Abstract
1,3-butadiene (BD)* is a known human carcinogen present in cigarette smoke and in automobile exhaust, leading to widespread exposure of human populations. BD requires cytochrome P450-mediated metabolic activation to electrophilic species, e.g. 3,4-epoxy-1-butene (EB), hydroxymethylvinylketone (HMVK), and 3,4-epoxy-1,2-diol (EBD), which form covalent adducts with DNA. EB, HMVK, and EBD can be conjugated with glutathione and ultimately excreted in urine as monohydroxybutenyl mercapturic acid (MHBMA), dihydroxybutyl mercapturic acid (DHBMA), and trihydroxybutyl mercapturic acid (THBMA), respectively, which can serve as biomarkers of BD exposure and metabolic processing. While MHBMA and DHBMA have been found in smokers and non-smokers, THBMA has not been previously detected in humans. In the present work, an isotope dilution HPLC-ESI−-MS/MS methodology was developed and employed to quantify THBMA in urine of known smokers and non-smokers (19–27 per group). The new method has excellent sensitivity (LOQ, 1 ng/mL urine) and achieves accurate quantitation using a small sample volume (100 µl). Mean urinary THBMA concentrations in smokers and non-smokers were found to be 21.6 and 13.7 ng/mg creatinine, respectively, suggesting that there are sources of THBMA other than exposure to tobacco smoke in humans, as is also the case for DHBMA. However, THBMA concentrations are significantly greater in urine of smokers than that of non-smokers (p < 0.01). Furthermore, THBMA amounts in human urine declined 25–50 % following smoking cessation, suggesting that smoking is an important source of this metabolite in humans. The HPLC-ESI−-MS/MS methodology developed in the present work will be useful for future epidemiological studies of BD exposure and metabolism.
Keywords: 1,3-butadiene; smoking; urinary metabolite; mercapturic acids; THBMA
Introduction
1,3-Butadiene (BD) is a colorless gas with a gasoline-like odor that is widely used in the polymer industry for the production of rubber and plastics.1 BD is a multi-site carcinogen in laboratory animals, capable of inducing lymphocytic lymphoma and neoplasms of the heart, forestomach, Harderian gland, mammary gland, ovary, liver, and the lung.2 Based on epidemiological evidence for an increased risk of leukemia in occupationally exposed workers,3–5 BD is classified as a “known human carcinogen” in the Ninth Report on Carcinogens (2000) by the US National Toxicology Program1 and is categorized as a “human carcinogen” by the US EPA and IARC.6 BD is also present in automobile exhaust, urban air, and is abundant in cigarette smoke (16–75 µg in mainstream smoke and 205–361 µg in side-stream smoke),7 leading to widespread exposure of the general population. Toxicological risk analysis suggests that BD has the highest cancer risk index among all tobacco constituents,8 necessitating further studies of its mechanism of action.
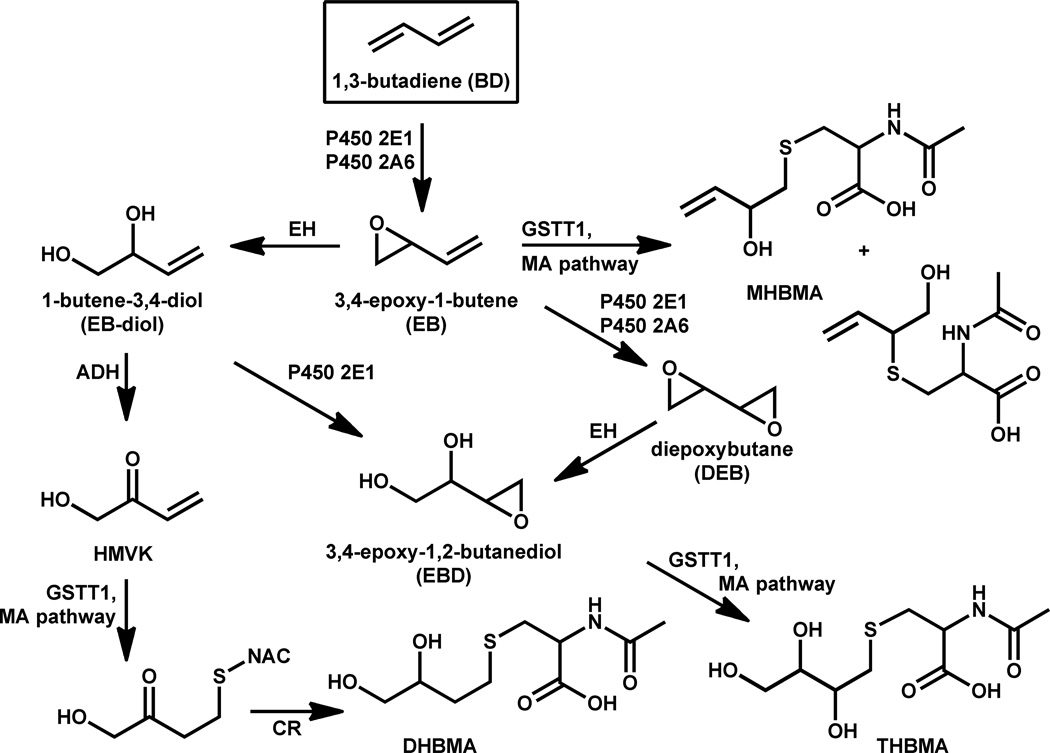
The genotoxic effects of BD are attributed to its epoxide metabolites.2,9 Cytochrome P450 monooxygenases P450 2E1 and P450 2A6 catalyze the initial epoxidation of BD to yield (R)- and (S)-3,4-epoxy-1-butene (EB) (Scheme 1).10,11 EB can be hydrolyzed to 1-butene-3,4-diol (EB-diol),12 which can be further epoxidized to form 3,4-epoxy-1,2-butanediol (EBD)13 or can undergo further metabolism to hydroxymethyl vinylketone (HMVK).14 Alternatively, EB can be subject to second oxidation catalyzed by cytochrome P450 2E1 and 3A to yield S,S, R,R, and meso-diepoxybutane (DEB).10,15
Scheme 1.
Metabolism of 1,3-butadiene to MHBMA, DHBMA and THBMA. ADH, alcohol dehydrogenase; CR, carbonyl reductase; DHBMA, N-acetyl-S-(3,4-dihydroxybutyl)-L-cysteine; EH, epoxide hydrolase; GST, glutathione-S-transferase; MA, mercapturic acid; MHBMA, (R)/(S)-N-acetyl-S-(1-hydroxymethyl)-2-propen-yl)-L-cysteine and (R)/(S)-N-acetyl-S-(2-hydroxy-3-butenyl)-L-cysteine; NAC, N-acetylcysteine; THBMA, N-acetyl-S-(2,3,4-trihydroxybutyl)-L-cysteine.
All three epoxides of BD, e.g. EB, DEB, and EBD, are direct-acting mutagens, with DEB exhibiting the most potent genotoxicity.9,16 If not detoxified by epoxide hydrolysis or glutathione conjugation, they can react with nucleophilic sites in DNA and proteins to form covalent adducts.17–20 DNA adducts induced by BD exposure include N-7-(2-hydroxy-3-buten-1-yl)guanine, N-7-(1-hydroxy-3-buten-2-yl)guanine, N-7-(2,3,4-trihydroxy-3-buten-2-yl)guanine,21 1,4-bis-(guan-7-yl)-2,3,-butanediol (bis-N7G-BD),22,23 1-(guan-7-yl)-4-(aden-1-yl)-2,3-butanediol (N7G-N1A-BD), 1-(guan-7-yl)-4-(aden-3-yl)-2,3-butanediol(N7G-N3A-BD), and 1-(guan-7-yl)-4-(aden-7-yl)-2,3-butanediol (N7G-N7A-BD).22 Several BD-induced hemoglobin adducts have been identified, e.g. N-(2-hydroxy-3-buten-1-yl)-valine (HB-Val), 1,2,3-trihydroxybutyl-valine (THB-Val), and N,N-(2,3-dihydroxy-1,4-butadiyl)-valine (Pyr-Val).24,25 DEB also induces DNA-protein cross-links that are hypothesized to interfere with DNA replication, transcription, and repair.26,27
BD is a potent carcinogen in mice,28 but is only a weak carcinogen in rats, requiring 300-fold higher concentrations to achieve the same tumor numbers.29 This can be explained by more efficient metabolic activation of butadiene to EB, DEB, and EBD, and an inefficient detoxification of butadiene epoxides in the mouse.30–32 Indeed, the concentrations of butadiene-induced DNA and protein adducts are greater in tissues of mice than in rats exposed to the same butadiene concentration.33–37
BD metabolites are excreted in urine as mercapturic acids. Glutathione conjugates of EB are processed via the mercapturic acid pathway to yield 2-(N-acetyl-L-cystein-S-yl)-1-hydroxybut-3-ene and 1-(N-acetyl-L-cystein-S-yl)-2-hydroxybut-3-ene, collectively called MHBMA (Scheme 1).38,39 HMVK undergoes glutathione conjugation followed by reduction and is excreted as 4-(N-acetyl-L-cystein-S-yl)-1,2-dihydroxybutane (DHBMA).14,40 EBD undergoes similar glutathione conjugation and metabolic conversion to form another mercapturic acid, 4-(N-acetyl-L-cystein-S-yl)-1,2,3-trihydroxybutane (THBMA).38,41 N-acetylcysteine conjugates of EB (MHBMA in Scheme 1), HMVK (DHBMA), and EBD (THBMA) can serve as useful indicators of BD exposure and metabolic activation to ultimate genotoxic species in smokers and occupationally exposed individuals, allowing for human biomonitoring and risk assessment from BD exposure.38–40,42,43
While MHBMA and DHBMA have been found in smokers and non-smokers, to our knowledge, THBMA has not been previously detected in humans. THBMA has been found in urine of rats and mice exposed to 200 ppm of radiolabelled 14C-1,3-BD for 6 hours. THBMA accounted for about 4.1% and 6.7% of the total BD dose in the rat and in mice, respectively.41 Van Sittert et. al.38 have attempted to quantify the amounts of THBMA in human urine using a GC-NECI-MS/MS method. In their approach, THBMA was isolated from smoker’s urine (1 mL) by strong anion exchange SPE and derivatized with pentafluorobenzyl bromide, followed by GC-NECI-MS/MS analysis. However, THBMA could not be quantified due to high interferences from sample matrix.38
The goal of the present study was to develop a sensitive, accurate and reliable isotope dilution HPLC-ESI−-MS/MS method for quantification of THBMA in human urine and evaluate its potential as a biomarker of exposure to BD in cigarette smoke and its metabolism to DNA-reactive species. The novel method was employed to determine the concentrations of THBMA in urine of current smokers and non-smokers and to analyze the effects of smoking cessation on THBMA levels.
Materials and Methods
Note: 1,3-butadiene and its epoxide metabolites are carcinogenic and should be handled with extreme care.
Materials
HPLC-MS grade acetic acid, methanol, acetonitrile, and d,l DEB were purchased from Sigma Aldrich (St. Louis, MO). HPLC grade acetonitrile and methanol were obtained from Fisher Scientific (Pittsburgh, PA). THBMA and its deuterated analog, d3-THBMA, were synthesized in our laboratory as described below. d3-N-acetyl- L- cysteine was purchased from Toronto Research Chemicals (Ontario, Canada). Isolute ENV+ 50 mg/1 mL SPE cartridges were purchased from Biotage (Charlotte, NC). All other materials and solvents were from Sigma-Aldrich (St. Louis, MO)
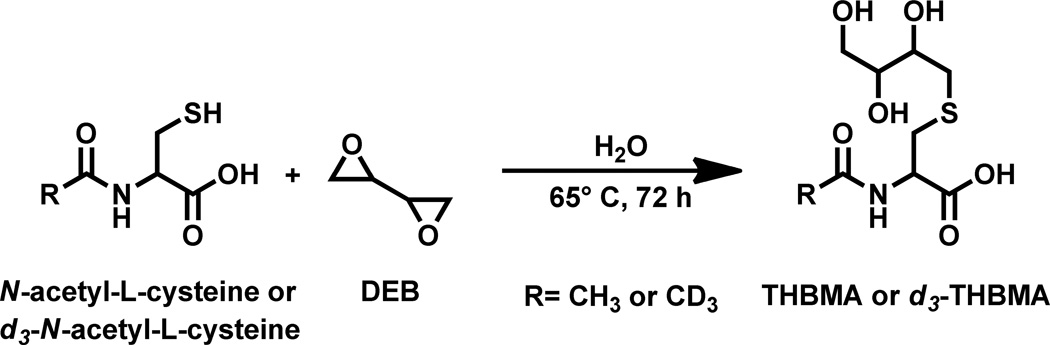
Synthesis of 4-(N-acetyl-L-cystein-S-yl)-1,2,3-trihydroxybutane (THBMA) and its deuterated analogue (d3-THBMA)
THBMA and d3-THBMA were prepared as shown in Scheme 2. N-acetyl-L-cysteine (338 mg) was dissolved in 1 mL of water. Racemic DEB (155 EL) was added, followed by mixing and incubation at 65°C for 72 h. THBMA was isolated by semi-preparative HPLC. HPLC purification was performed on an Agilent 1100 HPLC system equipped with a DAD UV detector (Agilent Technologies, Santa Clara, CA) using an Agilent Eclipse XDB C18 column (10 mm × 250 mm; 5 µm). The buffer system consisted of 5 mM N, N, dimethylhexylamine adjusted to pH 9.2 with acetic acid (A) and 3:1 methanol: acetonitrile containing 25% of 5 mM N, N, dimethylhexylamine (pH 9.2) (B). The flow rate was 3 mL/min, and the solvent composition was changed linearly from 1 to 13% (B) in the first 12 min. The gradient was ramped up to 50% (B) in the next 2 minutes and held at that composition for 3 minutes. The solvent composition was brought back to the initial composition of 1% (B) in the next three minutes and equilibrated at those conditions for 12 minutes. THBMA eluted as a broad peak at 14 minutes.
Scheme 2.
Synthetic scheme for preparation of THBMA and d3-THBMA.
HPLC fractions containing THBMA were collected, concentrated under vacuum, and re-purified with the same column and solvent system using a slower gradient. For the second round of purification, the flow rate was 3 mL/min, and the solvent composition was linearly changed from 8 to 30% (B) in 20 minutes. The system was brought back to 8% (B) in 1 minute and equilibrated at these conditions for 11 minutes. Under these conditions, THBMA eluted at 10.5 minutes. HPLC peaks corresponding to THBMA were collected and concentrated under vacuum (estimated reaction yield, 1%).
d3-THBMA was synthesized analogously starting with N-acetyl- d3- L- cysteine (Scheme 2). The isotopic purity of d3-THBMA as determined by mass spectrometry was as follows: THBMA-d3 (91%), THBMA-d2 (7.7%), THBMA-d1 (1.1%), THBMA-d0 (0.2%).
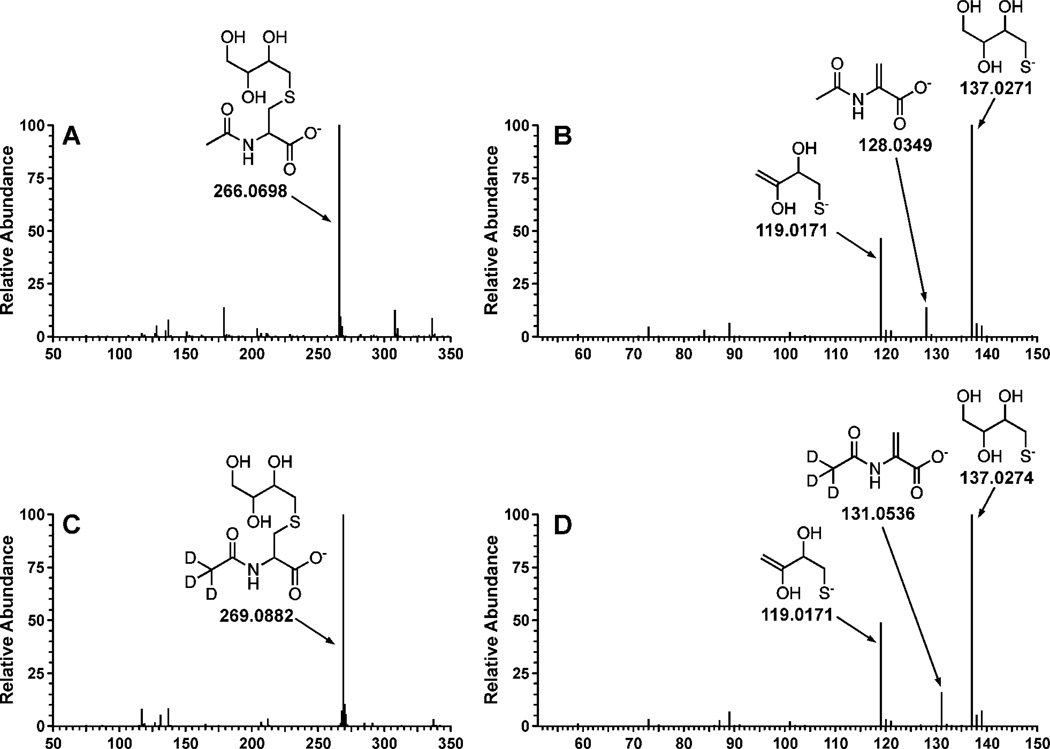
THBMA and d3-THBMA (Scheme 2) were structurally characterized by high resolution tandem mass spectrometry on Waters Acquity UPLC/Synapt G2 QTOF mass spectrometer and NMR. The HPLC conditions for accurate mass measurements included a Waters Acquity UPLC HSS T3 column (2.1 × 100, 1.8µ) eluted at a flow rate of 0.4 ml/min using 97:3 0.1% formic acid in water: 0.1% formic in ACN as the mobile phase. The sample was introduced into the mass spectrometer using “Lockspray” dual electrospray ion source (Agilent ESI tune mix as calibrant) and analyzed in the negative ionization mode with a m/z scan range of 50–300. MS/MS fragmentation patterns were as follows: THBMA (ESI−-MS : 266.0698 [M-H]−, MS/MS: 137.0271, 128.0349 and 119.0171); d3-THBMA (ESI−-MS : 269.0882 [M-H]−, MS/MS: 137.0274, 131.0536, 119.0171) (Figure 1). 1H NMR of THBMA: δ 7.9 (1H, s, cys-NH), 4.2 (1H, m, αC-cys), 3.5-3.3 (4H, m, OCH2, OCH), 2.9-2.5 (4H, m, SCH2), 1.8 (3H, s, COCH3) (Supplementary Figure S-1).
Figure 1.
Structural characterization of THBMA and d3-THBMA by accurate mass measurements with a Waters Acquity UPLC/Synapt G2 QTOF mass spectrometer. Shown are the full scan accurate mass ESI− TOF spectrum (A) and ESI−-TOF-MS/MS product ion spectrum of THBMA (B) and the corresponding spectra of d3-THBMA (C,D).
THBMA stock solution concentrations were determined by proton NMR. Toluene (1.12 µmol) was added to THBMA standard dissolved in DMSO (Supplementary Figure S-1). The ratio of 1H NMR integrations corresponding to aromatic protons of toluene (5H, 7.2 ppm) and a C-H proton of THBMA (1H, 4.2 ppm) was used to determine THBMA concentration (Supplementary Figure S-1). d3-THBMA concentrations were determined by capillary HPLC-UV using THBMA as an external standard and confirmed by HPLC-ESI-MS/MS analysis of solutions spiked with known amounts of unlabeled THBMA.
Sample Preparation
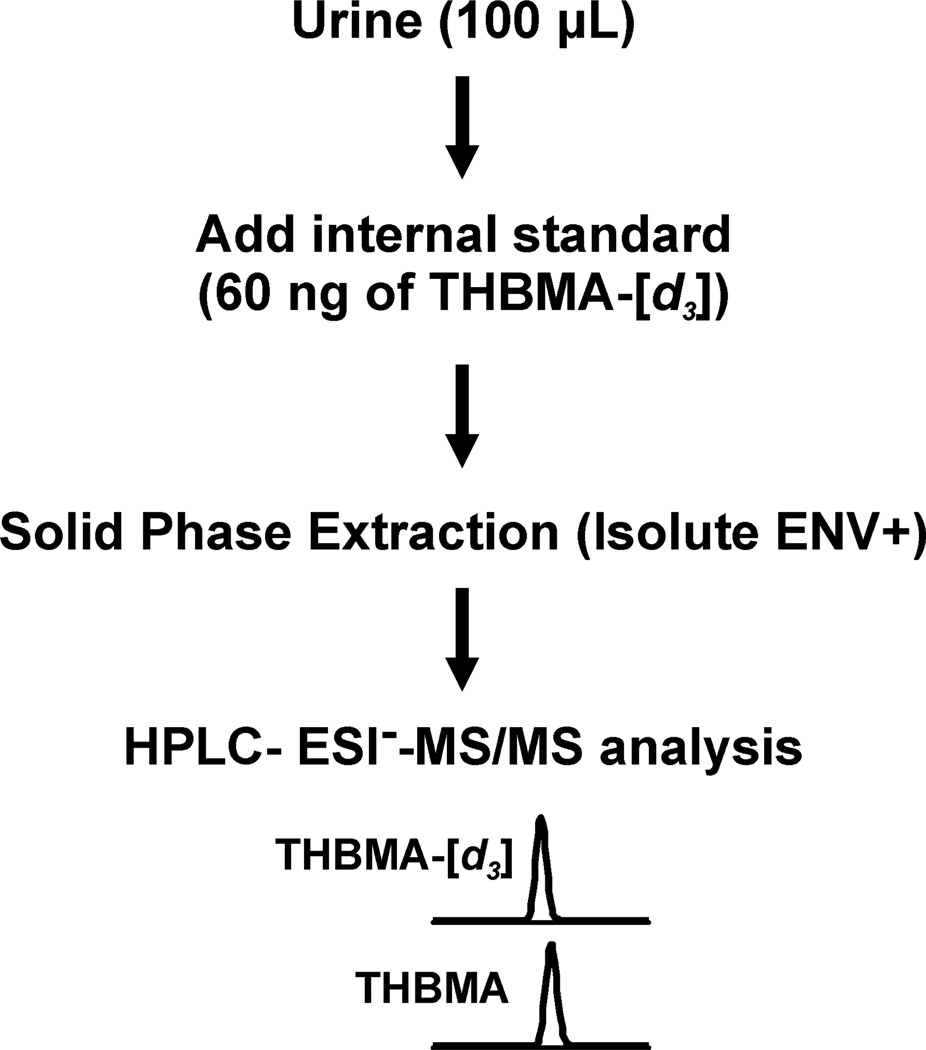
Solid phase extraction (SPE) was performed using Isolute ENV+ cartridges (1 mL / 50 mg) obtained from Biotage (Charlotte, NC, USA). Human urine (100 µL) was mixed with 100 µL of 50 mM ammonium formate buffer (pH 2.5), 10 µL of formic acid (98%), and 60 ng of d3-THBMA. Samples were vortexed repeatedly and centrifuged at 13,000 rpm for 15 minutes. The supernatant was loaded onto SPE cartridges pre-conditioned with methanol and 0.3% formic acid (3 ml each). The cartridges were sequentially washed with 1.5 mL of 0.3% formic acid and 0.75 mL of 5% aqueous methanol containing 0.3% formic acid and allowed to dry completely under vacuum for 20 minutes. THBMA and d3-THBMA were eluted with 1.2 mL of 2% formic acid in methanol, dried under vacuum, and reconstituted with 20 µL of 0.2% acetic acid for HPLC-ESI-MS/MS analysis.
Liquid chromatography-Tandem Mass spectrometry
HPLC-ESI−-MS/MS analysis was conducted using an Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA) coupled with a Thermo-Finnigan TSQ Quantum Discovery mass spectrometer (Thermo Scientific Corp., Waltham, MA). A SIELC Primesep B2 column (5 µm, 2.1 × 50 mm) was equipped with a guard column of the same packing (5 µm, 2.1 × 10 mm). The column was maintained at 50° C and eluted with a gradient of 0.2% acetic acid (A) and acetonitrile (B) at a flow rate of 150 µl/min. The solvent composition was maintained at 1% B for the first six minutes and then increased to 50% B in the next three minutes. The column was eluted with 50% B for three more minutes, and the solvent composition was brought back to the initial conditions of 1% B in the next three minutes. The column was equilibrated at 1% B for 10 minutes before a new injection was made. Under these conditions, THBMA and d3-THBMA eluted at 5.12 and 5.18 minutes, respectively. The total HPLC-ESI−-MS/MS run time was 25 minutes.
The mass spectrometer was operated in the ESI− mode, and typical MS settings were as follows: spray voltage, − 3500 V; sheath gas pressure, 50 psi; capillary temperature, 250° C; collision energy, 16; source CID, −9 V; collision gas pressure, 1.0 mTorr; Q1 (FWHM), 0.4; Q3 (FWHM), 0.7; scan width, 0.4 m/z; and scan time, 0.4 s. The mass spectrometer settings were optimized upon direct infusion of authentic standards and may vary slightly between analyses. Quantification of THBMA was performed in the selected reaction monitoring (SRM) mode by isotope dilution with d3-THBMA. The SRM transitions used for quantitation were: m/z 266.1 → 137.1 (THBMA) and m/z 269.1 → 137.1 (d3-THBMA). An additional SRM transition (m/z 266.1 → 128.1 for THBMA and 269.1→131.1 for d3-THBMA) was used to confirm the identity of the THBMA peak.
Calibration curves were constructed by injecting standard solutions containing fixed amount of d3-THBMA (25 ng) and varying amounts of THBMA (0.05, 0.25, 0.5, 1, 1.5, 2.5, 5 and 12.5 ng), in triplicate (on column). Regression analyses were conducted to determine the correlation between the theoretical molar ratio and the HPLC-ESI−-MS/MS response ratio (ratio of THBMA and d3-THBMA peak areas).
Method Validation
Eight validation standards were prepared by spiking non-smoker urine (100 µL) with increasing amounts of THBMA (0.1, 0.5, 1, 2, 3, 5, 10 and 25 ng) and a fixed amount of d3-THBMA (50 ng), in triplicate. These samples were processed by SPE and analyzed by HPLC-ESI−-MS/MS as described above. Regression analysis was conducted to compare the measured and the theoretical THBMA amounts.
Determination of LOD/LOQ
Artificial urine (100 µL) was spiked with 100, 50, 25 or 10 pg of THBMA and 60 ng of d3-THBMA, and the samples were processed by SPE and analyzed by HPLC-ESI−-MS/MS as described above. Limit of quantification (LOQ) was estimated as the amount of analyte which gave the signal to noise ratio (S/N) greater than 10 (Thermo Xcalibur, ICIS algorithm) and % CV < 15%. Limit of Detection (LOD) was determined as the amount of analyte which produced the signal to noise ratio > 3.
Precision and Accuracy
To determine the intraday and interday precision and accuracy of the new method, artificial urine (100 µL) was spiked with 1, 3, or 6 ng of THBMA and 60 ng of d3-THBMA. The samples were subjected to SPE as described above and analyzed by HPLC-ESI−-MS/MS three times per day on three consecutive days.
Extraction Recovery
Extraction recovery was determined by analyzing blank urine samples spiked with 2 ng of THBMA prior to SPE and 60 ng of internal standard post-extraction (n = 3) and the same urine sample spiked with both the analyte and the internal standard post SPE (n = 3).
Freeze-thaw stability
Stock solutions containing either 5 ng/ml or 30 ng/ml of THBMA were analyzed before and after three freeze-thaw cycles. The solutions were stored at − 20° C for 23 hours and thawed for 1 hour at room temperature, after which a fixed amount of internal standard (60 ng) was added, and the mixture was analyzed by HPLC-ESI−-MS/MS using standard methods. This procedure was repeated for 3 days.
A similar procedure was used to assess analyte stability in urine. Blank human urine sample (1 ml) was spiked with 5 ng or 30 ng of THBMA standard. The spiked urine samples were stored at −20° C for 23 hours and thawed for 1 hour at room temperature. Three 100 µL aliquots from each of the tubes were drawn and following the addition of d3-THBMA internal standard and SPE cleanup, HPLC-ESI−-MS/MS was used to quantify THBMA. The procedure was repeated for 3 consecutive days.
Matrix effects
Urine samples from a confirmed non-smoker and a confirmed smoker (100 µl each) were processed by SPE and spiked with THBMA (2 ng) and d3-THBMA (60 ng), followed by HPLC-ESI−-MS/MS analysis. HPLC-ESI−-MS/MS peak areas were compared for spiked samples and pure standard mixtures containing 2 ng of THBMA and 60 ng of internal standard to identify any potential analyte suppression by the biological matrix.
Human Urine Samples
Urine samples from confirmed smokers (N = 27) and non-smokers (N = 19) were obtained from the University of Minnesota Tobacco Research Programs and stored at −20° C. Urine samples (100 µL) were processed by SPE and analyzed by HPLC-ESI−-MS/MS methods as described above. THBMA amounts were normalized to creatinine, which was determined using the VITROS CREA kit and VITROS 350 system (Ortho-clinical Diagnostics, Rochester, New York).
Smoking cessation samples were obtained from the previously described study.40 In brief, 5 smokers from Minneapolis were asked to continue smoking for 2 weeks (baseline) and 24 hour urine samples were collected 14 and 7 days prior to smoking cessation. Following two week baseline period, the smokers were asked to quit smoking. Urine samples were collected on days 3, 28 and 56 post smoking cessation and analyzed by HPLC-ESI−-MS/MS as described above.
Statistical Analysis
Method calibration and validation curves were constructed in Microsoft Excel. Linear regression analysis was performed and residual plots were constructed to determine the homogeneity of the error. An unpaired t test was compiled to look at the differences in levels of THBMA between smokers and non-smokers. A p-value < 0.01 was considered statistically significant.
Results
Synthesis and structural characterization of THBMA
Authentic THBMA and its deuterated internal standard (d3-THBMA) were synthesized by reacting N-acetyl-L- cysteine and d3-N-acetyl-L-cysteine, respectively, with commercial d,l DEB (Scheme 2). Although multiple stereoisomers of THBMA are possible, they were not resolved by our HPLC methods, and both standards were isolated as racemic mixtures.
The structural identities of THBMA and d3-THBMA were confirmed by high resolution mass spectrometry, tandem mass spectrometry, and proton NMR spectroscopy (Figure 1, Supplemental Figure S-1). Accurate masses of THBMA and d3-THBMA as determined by Q-TOF MS (m/z 266.0698 and 269.0882; M-H) (Figure 1A, 1C) were consistent with the theoretical values (m/z 266.0698 and 269.0887, respectively). Tandem mass spectrometry analysis of THBMA (m/z 266.0698 (M-H)−) generated product ions at 137.0271 (2,3,4-trihydroxybutane-1-thiolate formed following the C-S bond cleavage), 128.349 (2-acetamidoacrylate, the other fragment formed as a result of C-S bond cleavage) and 119.0171 (the loss of water from 2,3,4-trihydroxybutane-1-thiolate) (Figure 1B). The corresponding ions for d3-THBMA were observed at m/z 269.0882 (M-H)−, 137.0274, 131.0536 and 119.0171 (Figure 1D) and were consistent with theoretical values (< 5 ppm error). The isotopic composition of synthetic d3-THBMA standard was as follows: THBMA-d3 (91%), THBMA-d2 (7.7%), THBMA-d1 (1.1%), THBMA-d0 (0.2%).
SPE Method Development for THBMA
THBMA is a highly polar metabolite that is not well retained on reverse phase HPLC stationary phases, complicating SPE method development. Due to the presence of a carboxylic acid functionality in its structure (pKa = 3.5), THBMA can potentially be retained via the anion exchange mechanism. Several types of SPE packing have been tested for solid phase extraction of THBMA from human urine, including reversed phase (Strata X, Bond Elut C18), weak anion exchange (Strata X-AW), strong anion exchange (Isolute SAX, Baker SAX, Agilent SAX), and mixed mode reversed phase/anion exchange (Oasis MAX, Oasis HLB), but the recoveries were insufficient (below 5%). The best results were achieved with ENV+ cartridges (Biotage), which are packed with a stationary phase composed of highly cross-linked hydroxylated polystyrene divinylbenzene copolymer. Since analyte retention on this packing is primarily due to hydrophobic interactions between the analyte and the stationary phase, samples were loaded under acidic conditions (formic acid) to neutralize the carboxylate group of THBMA, resulting in stronger binding of the analyte to the stationary phase. ENV+ cartridges have been previously employed by Eckert et. al. for solid phase extraction of six hydroxylalkyl mercapturic acids, including MHBMA and DHBMA, from human urine.44 The published method44 was modified to enable the isolation of THBMA, which has a greater polarity than the other two BD-mercapturic acids due to the presence of an additional hydroxyl group (Scheme 1). Small SPE cartridges (1 mL, 50 mg) were employed for processing 100 µL urine samples. Our optimized SPE method has an estimated recovery of 54.5% (Table 1).
Table 1.
Analytical detection limits and extraction recovery for HPLC-ES−-MS/MS analysis of THBMA.
| Analyte | Range (ng/mL) |
LOD (ng/mL) |
LOQ (ng/mL) |
Extraction Recovery (%) |
|---|---|---|---|---|
| THBMA | 1.0–250 | < 0.1 | 1.0 | 54.5 |
HPLC-ESI-MS/MS Method Development
As discussed above, the ESI− MS/MS spectrum of THBMA is characterized by the main product ion at m/z 137.0271 corresponding to C-S bond cleavage and the formation of trihydroxybutyl thiolate anion (Figure 1A). The second most abundant fragment (m/z 128.0349) corresponds to other half of the molecule following C-S bond cleavage and negative charge retention on the carboxylate functionality. The d3-THBMA internal standard produces analogous fragments at m/z 137.0274 and 131.0536 (Figure 1B). Similar fragments are observed with a triple quadrupole mass spectrometer (results not shown). Our selected reaction monitoring method is therefore based on the transitions 266.1 → 137.1 (THBMA) and 269.1 → 137.1 (d3-THBMA). The secondary fragmentation pathway 266.1 → 128.1 (THBMA) and 269.1 → 131.1 (d3-THBMA) was used for confirmation purposes (see below).
HPLC-ESI-MS/MS method development for THBMA was complicated by its poor retention on standard HPLC columns and ESI− signal suppression due to the presence of other polar compounds in human urine samples. Our initial method employed a capillary Thermo Hypercarb column. Although excellent sensitivity was achieved for pure standards, this method was not robust when analyzing multiple urine samples due to a sample-dependent retention time shift, complicating peak identification (results not shown). Therefore, a different HPLC method was sought.
Multiple HPLC columns were evaluated, including Thermo BetaMax Acid (2.1 × 50 mm), Waters Xterra MS C18 (4.6 × 50 mm), and Synergi Hydro RP (2.1 × 100 mm). The best results were achieved with the Primesep B2 column (SIELC), a mixed mode column containing C12 alkyl chains and weak ion exchange groups. Various solvent combinations, flow rates, and temperatures were evaluated in an effort to achieve good analyte retention, baseline resolution of the THBMA peaks from matrix components, and improved HPLC peak shape. Solvent composition consisting of 0.2% acetic acid (A) and acetonitrile (B) proved to be very efficient, and a temperature of 50° C was necessary to successfully resolve THBMA from the interfering matrix peaks (see below). THBMA was eluted at 1% of acetonitrile. This is not uncommon as many of the researchers including Boettcher et. al.45 reported the use of low percentages of organic solvents to achieve efficient separation of polar metabolites. Under our optimized conditions, THBMA and d3-THBMA have a similar retention time (5.0–5.2 min) and are resolved from the impurities present in human urine (see below). The total HPLC-ESI−-MS/MS run time is 25 minutes to allow for efficient column cleaning and equilibration prior to the next injection.
Method Validation
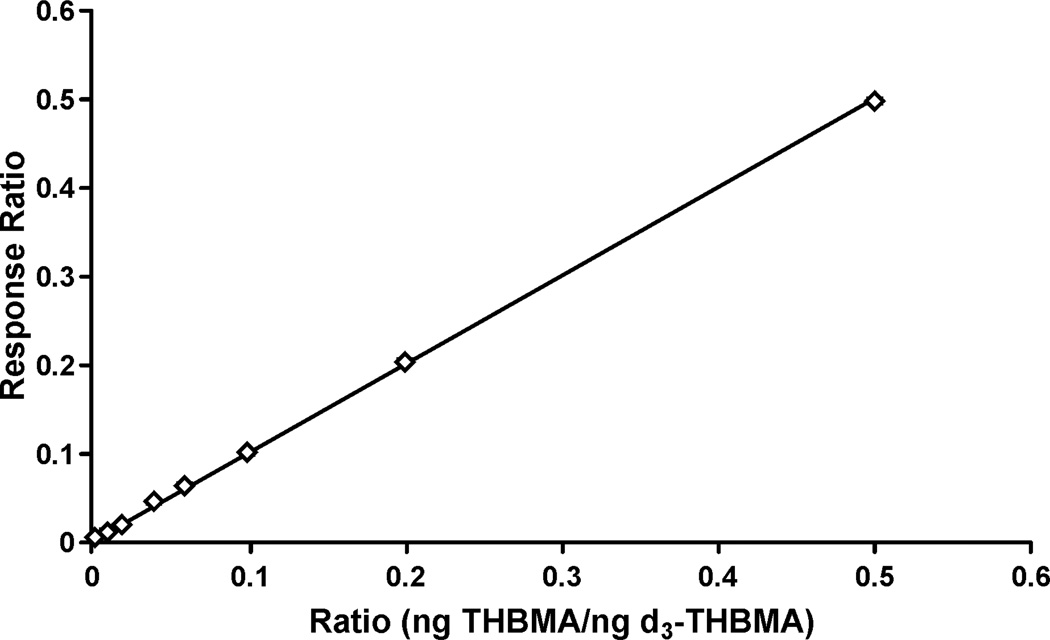
Calibration curves covering a broad THBMA concentration range were constructed by analyzing standard solutions containing fixed amounts of d3-THBMA (50 ng) and increasing amounts of THBMA (0.1–25 ng) (8 points) and plotting the ratios of the observed HPLC-ESI-MS/MS peak areas against the theoretical THBMA/ d3-THBMA molar ratios. A linear curve with a slope of 1 and an R2 value > 0.99 was obtained (Figure 2). The LOQ value for pure THBMA standard was less than 1 pg (on column).
Figure 2.
Calibration curve for HPLC-ESI−-MS/MS analysis of THBMA by isotope dilution with d3-THBMA. The curve was obtained by analyzing standard solutions containing 25 ng d3-THBMA and 0.05–12.5 ng THBMA (on column) and plotting the HPLC-ESI−-MS/MS peak area ratios (response ratios) against the actual THBMA/ d3-THBMA molar ratios. The equation for the best fit line was Y = 1.0013X (R2 = 0.9995).
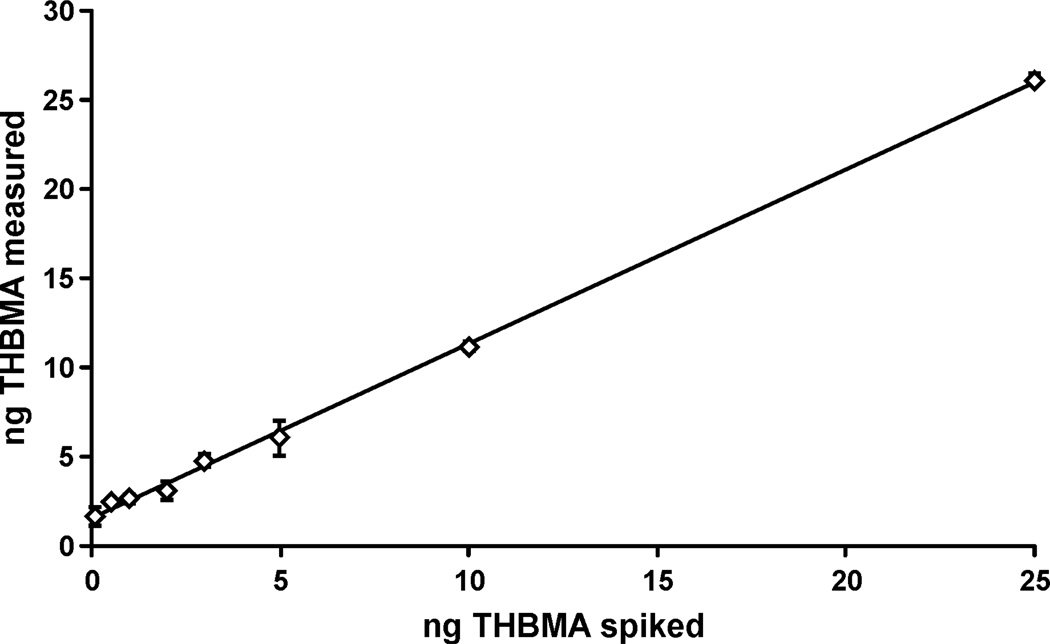
The HPLC-ESI−-MS/MS method was validated by analyzing non-smoker urine (100 µL) spiked with known amounts of THBMA (8 concentrations, 0.1–25 ng) and d3-THBMA (50 ng). Spiked samples were processed by SPE and analyzed by HPLC-ESI−-MS/MS as described above. The results were plotted as the amount of THBMA spiked versus the amount measured by HPLC-ESI−-MS/MS. The resulting method validation curve (Figure 3) has a slope of 0.98 and an R2 > 0.99, confirming that THBMA can be accurately quantified in human urine. The y-intercept of the validation curve is 1.54, corresponding to ~15.4 ng/mL of THBMA present in non-smoker urine (see below). Linear regression analysis46 showed that the error was homogenous (homoscedasticity), and hence no weighing factor was applied for plotting the validation curve.
Figure 3.
Correlation between the actual and measured concentrations of THBMA in non-smoker urine (100 µl) spiked with known amounts of THBMA (0.1–25 ng) and d3-THBMA (50 ng), followed by SPE and HPLC-ESI-MS/MS analysis using standard methods. The equation for the best fit line was Y = 0.9767X + 1.5458 (R2 = 0.9986).
Linearity, limits of detection, and extraction recovery
The linearity of the HPLC-ESI−-MS/MS method for THBMA has been demonstrated over a broad range of concentrations (1 to 250 ng/ml) (Figure 3). To determine the sensitivity of the method, artificial urine (100 µL) was spiked with 10–100 pg of THBMA. Artificial urine was used due to the presence of THBMA in urine of both smokers and non-smokers (see above). The spiked samples were processed by standard methods and analyzed by HPLC- ESI−-MS/MS. Based on the signal to noise ratio > 10 and % CV < 15%, the LOQ was found to be 1 ng/mL urine. The LOD for the HPLC- ESI−-MS/MS assay was estimated to be as 0.1 ng/mL urine (S/N > 3). The optimized extraction recovery was determined to be ~ 54.5% due to the polar nature of the analyte.
Precision and Accuracy
The precision and accuracy of our HPLC-ESI−-MS/MS assay were determined using artificial urine (100 µL) spiked with 1, 3, or 6 ng of THBMA. The samples were analyzed in triplicate on three consecutive days. The mean accuracy for THBMA was 85–115% at all the three concentration levels, while the intra-day and inter-day precision was < 15%. The precision and accuracy data for THBMA are reported in Table 2.
Table 2.
Precision and accuracy data for HPLC-ESI−-MS/MS analysis of THBMA.
| Concentration (ng/ml) |
Intraday Precision RSD (%) |
Interday Precision RSD (%) |
Accuracy (%) |
|---|---|---|---|
| 10 | 4.58 | 4.87 | 101.73 ± 4.75 |
| 30 | 6.55 | 7.85 | 106.67 ± 6.16 |
| 60 | 0.88 | 1.63 | 102.42 ± 1.67 |
Freeze-thaw Stability and matrix effects
Stability experiments determined that THBMA concentrations were essentially unchanged upon several freeze-thaw cycles, both in stock solutions and in spiked human urine. Analyte losses were < 3% over three freeze-thaw cycles at concentration levels of 5 ng/ml and 30 ng/ml.
A direct comparison of HPLC-ESI−-MS/MS peak areas corresponding to pure THBMA in buffer and THBMA mixed with SPE-processed human urine (smoker or non-smoker, 100 µl) revealed no significant signal suppression by the matrix, with peak areas remaining within 5% of the original value (results not shown).
Quantification of THBMA in urine from smokers and nonsmokers
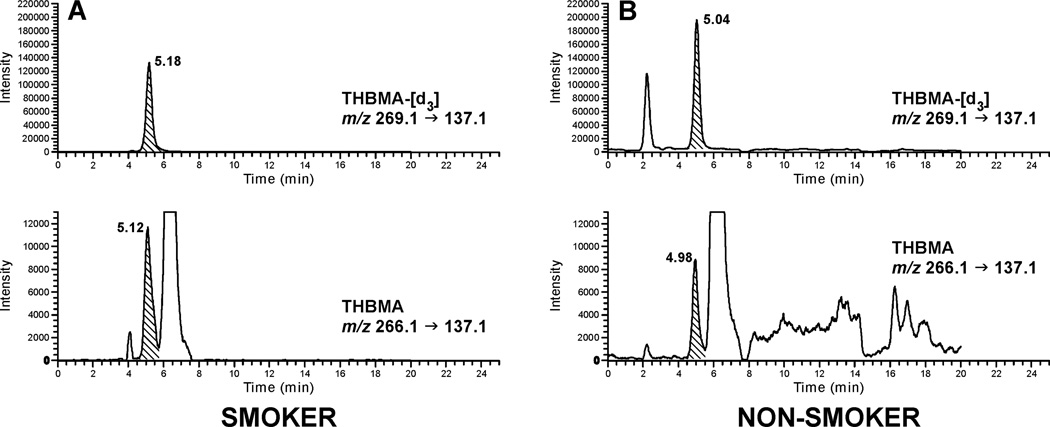
To determine the relationship between smoking and urinary THBMA concentrations, urine samples from 27 confirmed smokers and 19 non-smokers (100 µL) were analyzed. Representative HPLC-ESI−-MS/MS extracted ion chromatograms are shown in Figure 4. Interestingly, the HPLC-ESI-MS/MS retention time of THBMA was reproducibly 0.05–0.06 minutes shorter than that of the d3-internal standard. This is unusual since the non-deuterated compound is expected to have the same retention time as its non-deuterated analog or elute slightly later due to stronger van der Waals interactions between the C-H bonds and the stationary phase as compared to the C-D bonds.47 To confirm that the peak of interest indeed corresponded to THBMA, sample was spiked with 6 ng/mL of authentic THBMA and re-analyzed. HPLC-ESI-MS/MS analysis confirmed that authentic THBMA co-eluted with the peak observed in human samples, which was accompanied by proportional increase in the HPLC-ESI-MS/MS peak area corresponding to concentration increase by 6 ng/mL (Supplementary Figure S-2).
Figure 4.
Representative traces for HPLC-ESI−-MS/MS analysis of THBMA in urine of a smoker (A) and a non-smoker (B).
To obtain additional evidence for the presence of THBMA in human urine, the same sample was re-analyzed on a different HPLC column, Primesep D (2.1 × 100 mm) using a pH gradient method comprising of 0.5% acetic acid and 15 mM ammonium acetate in methanol. Similar results were obtained, with THBMA peak eluting 0.05 min before its deuterated standard (Figure S-3). The calculated amounts of THBMA were the same for the sample analyzed by two different HPLC-ESI-MS/MS methods.
Finally, a sample obtained from a larger volume of urine (500 µL) was analyzed in the product ion scan mode to reveal additional MS/MS fragments. These experiments revealed that the THBMA peak present in human samples produced fragment ions at m/z 137.1, 128.1, and 119.1, which are consistent with the structure of THBMA (Supplementary Figure S-4, Figure 1). SRM analysis using two transitions (266.1→137.1 and 266.1→128.1) revealed HPLC-ESI-MS/MS peaks in both ion channels (Supplementary Figure S-5). THBMA amounts calculated using the two SRM transitions were identical, further confirming the identity of the peak observed in human urine sample.
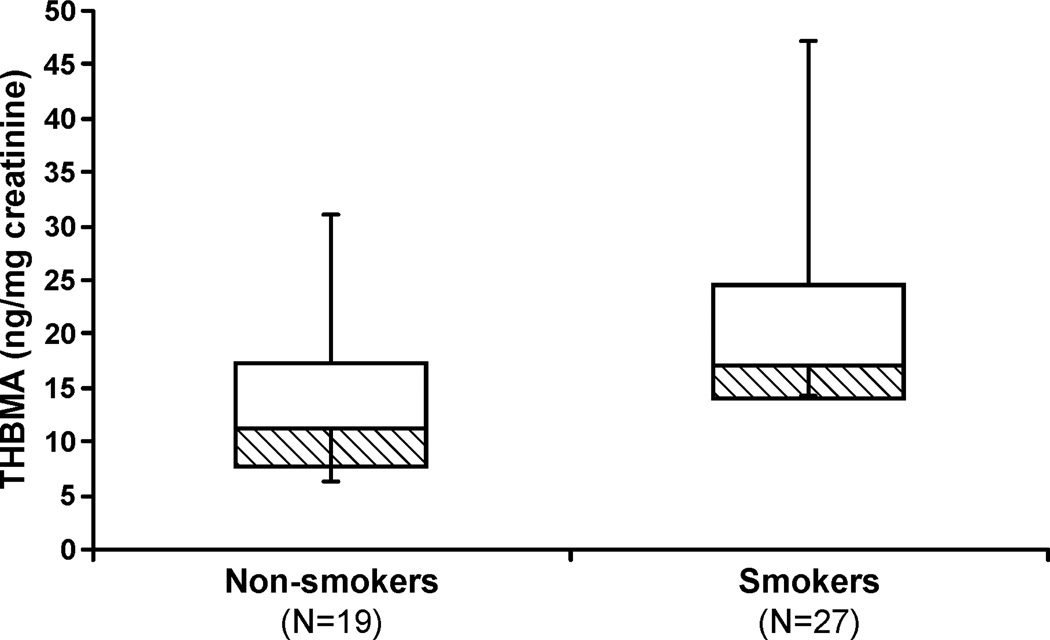
The new method was then employed to determine the effects of smoking on urinary THBMA concentrations. A box plot showing the creatinine-corrected THBMA concentrations in urine of non-smokers (N = 19) and smokers (N = 27) is shown in Figure 5. Smokers contained significantly higher concentrations of THBMA than nonsmokers (21.6 ± 10.2 versus 13.7 ± 7.9 ng THBMA/mg creatinine; p < 0.01 using unpaired two sample t-test). Importantly, THBMA concentration varied widely within each group (11.3 to 47.2 ng/mg creatinine in smokers, and 2.9 −31.0 ng/mg creatinine in non-smokers), revealing significant inter-individual differences.
Figure 5.
Creatinine-corrected concentrations of THBMA in urine from 19 non-smokers and 27 smokers. Boxes represent the 25th and 50th percentiles; lines represent the minimum and maximum. The difference between THBMA concentrations in smokers (21.6 ± 10.2 ng/mg creatinine) and non-smokers (13.7 ± 7.9 ng/mg creatinine) is statistically significant (p < 0.01).
The presence of THBMA in samples from non-smokers suggests that sources other than smoking contribute to its formation in human urine. For example, environmental exposures to BD from automobile exhaust and urban air may contribute to THBMA formation. Furthermore, the metabolic precursor of THBMA, EB-diol, has been hypothesized to form endogenously as a product of catabolism of carbohydrates.48
Smoking cessation study
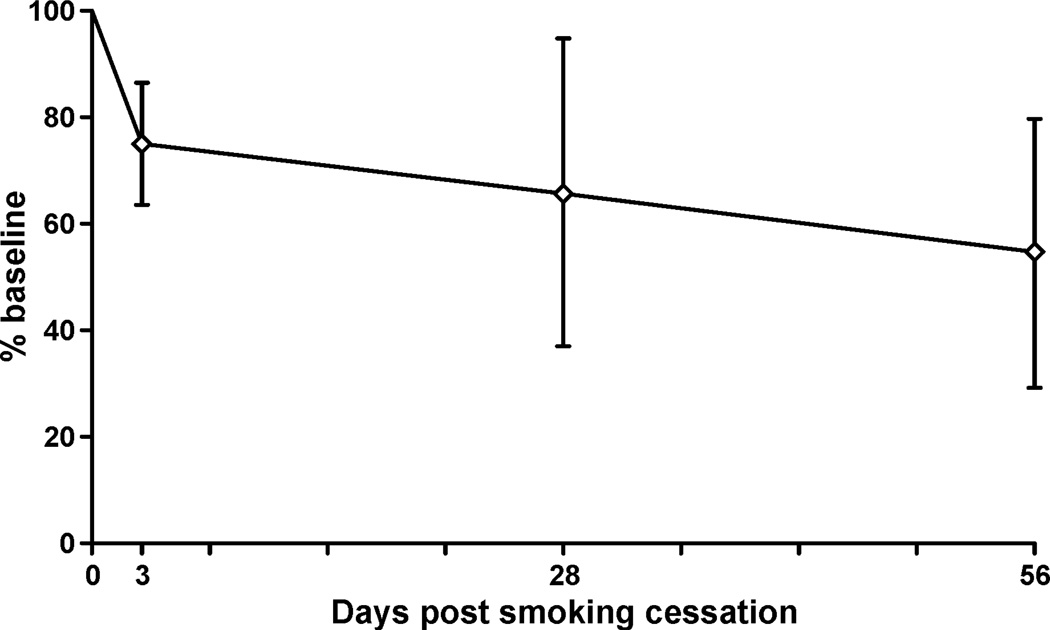
To investigate the effect of smoking on THBMA concentrations in human urine, samples were obtained from five individuals participating in a smoking cessation study. Five urine samples from each smoker were considered: the first two represented baseline values (−14th day and −7th day) prior to smoking cessation, and an additional three samples were obtained following termination of smoking (3, 28, and 56 days post cessation). Urine samples (100 µl) were processed by SPE and analyzed by HPLC-ESI−-MS/MS as described above. THBMA concentrations measured post smoking cessation were normalized to the baseline value to reveal any changes in urinary THBMA levels following the termination of smoking (Figure 6). As shown in Figure 6, the mean urinary THBMA levels decreased by 25–50% following smoking cessation, confirming that a significant portion of human exposure to BD can be attributed to exposure to cigarette smoke.
Figure 6.
Percent reduction in THBMA concentrations in urine of smokers following smoking cessation (N = 5). Samples were collected 2 weeks (−14th day) and 1 week (−7th day) before smoking cessation and 3, 28, and 56 days following smoking cessation. The day when the smokers quit smoking is designated at day 0.
Discussion
A recognized critical step in BD-mediated carcinogenesis is the chemical modification of DNA by its epoxy metabolites to form covalent nucleobase adducts.17–20 Because of the requirement for metabolic activation of BD to DNA-reactive epoxides, enzymes that are involved in the formation and detoxification of butadiene epoxides largely determine the individual sensitivity to BD-mediated mutagenesis and carcinogenesis. Therefore, quantitative analysis of butadiene metabolites in human samples may provide important information in regard to BD exposure and metabolic activation in a given individual.
Several laboratories previously quantified MHBMA and DHBMA (Scheme 1) in human urine using liquid chromatography-tandem mass spectrometry40,43,49–52 and gas chromatography-tandem mass spectrometry techniques.38,39 The concentrations of MHBMA have been reported as 1–132 ng/mL (smokers) and 1–73.4 ng/mL (non-smokers), while the corresponding values for DHBMA are 16–1,959 ng/mL (smokers) and 20–1000 (non-smokers).40,43,49–52 Following smoking cessation, the levels of MHBMA in human urine decreased from 66.1 ± 69.4 nmol/24 h (baseline) to 3.66 ± 2.41 nmol/24 h (56 days post cessation); while the concentrations of DHBMA dropped from 1038 ± 514 nmol/24 h (baseline) to 662 ± 248 nmol/24 h (56 days post cessation).40 In contrast, little is known about THBMA excretion in urine of smokers and non-smokers, despite the fact that it is produced from EBD, the most abundant epoxide metabolite of BD in humans.6
In the present study, a highly sensitive and specific HPLC-ESI−-MS/MS method was developed for quantitative analyses of THBMA in human urine (LOQ, 1 ng/mL). Our methodology (Scheme 3) incorporates isotope dilution with d3-THBMA, sample processing via SPE on ENV+ cartridges, and HPLC-ESI−-MS/MS analysis using a Primesep B2 column. The new method is reproducible (Table 2) and reasonably fast, with an average analysis time of 25 minutes. We also found out that the analyte concentrations in urine are essentially unchanged upon multiple freeze-thaw cycles.
Scheme 3.
Experimental procedure for HPLC-ESI−-MS/MS analysis of THBMA in human urine.
Several laboratories previously employed ENV+ solid phase extraction for isolation of mercapturic acids from human urine.44,45,53–55 However, lower SPE recoveries have been reported as analyte polarity increased.44,45,53–55 While percent recoveries for SPE of MHBMA and DHBMA using ENV+ cartridges have been reported as 81 and 67%, respectively,44 THBMA is significantly more polar than MHBMA and DHBMA due to the presence of an additional hydroxyl group in its structure (Scheme 1). To maximize THBMA recovery from small urine samples (100 µL), samples were loaded onto ENV+ cartridges (50 mg) under acidic conditions and eluted with 2% formic acid in methanol following several washing steps to remove impurities. We found that THBMA recovery decreased and interferences increased when stronger solvents such as acetonitrile were employed.
Our HPLC-ESI-MS/MS method development for THBMA was complicated by its high polarity, leading to poor analyte retention on typical reverse phase HPLC columns. Previous researchers have employed reversed phase HPLC with polar embedded groups to improve the retention of mercapturic acids and took advantage of reduced-length bonded phase chains (C8 and C12) to prevent stationary phase collapse under highly aqueous conditions required for their separation. For example, Carmella et. al. utilized Synergi MAX-RP column with a C12 bonded phase for the analysis of a series of mercapturic acids including MHBMA and DHBMA in human urine.40 Boettcher et. al.45 and Schettgen et. al.55 used Luna C8(2) column for the analysis of BD mercapturic acids. Eckert et. al. employed hydrophilic interaction liquid chromatography (HILIC) for analyses of various mercapturic acids.44 We have selected a mixed mode Primesep B2 column with C12 bonded phase length and embedded basic ion pairing groups, which in our hands provided the best separation of THBMA from other sample components (Figure 4).
The optimized HPLC-ESI−-MS/MS method was applied to quantify THBMA in urine samples from 27 smokers and 19 non-smokers (Figure 5). We found that THBMA concentrations in urine of smokers (30.7 ng/mL) were significantly greater than in samples from non-smokers (16.3 ng/mL, ~ 45% difference, p < 0.001). This difference was less pronounced when THBMA amounts were normalized to urinary creatinine (36%) due to the higher creatinine levels in urine of smokers as compared to non-smokers. However, the differences between creatinine-adjusted concentrations of THBMA in smokers and non-smokers were still statistically significant (p < 0.01).
To our knowledge, THBMA had not been previously quantified in human urine. However, the corresponding N-terminal valine hemoglobin adducts, THB-Val, have been quantified in humans.24,25 Most recently, Vacek et. al.24 compared the levels of BD-hemoglobin adducts in males and females with different smoking status. The mean concentrations of THB-Val adducts in male smokers were reported as 501.9 pmol/g hemoglobin (N = 7) and 179.1 pmol/g hemoglobin in male non-smokers (N = 15). THB-Val concentrations in smoking females were 189.2 pmol/g hemoglobin (N = 6) as compared 180.2 pmol/g hemoglobin in non-smoking females (N = 19).24 Since both THBMA and THB-Val are produced from the same metabolite of BD (EBD, Scheme 1), these results are consistent with our data (Figure 5), which reveal that both smokers and non-smokers excrete significant amounts of THBMA.
The urinary concentrations of THBMA observed in the present study are 2–3 fold higher than the published concentrations of MHBMA in smokers and 20–30 fold lower than previously observed concentrations of DHBMA. For example, Urban et. al.49 reported that the mean MHBMA levels in smokers were 86.4 µg/24 h (N = 10) while the corresponding levels in non-smokers were 12.5 µg/ 24 h (N = 10). DHBMA levels in smokers and non-smokers were 644 and 459 µg/ 24h (N = 10), respectively.49 Several studies, including the most recent one by Carmella et. al.40 concluded that MHBMA is a better biomarker of exposure to BD as compared to DHBMA. Existence of alternate source of exposure to the precursors to DHBMA other than BD has been speculated.40 Similar to THBMA, MHBMA and DHBMA are present in significant levels in the urine of non-smokers, which may be a result of background exposure to BD due to passive smoking and environmental exposure to BD.56
Richardson et. al. previously employed HPLC with radioflow detection to analyze THBMA in urine of rats and mice exposed to radiolabelled 14C-1,3-butadiene (200 ppm of for 6 hours).41 Their analyses revealed 2 peaks corresponding to THBMA. The main peak was identified as 4-(N-acetyl-L-cystein-S-yl)-1,2,3-trihydroxybutane based on co-chromatography with a synthetic standard. The authors inferred that the second peak was the other regioisomer of THBMA, 3-(N-acetyl-L-cystein-S-yl)-1,2,4-trihydroxybutane, which would form via glutathione conjugation to an internal carbon of EBD.41 However, synthetic standard for 3-(N-acetyl-L-cystein-S-yl)-1,2,4-trihydroxybutane was not available to validate this result.41 An attempt to synthesize all possible isomers of THBMA yielded only the diastereoisomers of 4-(N-acetyl-L-cystein-S-yl)-1,2,3-trihydroxybutane, but no 3-(N-acetyl-L-cystein-S-yl)-1,2,4-trihydroxybutane.57 In the current study, only 4-(N-acetyl-L-cystein-S-yl)-1,2,3-trihydroxybutane was observed both in reactions of N-acetylcysteine with d,l DEB and in human samples. However, it is possible that our HPLC-MS methods cannot resolve the THBMA regioisomers, therefore the potential formation of 3-(N-acetyl-L-cystein-S-yl)-1,2,4-trihydroxybutane cannot be excluded.
In summary, we have successfully developed and validated a sensitive and reproducible HPLC-ESI−-MS/MS method for quantification of THBMA in human urine. Our results provide evidence for the presence of THBMA in urine of smokers and non-smokers, with smokers excreting significantly higher concentrations of this metabolite. Smoking cessation results indicated that a significant portion of THBMA found in human urine is due to exposure of BD in cigarette smoke. However, the presence of any other endogenous and exogenous sources of THBMA in humans requires further investigation.
Supplementary Material
Acknowledgment
Funding Support
This work is supported by a program project grant from the NCI (CA-138338). Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center which is supported in part by Grant CA-77598 from the National Cancer Institute.
We thank Prof. Stephen S. Hecht and Dr. Steven Carmella (University of Minnesota Cancer center) for their helpful comments on this manuscript and their valuable advice throughout this project. We are thankful to Uthpala Seneviratne and Susith Wickramaratne for quantitative analysis of THBMA using proton NMR. We are grateful to Bob Carlson for preparing figures for this paper. We also would like to acknowledge Gregory C. Janis, Director, R&D, Medtox Laboratories, for his recommendations in regard to HPLC and SPE method development. We are also thankful to Joseph Dalluge, Director, Mass spectrometry facility, Department of Chemistry at the University of Minnesota for help with the accurate mass determination. We are grateful to Martin Cherrier (Technical sales specialist, Biotage) and Tammy Argentine (Senior Chemistry Specialist, Waters) for providing trial SPE cartridges and HPLC columns, respectively, during the initial stages of method development.
Footnotes
BD, 1,3-butadiene; EB, 3,4-epoxy-1-butene; HMVK, hydroxymethylvinylketone; EBD, 3,4-epoxy-1,2-diol; DEB, 1,2,3,4-diepoxybutane; MHBMA, monohydroxybutenyl mercapturic acid [2-(N-acetyl-L-cystein-S-yl)-1-hydroxybut-3-ene + 1-(N-acetyl-L-cystein-S-yl)-2-hydroxybut-3-ene]; DHBMA, dihydroxybutyl mercapturic acid [4-(N-acetyl-L-cystein-S-yl)-1,2-dihydroxybutane]; THBMA, trihydroxybutyl mercapturic acid [4-(N-acetyl-L-cystein-S-yl)-1,2,3-trihydroxybutane]; bis-N7G-BD, 1,4-bis-(guan-7-yl)-2,3,-butanediol; N7G-N1A-BD, 1-(guan-7-yl)-4-(aden-1-yl)-2,3-butanediol; N7G-N3A-BD, 1-(guan-7-yl)-4-(aden-3-yl)-2,3-butanediol; N7G-N7A-BD, 1-(guan-7-yl)-4-(aden-7-yl)-2,3-butanediol; HB-Val, N-(2-hydroxy-3-buten-1-yl)-valine; THB-Val, 1,2,3-trihydroxybutyl-valine; Pyr-Val, N,N-(2,3-dihydroxy-1,4-butadiyl)-valine; HPLC-ESI−-MS/MS, high performance liquid chromatography-electrospray ionization tandem mass spectrometry; SPE, solid phase extraction; GC-NECI-MS/MS, gas chromatography-electron capture ionization tandem mass spectrometry; TSQ, triple stage quadrupole; SRM, selected reaction monitoring; LOD, limit of detection; LOQ, limit of quantification; NMR, nuclear magnetic resonance; US EPA, United States Environmental Protection Agency; IARC, International Agency for Research on Cancer
Reference List
- 1.US Department of Health and Human Services, National Toxicology Program. 1,3-Butadiene. [accessed December 15, 2010];Report on carcinogens. (11th edn.). 2008 http://ntp.niehs.nih.gov /ntp/roc/eleventh/profiles/s025buta.pdf.
- 2.Himmelstein MW, Acquavella JF, Recio L, Medinsky MA, Bond JA. Toxicology and epidemiology of 1,3-butadiene. Crit Rev. Toxicol. 1997;27:1–108. doi: 10.3109/10408449709037482. [DOI] [PubMed] [Google Scholar]
- 3.Cheng H, Sathiakumar N, Graff J, Matthews R, Delzell E. 1,3-Butadiene and leukemia among synthetic rubber industry workers: exposure-response relationships. Chem. Biol. Interact. 2007;166:15–24. doi: 10.1016/j.cbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Delzell E, Sathiakumar N, Hovinga M, Macaluso M, Julian J, Larson R, Cole P, Muir DC. A follow-up study of synthetic rubber workers. Toxicology. 1996;113:182–189. doi: 10.1016/0300-483x(96)03443-9. [DOI] [PubMed] [Google Scholar]
- 5.Sathiakumar N, Graff J, Macaluso M, Maldonado G, Matthews R, Delzell E. An updated study of mortality among North American synthetic rubber industry workers. Occup. Environ. Med. 2005;62:822–829. doi: 10.1136/oem.2004.018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swenberg JA, Bordeerat NK, Boysen G, Carro S, Georgieva NI, Nakamura J, Troutman JM, Upton PB, Albertini RJ, Vacek PM, Walker VE, Sram RJ, Goggin M, Tretyakova N. 1,3-Butadiene: Biomarkers and application to risk assessment. Chem. Biol. Interact. 2011;192:150–154. doi: 10.1016/j.cbi.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunnemann KD, Kagan MR, Cox JE, Hoffmann D. Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection. Carcinogenesis. 1990;11:1863–1868. doi: 10.1093/carcin/11.10.1863. [DOI] [PubMed] [Google Scholar]
- 8.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirman CR, Albertini RA, Gargas ML. 1,3-Butadiene: III. Assessing carcinogenic modes of action. Crit Rev. Toxicol. 2010;40 Suppl 1:74–92. doi: 10.3109/10408444.2010.507183. [DOI] [PubMed] [Google Scholar]
- 10.Csanady GA, Guengerich FP, Bond JA. Comparison of the biotransformation of 1,3-butadiene and its metabolite, butadiene monoepoxide, by hepatic and pulmonary tissues from humans, rats and mice. Carcinogenesis. 1992;13:1143–1153. doi: 10.1093/carcin/13.7.1143. [DOI] [PubMed] [Google Scholar]
- 11.Duescher RJ, Elfarra AA. Human liver microsomes are efficient catalysts of 1,3-butadiene oxidation: evidence for major roles by cytochromes P450 2A6 and 2E1. Arch. Biochem. Biophys. 1994;311:342–349. doi: 10.1006/abbi.1994.1246. [DOI] [PubMed] [Google Scholar]
- 12.Krause RJ, Sharer JE, Elfarra AA. Epoxide hydrolase-dependent metabolism of butadiene monoxide to 3-butene-1,2-diol in mouse, rat, and human liver. Drug Metab Dispos. 1997;25:1013–1015. [PubMed] [Google Scholar]
- 13.Malvoisin E, Roberfroid M. Hepatic microsomal metabolism of 1,3-butadiene. Xenobiotica. 1982;12:137–144. doi: 10.3109/00498258209046787. [DOI] [PubMed] [Google Scholar]
- 14.Sprague CL, Elfarra AA. Mercapturic acid urinary metabolites of 3-butene-1,2-diol as in vivo evidence for the formation of hydroxymethylvinyl ketone in mice and rats. Chem. Res. Toxicol. 2004;17:819–826. doi: 10.1021/tx049949f. [DOI] [PubMed] [Google Scholar]
- 15.Krause RJ, Elfarra AA. Oxidation of butadiene monoxide to meso- and (+/−)-diepoxybutane by cDNA-expressed human cytochrome P450s and by mouse, rat, and human liver microsomes: evidence for preferential hydration of meso-diepoxybutane in rat and human liver microsomes. Arch. Biochem. Biophys. 1997;337:176–184. doi: 10.1006/abbi.1996.9781. [DOI] [PubMed] [Google Scholar]
- 16.Cochrane JE, Skopek TR. Mutagenicity of butadiene and its epoxide metabolites: I. Mutagenic potential of 1,2-epoxybutene, 1,2,3,4-diepoxybutane and 3,4-epoxy-1,2-butanediol in cultured human lymphoblasts. Carcinogenesis. 1994;15:713–717. doi: 10.1093/carcin/15.4.713. [DOI] [PubMed] [Google Scholar]
- 17.Citti L, Gervasi PG, Turchi G, Bellucci G, Bianchini R. The reaction of 3,4-epoxy-1-butene with deoxyguanosine and DNA in vitro: synthesis and characterization of the main adducts. Carcinogenesis. 1984;5:47–52. doi: 10.1093/carcin/5.1.47. [DOI] [PubMed] [Google Scholar]
- 18.Elfarra AA, Krause RJ, Selzer RR. Biochemistry of 1,3-butadiene metabolism and its relevance to 1,3-butadiene-induced carcinogenicity. Toxicology. 1996;113:23–30. doi: 10.1016/0300-483x(96)03423-3. [DOI] [PubMed] [Google Scholar]
- 19.Tretyakova NY, Sangaiah R, Yen TY, Swenberg JA. Synthesis, characterization, and in vitro quantitation of N-7-guanine adducts of diepoxybutane. Chem. Res. Toxicol. 1997;10:779–785. doi: 10.1021/tx970004q. [DOI] [PubMed] [Google Scholar]
- 20.Park S, Anderson C, Loeber R, Seetharaman M, Jones R, Tretyakova N. Interstrand and intrastrand DNA-DNA cross-linking by 1,2,3,4-diepoxybutane: role of stereochemistry. J. Am. Chem. Soc. 2005;127:14355–14365. doi: 10.1021/ja051979x. [DOI] [PubMed] [Google Scholar]
- 21.Bolt HM, Jelitto B. Biological formation of the 1,3-butadiene DNA adducts 7-N-(2-hydroxy-3-buten-1-yl)guanine, 7-N-(1-hydroxy-3-buten-2-yl)guanine and 7-N-(2,3,4-trihydroxy-butyl)guanine. Toxicology. 1996;113:328–330. doi: 10.1016/0300-483x(96)03467-1. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Hodge J, Anderson C, Tretyakova N. Guanine-adenine DNA cross-linking by 1,2,3,4-diepoxybutane: potential basis for biological activity. Chem. Res. Toxicol. 2004;17:1638–1651. doi: 10.1021/tx0498206. [DOI] [PubMed] [Google Scholar]
- 23.Goggin M, Loeber R, Park S, Walker V, Wickliffe J, Tretyakova N. HPLC-ESI+-MS/MS analysis of N7-guanine-N7-guanine DNA cross-links in tissues of mice exposed to 1,3-butadiene. Chem. Res. Toxicol. 2007;20:839–847. doi: 10.1021/tx700020q. [DOI] [PubMed] [Google Scholar]
- 24.Vacek PM, Albertini RJ, Sram RJ, Upton P, Swenberg JA. Hemoglobin adducts in 1,3-butadiene exposed Czech workers: female-male comparisons. Chem. Biol. Interact. 2010;188:668–676. doi: 10.1016/j.cbi.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Swenberg JA, Christova-Gueorguieva NI, Upton PB, Ranasinghe A, Scheller N, Wu KY, Yen TY, Hayes R. 1,3-butadiene: cancer, mutations, and adducts. Part V: Hemoglobin adducts as biomarkers of 1,3-butadiene exposure and metabolism. Res. Rep. Health Eff. Inst. 2000:191–210. [PubMed] [Google Scholar]
- 26.Loeber R, Rajesh M, Fang Q, Pegg AE, Tretyakova N. Cross-linking of the human DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of 1,2,3,4-diepoxybutane. Chem. Res. Toxicol. 2006;19:645–654. doi: 10.1021/tx0600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaelson-Richie ED, Loeber RL, Codreanu SG, Ming X, Liebler DC, Campbell C, Tretyakova NY. DNA-protein cross-linking by 1,2,3,4-diepoxybutane. J. Proteome. Res. 2010;9:4356–4367. doi: 10.1021/pr1000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melnick RL, Huff J, Chou BJ, Miller RA. Carcinogenicity of 1,3-butadiene in C57BL/6 x C3H F1 mice at low exposure concentrations. Cancer Res. 1990;50:6592–6599. [PubMed] [Google Scholar]
- 29.Owen PE, Glaister JR, Gaunt IF, Pullinger DH. Inhalation toxicity studies with 1,3-butadiene. 3. Two year toxicity/carcinogenicity study in rats. Am. Ind. Hyg. Assoc. J. 1987;48:407–413. doi: 10.1080/15298668791384959. [DOI] [PubMed] [Google Scholar]
- 30.Jelitto B, Vangala RR, Laib RJ. Species differences in DNA damage by butadiene: role of diepoxybutane. Arch. Toxicol. Suppl. 1989;13:246–249. doi: 10.1007/978-3-642-74117-3_42. [DOI] [PubMed] [Google Scholar]
- 31.Henderson RF, Thornton-Manning JR, Bechtold WE, Dahl AR. Metabolism of 1,3-butadiene: species differences. Toxicology. 1996;113:17–22. doi: 10.1016/0300-483x(96)03422-1. [DOI] [PubMed] [Google Scholar]
- 32.Henderson RF, Barr EB, Belinsky SA, Benson JM, Hahn FF, Menache MG. 1,3-butadiene: cancer, mutations, and adducts. Part I: Carcinogenicity of 1,2,3,4-diepoxybutane. Res. Rep. Health Eff. Inst. 2000:11–43. [PubMed] [Google Scholar]
- 33.Swenberg JA, Boysen G, Georgieva N, Bird MG, Lewis RJ. Future directions in butadiene risk assessment and the role of cross-species internal dosimetry. Chem. Biol. Interact. 2007;166:78–83. doi: 10.1016/j.cbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Boysen G, Georgieva NI, Upton PB, Jayaraj K, Li Y, Walker VE, Swenberg JA. Analysis of diepoxide-specific cyclic N-terminal globin adducts in mice and rats after inhalation exposure to 1,3-butadiene. Cancer Res. 2004;64:8517–8520. doi: 10.1158/0008-5472.CAN-04-3184. [DOI] [PubMed] [Google Scholar]
- 35.Koc H, Tretyakova NY, Walker VE, Henderson RF, Swenberg JA. Molecular dosimetry of N-7 guanine adduct formation in mice and rats exposed to 1,3-butadiene. Chem. Res. Toxicol. 1999;12:566–574. doi: 10.1021/tx980265f. [DOI] [PubMed] [Google Scholar]
- 36.Thornton-Manning JR, Dahl AR, Bechtold WE, Griffith WC, Jr, Henderson RF. Disposition of butadiene monoepoxide and butadiene diepoxide in various tissues of rats and mice following a low-level inhalation exposure to 1,3-butadiene. Carcinogenesis. 1995;16:1723–1731. doi: 10.1093/carcin/16.8.1723. [DOI] [PubMed] [Google Scholar]
- 37.Goggin M, Swenberg JA, Walker VE, Tretyakova N. Molecular dosimetry of 1,2,3,4-diepoxybutane-induced DNA-DNA cross-links in B6C3F1 mice and F344 rats exposed to 1,3-butadiene by inhalation. Cancer Res. 2009;69:2479–2486. doi: 10.1158/0008-5472.CAN-08-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Sittert NJ, Megens HJ, Watson WP, Boogaard PJ. Biomarkers of exposure to 1,3-butadiene as a basis for cancer risk assessment. Toxicol. Sci. 2000;56:189–202. doi: 10.1093/toxsci/56.1.189. [DOI] [PubMed] [Google Scholar]
- 39.Boogaard PJ, van Sittert NJ, Megens HJ. Urinary metabolites and haemoglobin adducts as biomarkers of exposure to 1,3-butadiene: a basis for 1,3-butadiene cancer risk assessment. Chem. Biol. Interact. 2001:135–136. doi: 10.1016/s0009-2797(01)00205-8. 695-701. [DOI] [PubMed] [Google Scholar]
- 40.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem. Res. Toxicol. 2009;22:734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson KA, Peters MM, Wong BA, Megens RH, van Elburg PA, Booth ED, Boogaard PJ, Bond JA, Medinsky MA, Watson WP, van Sittert NJ. Quantitative and qualitative differences in the metabolism of 14C-1,3-butadiene in rats and mice: relevance to cancer susceptibility. Toxicol. Sci. 1999;49:186–201. doi: 10.1093/toxsci/49.2.186. [DOI] [PubMed] [Google Scholar]
- 42.Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk RA, Mendes PE. Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine. Tob. Res. 2009;11:1216–1225. doi: 10.1093/ntr/ntp126. [DOI] [PubMed] [Google Scholar]
- 43.Sapkota A, Halden RU, Dominici F, Groopman JD, Buckley TJ. Urinary biomarkers of 1,3-butadiene in environmental settings using liquid chromatography isotope dilution tandem mass spectrometry. Chem. Biol. Interact. 2006;160:70–79. doi: 10.1016/j.cbi.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Eckert E, Drexler H, Goen T. Determination of six hydroxyalkyl mercapturic acids in human urine using hydrophilic interaction liquid chromatography with tandem mass spectrometry (HILIC-ESI-MS/MS) J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010;878:2506–2514. doi: 10.1016/j.jchromb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Boettcher MI, Angerer J. Determination of the major mercapturic acids of acrylamide and glycidamide in human urine by LC-ESI-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;824:283–294. doi: 10.1016/j.jchromb.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 46.Almeida AM, Castel-Branco MM, Falcao AC. Linear regression for calibration lines revisited: weighting schemes for bioanalytical methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;774:215–222. doi: 10.1016/s1570-0232(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 47.Baweja R. Application of reversed-phase high-performance liquid-Chromatography for the separation of deuterium and hydrogen analogs of aromatic-hydrocarbons. Analytica Chimica Acta. 1987;192:345–348. [Google Scholar]
- 48.Fustinoni S, Soleo L, Warholm M, Begemann P, Rannug A, Neumann HG, Swenberg JA, Vimercati L, Colombi A. Influence of metabolic genotypes on biomarkers of exposure to 1,3-butadiene in humans. Cancer Epidemiol. Biomarkers Prev. 2002;11:1082–1090. [PubMed] [Google Scholar]
- 49.Urban M, Gilch G, Schepers G, van Miert E, Scherer G. Determination of the major mercapturic acids of 1,3-butadiene in human and rat urine using liquid chromatography with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;796:131–140. doi: 10.1016/j.jchromb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Fustinoni S, Perbellini L, Soleo L, Manno M, Foa V. Biological monitoring in occupational exposure to low levels of 1,3-butadiene. Toxicol. Lett. 2004;149:353–360. doi: 10.1016/j.toxlet.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 51.McDonald JD, Bechtold WE, Krone JR, Blackwell WB, Kracko DA, Henderson RF. Analysis of butadiene urinary metabolites by liquid chromatography-triple quadrupole mass spectrometry. J. Anal. Toxicol. 2004;28:168–173. doi: 10.1093/jat/28.3.168. [DOI] [PubMed] [Google Scholar]
- 52.Ding YS, Blount BC, Valentin-Blasini L, Applewhite HS, Xia Y, Watson CH, Ashley DL. Simultaneous determination of six mercapturic acid metabolites of volatile organic compounds in human urine. Chem. Res. Toxicol. 2009;22:1018–1025. doi: 10.1021/tx800468w. [DOI] [PubMed] [Google Scholar]
- 53.Urban M, Kavvadias D, Riedel K, Scherer G, Tricker AR. Urinary mercapturic acids and a hemoglobin adduct for the dosimetry of acrylamide exposure in smokers and nonsmokers. Inhal. Toxicol. 2006;18:831–839. doi: 10.1080/08958370600748430. [DOI] [PubMed] [Google Scholar]
- 54.Mascher DG, Mascher HJ, Scherer G, Schmid ER. High-performance liquid chromatographic-tandem mass spectrometric determination of 3-hydroxypropylmercapturic acid in human urine. J. Chromatogr. B Biomed. Sci. Appl. 2001;750:163–169. doi: 10.1016/s0378-4347(00)00385-6. [DOI] [PubMed] [Google Scholar]
- 55.Schettgen T, Musiol A, Kraus T. Simultaneous determination of mercapturic acids derived from ethylene oxide (HEMA), propylene oxide (2-HPMA), acrolein (3-HPMA), acrylamide (AAMA) and N,N-dimethylformamide (AMCC) in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008;22:2629–2638. doi: 10.1002/rcm.3659. [DOI] [PubMed] [Google Scholar]
- 56.Schettgen T, Musiol A, Alt A, Ochsmann E, Kraus T. A method for the quantification of biomarkers of exposure to acrylonitrile and 1,3-butadiene in human urine by column-switching liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2009;393:969–981. doi: 10.1007/s00216-008-2510-1. [DOI] [PubMed] [Google Scholar]
- 57.Richardson KA, Peters MM, Megens RH, van Elburg PA, Golding BT, Boogaard PJ, Watson WP, van Sittert NJ. Identification of novel metabolites of butadiene monoepoxide in rats and mice. Chem. Res. Toxicol. 1998;11:1543–1555. doi: 10.1021/tx970175v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.