 5.5 s | 10 s | 5 s | 18 s | 7 s | 9 s | 7 s |
| Effect from partial pressure of oxygen | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes |
| Effect from maltose | No | No | No | No | Yes | No | No | No | No |
| Manufacturer | Panasonic | Panasonic | Panasonic | Terumo | ARKRAY | GUNZE | ARKRAY | Terumo | ARKRAY |
| Distributor | ARKRAY | Bayer | ARKRAY | Terumo | ARKRAY | EIDIA | ARKRAY | Terumo | ARKRAY |
1. GLUCOCARD GT-Series
Jointly developed by Panasonic Co. and ARKRAY, Inc., this SMBG system was released in 1991 and is sold in a total of 40 countries including Japan, the United States, and countries in Europe and Asia. As the first widely adopted system using the electrode method, it has managed to maintain its reputation as a reliable brand for the past 20 years.
2. CONTOUR® Series
This is a system developed in 2003 by Panasonic Co. and sold overseas (excluding Japan) by Bayer AG. One feature of this system is the low interference from maltose through the use of FAD-GDH.
3. GLUCOCARD G-Series
This is a SMBG system developed by Panasonic Co. that uses the same sensor as the CONTOUR series but with a different meter design. This is sold by ARKRAY, Inc. in Japan, China, and Europe.
4. Medisafe® Mini
This is a SMBG system based on colorimetric principle and using GOx. It was launched in 2003 by Terumo Co. and showed significant improvement with its 50% reduction in measurement time and required sample volume compared to its predecessor, the Mediace.
5. GLUCOCARD X-Series
Developed by ARKRAY, Inc. in 2005, this system is sold worldwide. It features a short reaction time (5 seconds), FAD-GDH with excellent temperature stability, tiny sample volume (0.3 μl), and great precision (with a coefficient of variation of less than 3%).
6. G-Checker
Launched in 2005 by Gunze Ltd., this is an electrochemical-based system that uses GOx. It uses a platinum electrode with wide dynamic range (20–900 mg/dl).
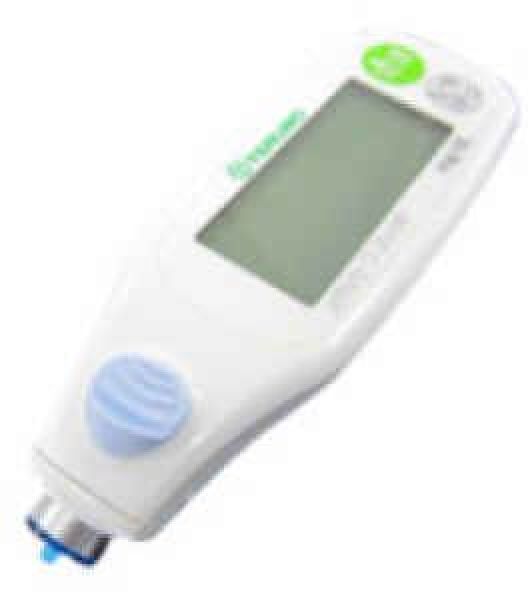
7. GLUCOCARD 01-Series
Developed by ARKRAY, Inc. in 2007, this system uses GOx and high precision in line with consumer needs. It is a low-priced alternative sold worldwide.
8. Medisafe Fit
This is a colorimetric, GOx-based SMBG system released by Terumo Co. in 2009. Although it does use a colorimetric method, it has a measurement precision and specifications comparable to electrochemical-based sensors together with a 0.8-μl sample volume and a 9-second measurement time.
9. GLUCOCARD V1-Series
This GOx-based SMBG system was developed by ARKRAY, Inc., in 2010. It is a low-cost system aimed at developing countries while maintaining the same high performance of the 01-Series.
Developmental Direction and Features of Japanese SMBG Systems
Japan has a history of fermentation technology as can be seen through the production of foods such as sake, miso, and soy sauce, which date back hundreds of years. From the 1970s onwards, this fermentation technology allowed Japan to take the lead in supplying enzymes for in vitro diagnostics to the world, which in turn, allowed Japan to take the global lead in the development of enzymatic biosensors.
In 1979, ARKRAY, Inc. pioneered electrochemical-based glucose measurement using enzymes by developing a fully automated benchtop system, the AUTO&STAT Glucose Analyzer, which combined a sensor with a GOx immobilized membrane and a hydrogen peroxide detection electrode.
In the 1980s, development of biosensors began to pick up steam in Japan, especially in academic circles. These technologies came to blossom especially for blood glucose test strips and in 1991, ARKRAY, Inc. and Panasonic Co.'s development collaboration resulted in the GLUCOCARD.
Japan was able to take the lead in electrochemical-based glucose monitoring systems because of the combination of its cutting edge enzyme technologies with design technologies behind their sensor strips/meters. This unique combination of technologies would then enable the use of capillary integrated test strips as well as buttonless meter configurations, resulting in an extremely easy to use system. This Japanese-style form of blood glucose monitoring system would go on to become the de facto standard throughout the world.
Additionally, key factors for a disposable test strip are achievement of highly precise manufacturing as well as perfect quality control in production. Japan has a high level of production technology sufficient for the development of a fully automated production line, enabling it to maintain the high quality of Japan-made glucose test strips. This enables them to be well accepted around the world and to provide safety and peace of mind to patients with diabetes.
Glossary
Abbreviations
- (FAD-GDH)
flavin adenine dinucleotide-dependent glucose dehydrogenase
- (GOx)
glucose oxidase
- (SMBG)
self-monitoring of blood glucose
References
- 1.Adams EC, Smeby RR, inventors Miles Laboratories, Inc., assignee. Diagnostic test device for blood sugar. United States patent US 3092465. 1963 Jun 4.
- 2.Cheah JS, Wong AF. A rapid simple blood sugar determination using the Ames reflectance meter and Dextrostix system: a preliminary report. SMJ. 1974;15(1):51–52. [PubMed] [Google Scholar]
- 3.Schersten B, Kuhl C, Hollender A, Ekman R. Blood glucose measurement with Dextrostix and new reflectance meter. Br Med J. 1974;3(5927):384–387. doi: 10.1136/bmj.3.5927.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moerman J, Van Crombrugge P, Sierens L. Self blood glucose monitoring: a comparative study of the Glucoscot/Glucopat and Reflolux/Haemo-Glukotest 20-800R system. Acta Clin Belg. 1985;40(5):285–291. doi: 10.1080/22953337.1985.11719095. [DOI] [PubMed] [Google Scholar]
- 5.Higgins IJ, Hill HAO, Plotkin EV, inventors Genetics International, Inc., assignee. Sensor for components of a liquid mixture. United States patent US 4545382. 1985 Oct 8.
- 6.Lui KF, Ng WY, Thai AC. An amperometric measurement–the ExacTech pen meter. Ann Acad Med Singapore. 1990;19(4):473–476. [PubMed] [Google Scholar]
- 7.Cass AE, Davis G, Francis GD, Hill HA, Aston WJ, Higgins IJ, Plotkin EV, Scott LD, Turner AP. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal Chem. 1984;56(4):667–671. doi: 10.1021/ac00268a018. [DOI] [PubMed] [Google Scholar]
- 8.Tsuya T, Ferri S, Fujikawa M, Yamaoka H, Sode K. Cloning and functional expression of glucose dehydrogenase complex of Burkholderia cepacia in Escherichia coli. J.Biotechnol. 2006;123:127–136. doi: 10.1016/j.jbiotec.2005.10.017. [DOI] [PubMed] [Google Scholar]