Abstract
Rationale
Human atherosclerotic plaques contain large numbers of cells deprived of O2. In murine atherosclerosis, because the plaques are small, it is controversial whether hypoxia can occur.
Objective
To examine if murine plaques contain hypoxic cells, and whether hypoxia regulates changes in cellular lipid metabolism and gene expression in macrophages.
Methods and Results
Aortic plaques from apolipoprotein-E-deficient mice were immunopositive for hypoxia-inducible transcription factor (HIF-1α) and some of its downstream targets. Murine J774 macrophages rendered hypoxic demonstrated significant increases in cellular sterol and triglycerides. The increase in sterol content in hypoxic macrophages correlated with elevated 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase activity and mRNA levels. In addition, when macrophages were incubated with cholesterol complexes, hypoxic cells accumulated 120% more cholesterol, predominately in the free form. Cholesterol-efflux assays showed that hypoxia significantly decreased efflux mediated by ATP binding cassette sub-family A member 1 (ABCA1), whose sub cellular localization was altered in both J774 and primary macrophages. Furthermore, in vivo expression patterns of selected genes from cells in hypoxic regions of murine plaques were similar to those from J774 and primary macrophages incubated in hypoxia. The hypoxia-induced accumulation of sterol and decreased cholesterol efflux was substantially reversed in vitro by reducing the expression of the hypoxia-inducible transcription factor, HIF-1α.
Conclusion
Hypoxic regions are present in murine plaques. Hypoxic macrophages have increased sterol content due to the induction of sterol synthesis and the suppression of cholesterol efflux, effects that are in part mediated by HIF-1α.
Keywords: hypoxia, atherosclerosis, mouse, macrophage, sterol synthesis, cholesterol efflux
Introduction
Atherosclerosis is the major cause of cardiovascular disease (CVD)1, which in turn is the leading cause of mortality worldwide.2 While a large body of research has shed insight into the biochemical and cellular mechanisms of atherosclerosis, further study of the processes that lead to the progression of arterial plaques is needed, especially for the development of future therapies. Atherosclerotic plaques in both humans and large animal models contain highly metabolically active cells whose distances from a blood supply exceed the 100-200 μm diffusion limit for oxygen,3,4 yet an incompletely explored area is the specific role of hypoxia in plaque biology and atherogenesis. That increased investigation of this area is clinically relevant and important is strongly suggested by the findings that people with hypoxia induced by obstructive sleep apnea have increased narrowing of their coronary arteries5 and increased risk of cardiovascular disease.6 In addition, chronic intermittent hypoxia in mice increased atherosclerosis.7
Hypoxic cells activate a number of pathways that affect cell signaling and gene regulation (for a recent review, see 8). One of the best described mechanisms by which hypoxic cells adapt to their hostile environment is through the activation of the hypoxia-inducible transcription factors, HIF-1 and HIF-2, which avoid degradation by the proteasome under hypoxic conditions.9 HIF-1 is a heterodimeric transcription factor consisting of an inducible α unit and a constitutively expressed β subunit. These transcription factors have been best studied in tumors, which are often hypoxic. For example, in mouse models of cancer, tumor hypoxia leads to a number of changes also relevant to atherosclerosis, such as increased angiogenesis, anaerobic metabolism, and monocyte recruitment.10-13 HIF-1 is also known to regulate additional genes relevant to atherosclerosis, including ones regulating glucose metabolism, apoptosis, nitric oxide metabolism,14 the inflammatory response,15 and intracellular reduction–oxidation (redox) homeostasis.16, 17 HIF-1α mRNA has been found to be expressed in both human and rabbit atherosclerotic plaques,4, 18 and HIF-1α in the arterial wall may be stabilized under normoxic conditions by oxidized low density lipoprotein (LDL),19 inflammation,20 and mechanical factors,21 such as cyclic strain at arterial branch points.22 In humans HIF-1α expression was not only observed in carotid and femoral endarterectomy specimens, but HIF-1α was associated with large extracellular lipid core and macrophages.23
While a deeper understanding of the mechanisms underlying atherosclerosis has come from the study of mouse models, because of the small size of murine plaques, it is controversial whether they contain significant areas of cells sufficiently distant from a blood supply to become hypoxic. The evidence that intermittently hypoxic mice have accelerated atherosclerosis is not adequate to address this issue, because it has been shown that these mice exhibit a worsening of their plasma lipoprotein profile (e.g., 24), which would be expected to be pro-atherogenic independent of the effects of hypoxia on cells in the plaques. In the present report, we provide the first direct evidence of the stabilization of HIF-1α protein and activation of HIF-1 target genes in the plaques of apolipoprotein E-deficient (apoE-/-) mice. Furthermore, studies of both a cell culture murine macrophage model (J774) and primary bone marrow derived macrophages (BMDM) demonstrate potent effects of hypoxia on lipid metabolism, including increased sterol content, decreased ATP binding cassette sub-family A member 1 (ABCA1)-mediated cholesterol efflux, and impaired sterol esterification, all of which are considered to be atherogenic in plaque macrophages. Hypoxia-like changes in lipid metabolism were observed in cultured macrophages when HIF-1α was over expressed under normoxic conditions, and were reversed in hypoxic macrophages with HIF-1α knockdown, thereby directly linking HIF-1-α to the lipid changes. These results not only argue for the presence of hypoxia in murine atherosclerotic plaques, but also suggest one molecular mechanism by which hypoxia may be atherogenic is through the effects of HIF-1α on macrophage lipid metabolism.
Methods-For detailed methods see online methods
All mouse experiments were approved by the NYU School of Medicine Institutional Animal Care Committee. J774 macrophages were cultured in 1g/L glucose (“normal glucose”) or 4.5 g/L (“high glucose”) containing DMEM. Bone marrow-derived macrophages were prepared from apoE-/- mice. Hypoxic incubations were carried out at 37°C in a Plas-Labs chamber maintained at 1% oxygen.
Results
HIF-1α and its target genes are expressed in mouse aortic atherosclerotic plaques
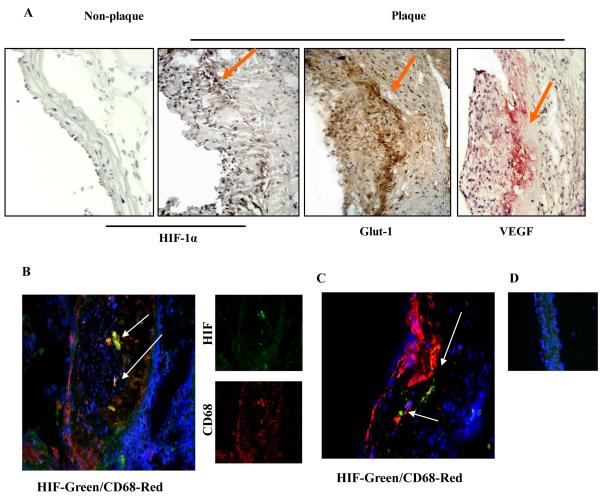
To determine if areas of significantly hypoxic cells exist in mouse aortic plaques we performed immunohistochemical staining for HIF-1α and two of its well-known transcriptional targets, Glucose transporter-1 (Glut-1) and Vascular endothelial growth factor (VEGF), using tissue sections obtained from apoE-/- mice fed Western diet for 16 weeks (Figure 1). We noted HIF-1α and its targets were prominently expressed in the central region of these plaques (Figure 1A), the area maximally distant from the blood supplies in the arterial lumen and adventitia.
Figure 1. HIF-1α and its target genes are expressed in mouse atherosclerotic plaques.
ApoE-/-mice were fed the Western diet for 16 weeks. Aortic roots and arches were harvested and frozen sections were prepared. A, Differential immunohistochemical staining for HIF-1(plaque and healthy vasculature) and two of its well known downstream targets, Glut-1 and VEGF. Staining intensity begins at approximately 100 microns from the vessel lumen; B-C, To examine macrophages in hypoxic areas, we performed double immunofluoresence studies using antibodies to HIF-1α (green) and CD68, a macrophage marker (red). Nuclei were stained with DAPI (blue). Two separate examples are shown, with typical results for the individual antibodies in the small images in panel B; D, Double immunofluoresence experiments were also performed on non-diseased areas of the aortic arch. No detectable signals were detected except for DAPI. Arrows highlight areas with representative staining.
To further characterize the plaque cells expressing HIF-1α, we performed double immunostaining experiments for it and the macrophage marker Cluster of Differentiation 68 (CD68) (Figure 1 B-D). The images (Figure 1B and C) revealed significant co-localization of CD68+ macrophages and HIF-1α, primarily in the central region of the plaque. In contrast, in apoE-/- mice there was no detectable staining for HIF-1α in either a segment of the ascending aortic arch (Figure 1D is a representative section) or in a non-diseased portion of the lesser curvature of the aortic arch (data not shown).
Effects of hypoxia on macrophage lipid content in vitro
We next examined the effects of hypoxia on macrophage lipid metabolism. We used J774 cells, a murine macrophage cell line and a standard in vitro model in atherosclerosis research. The cells were incubated in serum-containing medium at a level of oxygen of 1%, which has been previously demonstrated in intra-plaque regions of other species.4 Elevated glucose levels have been shown to blunt HIF-1α up-regulation and transactivation, perhaps due to reactive oxygen species-induced HIF-1α modification. 25, 26 Because hyperglycemia also accelerates foam cell formation independent of lipid levels,27 we decided to examine the interaction between hypoxia and glucose levels. Therefore, two different glucose concentrations were used for initial experiments: one physiologic (5.5 mM glucose, simulating serum glucose of ~100mg/dL, “normal”. This was similar to the average plasma level of glucose- 114 mg/dL- of the type of mice used for the tissue studies, and one simulating hyperglycemic conditions (25 mM, equivalent to a serum glucose of ~ 450mg/dL).
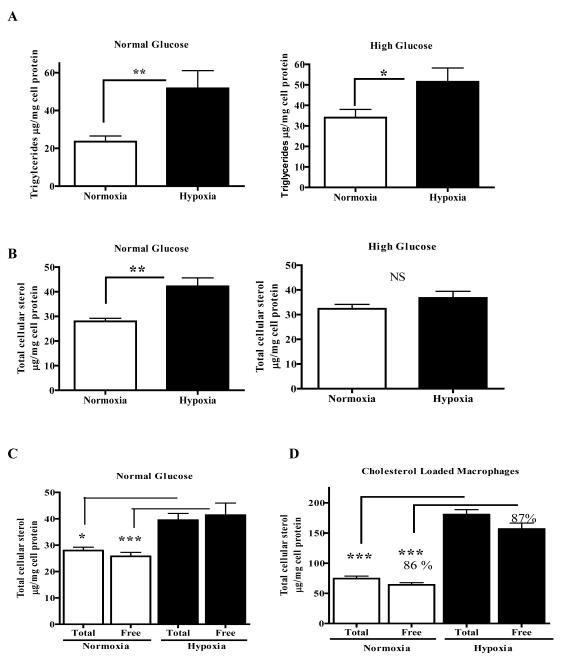
In normal glucose conditions, macrophages incubated in 1% oxygen showed an increased triglyceride content (~220% increased content relative to normoxic cells, p<0.01; Figure 2A). The effect was lower, but still statistically significant (p<0.05), under hyperglycemic conditions, with a 51% increase in triglyceride content in hypoxic macrophages (Figure 2A). This dampening may be related to the aforementioned inhibitory effects of high glucose concentrations on the hypoxic accumulation of HIF-1α,25, 26, 28 which we also noted by immunofluorescence (Online Figure I). The changes in triglyceride metabolism were also obtained by treating J774 macrophages with the hypoxia-mimetic CoCl229 in both glucose conditions (Online Figure II A, B).
Figure 2. J774 macrophage triglyceride and sterol contents are increased by hypoxia under normoglycemic conditions.
J774 macrophages were incubated for 24 h under normoxic or hypoxic (1% O2) conditions in either normal or hyperglycemic medium containing 15% FBS (panels A-C) or serum free (panel D). Lipid assays were performed and shown are the effects of hypoxia on cellular levels of: A, Triglycerides; B, Total sterol (desmosterol+cholesterol); C, Total and free sterol in non-cholesterol-loaded cells. Note that the assays for total and free sterol are independently performed, so that minor assay variations may appear as small differences, such as those in the hypoxic cells; D, Same as panel C, but in cholesterol-loaded cells. The majority of total sterol is in the free form (~86-87%) in both conditions, despite the massive increase in sterol content in hypoxia. All panels: These experiments were performed 3 times in triplicate. Statistical significance is * for p<0.05, ** for p<0.01, and *** p<0.001. NS- non significant.
Besides triglycerides, we were interested in the effects of hypoxia on sterol metabolism. In J774 cells the terminal sterol produced is desmosterol, not cholesterol, but the regulation of its synthesis and of sterol response element (SRE) mediated genes (such as 3-hydroxy-3-methyl-glutaryl-CoA (HMGCoA) reductase) is the same as for cholesterol.30 In addition, desmosterol is an efficient substrate for sterol esterification 31, and it is effluxed to apoA-1 and HDL.32 Though J774 cells do not synthesize cholesterol, in serum-containing medium the majority (85%) of their sterol mass is cholesterol, which slowly declines over 10 days in serum free medium to 20% of the cellular sterol pool.30
In addition to the dramatic increase in triglycerides in hypoxic macrophages, we also noted under normoglycemic conditions a highly significant (p<0.001) 50% increase in total cellular sterol (defined in this report as desmosterol plus cholesterol30) under normal glucose, the majority in the free form (Figure 2B left graph and Figure 2C). There was only a non-significant 14% increase in total cellular sterol in macrophages incubated in 1% O2 and the hyperglycemic condition (relative to macrophages incubated in the normoxic hyperglycemic condition; Figure 2B, right graph). These findings were replicated in cells incubated in normal and high glucose conditions that were treated with CoCl2 (Online Figure II C, D). Given the blunting effects produced by hyperglycemia, the remaining experiments were conducted in only the normoglycemic condition.
Because the majority of the sterol accumulation was in the free form (Figure 2C), we wanted to determine whether free or esterified cholesterol would accumulate when a large amount of exogenously supplied cholesterol was provided to hypoxic macrophages, which simulates the chronic exposure of plaque cells to high levels of atherogenic lipoproteins. J774 macrophages under normoxia or hypoxia were loaded with cyclodextrin-cholesterol (in serum-free medium) and the levels of free and esterified sterol were determined. As shown in Figure 2D, there was a ~2.5 fold (P<0.001) increase in total and free sterol (predominately derived from the exogenous cholesterol) in macrophages rendered hypoxic compared to cholesterol-loaded normoxic cells. We also observed that in both hypoxic and normoxic conditions, the percentages of total sterol in the free form (~86-87%) were similar.
Effects of hypoxia on sterol synthesis
Given the results above, we next explored the impact of hypoxia on cellular sterol synthesis. As noted above, J774 cells synthesize desmosterol, not cholesterol.30 This results from deficiency of sterol-delta24-reductase, which converts desmosterol to cholesterol, but importantly, the upstream regulation of the synthesis of both sterols is the same.
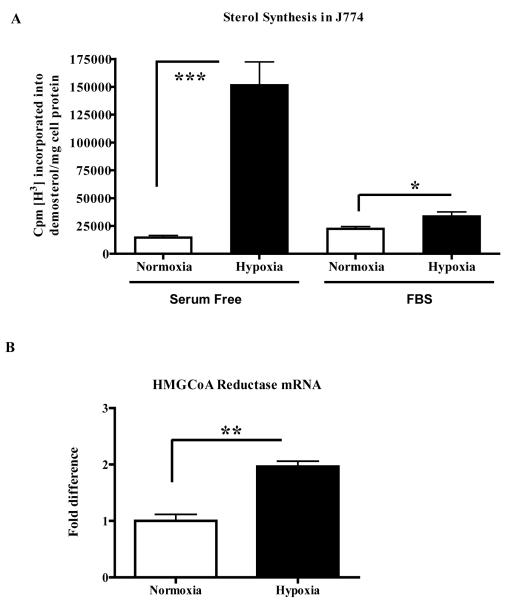
Desmosterol synthesis assays using H3-acetate33 were performed in both serum-free conditions and with serum. As shown in Figure 3A, in serum-free conditions desmosterol synthesis was ~6X greater in hypoxic J774 macrophages. Similar results were obtained using CoCl2 under serum-free conditions (Online Figure III). Under serum-containing conditions, perhaps more physiologically relevant, desmosterol synthesis significantly increased by approximately 50% in the hypoxic J774 macrophages (Figure 3A). The less dramatic increase was most likely related to inhibition of HMGCoA reductase activity by the cholesterol in the serum.
Figure 3. Hypoxia increases sterol synthesis and HMGCoA reductase mRNA in J774 macrophages.
J774 macrophages were incubated for 24h under normoxic or hypoxic (1% O2) conditions in either serum-free or rich (15% FBS) medium. A, Newly synthesized sterol was assessed by incorporation of radioactive acetate into desmosterol under the conditions indicated; B, HMGCoA reductase mRNA was assessed by qRT-PCR from cells in serum-containing medium. This is a representative of 3 experiments performed in triplicate. Statistical significance is indicated by *p<0.05, ** p<0.01, and *** p<0.001.
Overall, then, under both serum-free and serum-containing conditions, hypoxia stimulated sterol synthesis. To test whether this was from effects on HMGCoA reductase, we first performed synthesis assays in serum free medium under normoxic and hypoxic-mimicking (CoCl2) conditions in the presence of a statin, which directly inhibits the enzyme. There were ~80% reductions in desmosterol synthesis under both normoxic and hypoxic conditions, consistent with a requirement of HMGCoA reductase activity for the effects of hypoxia on sterol synthesis (Online Figure III). Next, we determined the effects of hypoxia on HMGCoA reductase mRNA abundance. An effect would be consistent with the sequence analysis of the promoter, which revealed 3 HIF-1 binding sites (at −6139, −5665 and −4769bp preceding the ATG start site). qRT-PCR analysis of RNA isolated from J774 macrophages incubated in the serum-containing, hypoxic condition showed a statistically significant ~2-fold (p< 0.05) increase in HMG-CoA reductase mRNA relative to the corresponding results in normoxic cells (Figure 3B). There was also a comparable increase in HMGCoA reductase protein by western blotting in hypoxic bone marrow-derived macrophages (data not shown).
Effects of hypoxia on cholesterol efflux and ABCA1 sub-cellular localization
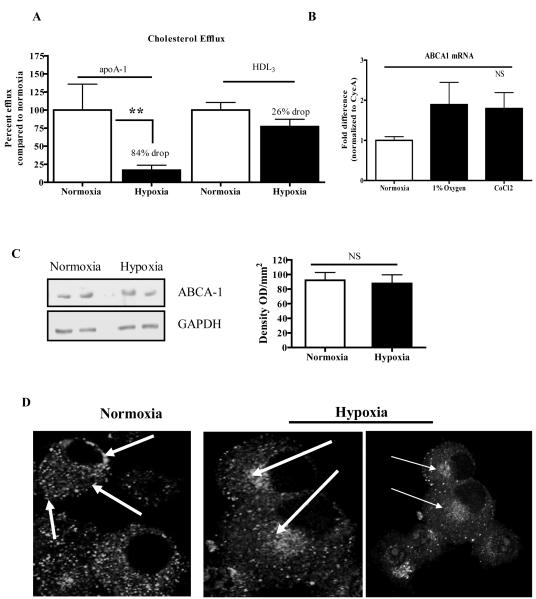
Although we found that hypoxic J774 macrophages had increased sterol synthesis (Figure 3A), they also had greater sterol accumulation after exposure to high levels of exogenous cholesterol (Figure 2D). This suggested that there was impaired cholesterol efflux as a result of hypoxia. To examine this directly, we determined the efflux of radio labeled cholesterol to the ABCA1-specific acceptor, apoA-1, or to the ATP-binding cassette sub-family G member 1 (ABCG-1) and SRB1 specific acceptor, high-density lipoprotein (HDL3). We noted a dramatic decrease (by ~84%, p<0.01) of ABCA1-mediated cholesterol efflux from hypoxic J774 macrophages (Figure 4A). In contrast, there was a non-significant 26% decrease in ABCG-1 and SRB1-mediated cholesterol efflux (Figure 4A). We repeated these efflux experiments in RAW264 cells (another standard murine macrophage cell line) and found a 77% decrease in cholesterol efflux to apoA-1 during hypoxia (Online Figure IV), suggesting that the decreased efflux through the ABCA1-mediated pathway was not macrophage cell-line specific. This was further confirmed in the bone marrow-derived macrophage (BMDM) experiments to be described below.
Figure 4. Cholesterol efflux from J774 macrophages is decreased by hypoxia concurrent with the sub-cellular redistribution of ABCA-1.
A, J774 macrophages were incubated for 24 h under normoxic and hypoxic (1% O2) conditions, and efflux of radio-labeled free cholesterol to lipid-poor apoAI (an ABCA-1 acceptor) or HDL3 (an ABCG1 or SR-BI acceptor) was measured (Methods); B, ABCA-1 mRNA abundance was measured under hypoxic or hypoxia-mimicking (CoCl2) conditions; C, ABCA-1 protein levels were detected by western blot (representative sample on left panel) and quantified by densitometry (right panel); D, Confocal microscopic images of indirect immunofluorescence of ABCA-1. The arrows highlight that the distribution of ABCA-1 shifted from the plasma membrane and in the cytoplasm under normoxic conditions to a juxtanuclear location in hypoxia. For the graphs, statistical significance is represented * for p<0.05 and ns for non significance. Efflux experiments were performed 3 times in triplicate and ABCA-1 expression levels were performed twice in duplicate.
Given the magnitude of the effect of hypoxia on ABCA1-mediated efflux and the previous report that ABCA1 accounts for the majority of transporter-mediated cholesterol efflux in cholesterol-enriched macrophages,34 we explored the mechanism for the decrease. First, qRT-PCR analyses of mRNA isolated from J774 macrophages incubated under hypoxic conditions for 24 h or with CoCl2 revealed no statistically significant changes in the level of ABCA1 mRNA compared to those in normoxic cells (Figure 4B). There was also no significant change in macrophage ABCA1 protein in hypoxic as compared to normoxic cells (Figure 4C).
Examination of the sub-cellular distribution of immunostained ABCA1 by laser-scanning confocal microscopy revealed, however, that the plasma membrane and widely dispersed cytoplasmic pools of ABCA1 seen in normoxic macrophages had an altered localization in hypoxic cells, where ABCA1 localized mainly to a juxtanuclear location (Figure 4D). These results suggested impairment of the trafficking of ABCA1 to the plasma membrane of newly made protein or of plasma membrane ABCA1 through the endosomal compartment (which also has a juxtanuclear distribution). In either case, the lack of ABCA1 at the plasma membrane would be expected to decrease cholesterol efflux to apoA-1, consistent with our findings (Figure 4A). The re-distribution of ABCA1 in hypoxic cells was confirmed in BMDM, as will be presented below.
HIF-1α modulates cellular sterol content and cholesterol efflux directly
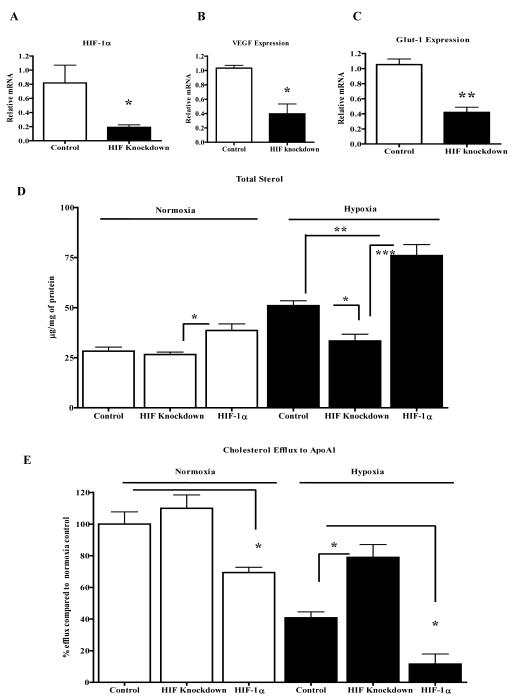
Though in hypoxic macrophages there were significant changes in sterol metabolism, suggesting a role for HIF-1α, this does not directly establish it as a mediator of these metabolic changes. Thus, we performed experiments using J774 cells with modulated HIF-1α expressing a previously validated HIF-1α expression. First, effects on sterol content were assessed. In cells stably expressing a previously validated HIF-1α shRNA,35 HIF-1α mRNA expression was reduced by ~75% (Figure 5A), which was accompanied by reduced expression (~60%) of the HIF-1α target genes VEGF and Glut-1 (Figure 5B, C). In addition, the increase of HMGCoA mRNA seen in hypoxic cells (Figure 3B) was no longer significant (Online Figure V). In hypoxic conditions, decreased HIF-1α expression led to lower cellular sterol levels compared to controls (Figure 5D). In fact, the level under hypoxia in the knockdown cells was comparable to the level in normoxic control cells (Figure 5D). In hypoxia, there was also a trend towards less triglyceride in the knockdown compared to control cells (Online Figure VI). To study effects on sterol content of HIF-1α over expression, J774 cells were transduced with a retrovirus expressing HIF-1α engineered to be stable in normoxic cells.36 Notably, even in normoxia, the transduced cells had increased sterol content, which was further increased in hypoxia (Figure 5D). Triglyceride content also was also increased relative to control cells when HIF-1α hypoxia (Online Figure VI).
Figure 5. HIF-1α plays a direct role in macrophage sterol metabolism A-C.
J774 cells were transfected to stably express HIF-1α shRNA or scrambled shRNA (“Control”) and were incubated under hypoxic conditions (1% O2). Shown are the resulting levels of the indicated mRNA species; D, J774 cells were stably transfected either with control plasmid (pBabe-puro), scrambled shRNA (“Control”), HIF-1α shRNA (“HIF Knockdown”) or a HIF-1α expression plasmid (“HIF-1α”), and desmosterol+cholesterol (“Total Sterol”) cellular content measured after incubation in normoxic (left) and hypoxic (right) conditions for 24 h; E, J774 cells were treated as in panel D, but now cholesterol efflux to apoAI, which is the acceptor for ABCA-1, was measured. Experiments were done twice in duplicate (A-C) or quadruplicates (D and E) and statistical significance is indicated by * for p<0.05, **p<0.01. The data with plasmid (pBabe-puro), and scrambled shRNA samples were essentially the same and were combined as one control group.
Next, we examined effects of varying HIF-1α expression on cholesterol efflux to apoA-1. As shown in Figure 5E, in hypoxia, cholesterol efflux in the knockdown cells was increased by 50% compared to controls. In addition, in hypoxia, HIF-1α over expression further reduced the impaired cholesterol efflux to ~25% of that in control cells. Even in normoxia, over expression of HIF-1α affected cholesterol efflux, decreasing it by more than 30%.
Hypoxia alters lipid metabolism in primary macrophages
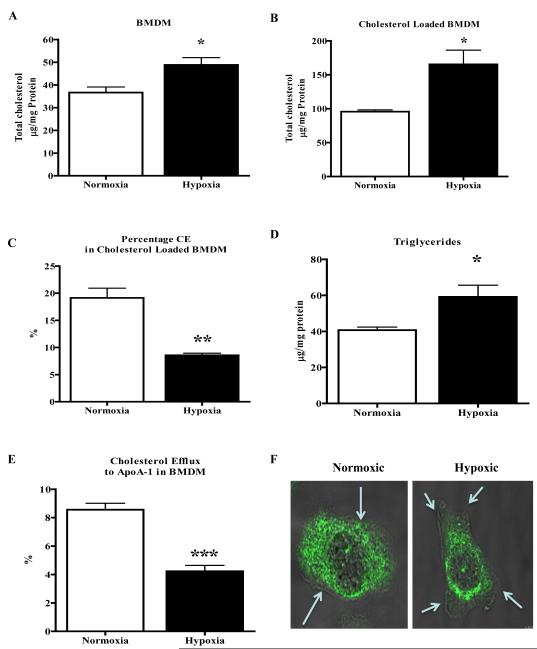
To ensure that the pattern of results were properties of not just macrophage cell lines, we repeated several of the key experiments in primary macrophages (BMDM) from apoE-/- mice. As shown in Figure 6A, hypoxia led to a 28% increase in cholesterol content, while cholesterol loading of these cells resulted in 65% increase in total cholesterol (Figure 6B). Additionally, CE content was significantly less (Figure 5C) under hypoxic condition (19 vs. 8% respectively). There was also a significant increase in TG content under hypoxic conditions (Figure 5D). Cholesterol efflux to apoA-1 was decreased (Figure 6E) in hypoxic BMDM (by ~80%). Finally, ABCA1 localization was altered in hypoxic cells, going from a diffuse distribution out to the plasma membrane to a more restricted one with enrichment in the juxtanuclear area (Figure 6F). Comparisons to the corresponding data from J774 and RAW macrophages show that the major results on lipid metabolism and ABCA1 function and distribution are independent of the type of murine macrophage (cell line vs. primary) studied.
Figure 6. Bone marrow derived macrophages (BMDM) show altered cholesterol metabolism under hypoxic conditions.
A-F, BMDM cells from apoE-/- mice were incubated under hypoxic conditions (1% O2) for 24h. Shown are the resulting levels of total cholesterol from cells that were non-loaded (A) or loaded with cholesterol-cyclodextrin complexes (B). C, The percentage of total cholesterol that was cholesteryl ester was decreased in BMDM loaded with cholesterol under hypoxic conditions. D, BMDM also showed elevated triglycerides under hypoxic conditions. E, BMDM cells were used to measure free cholesterol efflux to apoAI, which is the acceptor for ABCA-1, under normoxic and hypoxic conditions. F, BMDM cells rendered hypoxic were stained for ABCA-1 using confocal microscopy and overlaid with phase contrast image using 63X objective. The arrows indicate the plasma membrane boundary. Experiments were done twice in triplicate (A-E) and statistical significance is indicated by * for p<0.05, ** for p<0.01 and ***p<0.001.
Laser capture microdissection and gene expression analysis of murine aortic root plaques: relationships to results in vitro
To extend the physiological relevance of the cell culture data, we performed molecular analyses of cell samples laser-captured from apoE-/- mouse atherosclerotic plaques. As noted above, the plasma glucose concentration of the mice used for these studies (~110 mg/dL) was similar to the normal glucose condition in vitro.
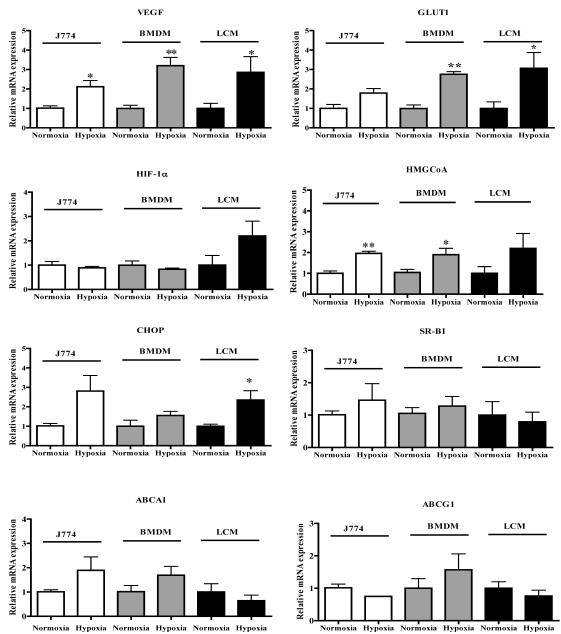
The differential immunohistochemical staining pattern shown in Figure 1 suggested that there were hypoxic areas in the plaques. To extend this finding, the RNA isolated from cells selected from the hypoxic (immunopositive for Glut-1 and VEGF) and non-hypoxic (the area between the endothelial layer and the hypoxic area) regions were subjected to qRT-PCR analysis to determine differential gene expression patterns (Figure 7). In addition, the expression of the genes measured in the mouse samples were compared with the corresponding results in normoxic and hypoxic J774 cells and BMDM.
Figure 7. Hypoxic regions of atherosclerotic plaques show a gene expression pattern similar to those in macrophages incubated under hypoxic conditions.
ApoE-/- mice were fed the Western diet for 16 weeks and aortic root sections were prepared for laser capture microdissection. Samples were isolated from normoxic and hypoxic regions (positive for both VEGF and GLUT-1 staining; on-line Methods). J774 macrophages and BMDM were exposed to hypoxia in vitro for 24 h. RNA was isolated from laser-captured samples and cultured macrophages, and mRNA abundances was measured using qRT-PCR with resulted normalized to Cyclophilin A. Note that the graphs for ABCA-1 and HMGCoA reductase are repeated from Figures 4B and 3B, respectively. Statistical significance is indicated by * for p<0.05 and ** for p<0.01. The in vitro experiments were performed 3 times in triplicate and the LCM samples were isolated from 6 animals.
As shown in Figure 7, in hypoxic regions, there were significant increases in the gene expression of the classical HIF-1α targets Glut-1 and VEGF, in agreement with immunostaining (Figure 1 and data not shown). Also significantly increased in the hypoxic regions was the expression of C/EBP-homologous protein (CHOP), a hypoxia-inducible ER-stress marker.37 There was also a trend of increased expression of HIF-1α and HMGCoA reductase. The gene expression of the cholesterol efflux factors, SR-BI, ABCA1, and ABCG1, were not significantly affected by hypoxia, though there was a trend of decreased ABCA1 mRNA. The pattern of gene expression data in the hypoxic plaque regions was similar to those in macrophages (J774 and BMDM) that were rendered hypoxic with 1% O2 and in J774 cells treated with CoCl2 (data not shown). There were some differences, however, as shown in Figure 7. For example, HIF-1α mRNA was unchanged in cultured macrophages, though it tended to increase in hypoxic regions. Also, there was no trend towards lower ABCA1 gene expression in the hypoxic macrophages. These differences could have reflected variations between in vitro and in vivo states, or that not all of the laser-captured cells were macrophages (Figure 1 and on-line Methods).
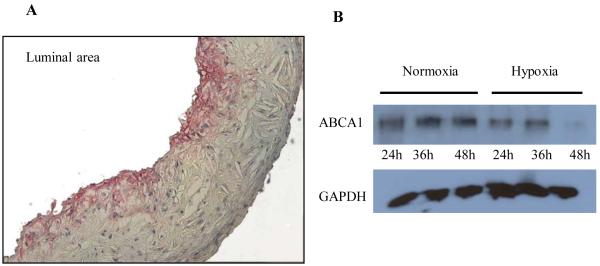
To gain more insight into the gene expression laser capture results for ABCA1, we also examined the pattern of ABCA1 immunostaining in plaques. As shown in Figure 8A, there was a gradient, with little protein found in the deeper region. Indeed, others have also shown decreased ABCA1 protein in deeper plaque regions.38 In the in vitro observations, ABCA1 protein levels were not significantly altered by 24 h of hypoxia (Figure 4). If the areas of decreased ABCA1 protein in the deeper areas of plaques reflected the consequences of prolonged hypoxia, we reasoned that subjecting the cells to longer periods of hypoxia would produce a similar result. Macrophages were incubated under normoxic and hypoxic conditions for 24 and 48 h. As shown in Fig. 8B, after 24 h of hypoxia ABCA1 protein levels were similar in macrophages incubated under normoxic and hypoxic conditions, but after 48h ABCA1 protein levels were decreased by 65%. This was not due to general cell toxicity based on no differences in the release of lactate dehydrogenase or uptake of the dye 7AAD in BMDM incubated for 48 h in either normoxia or hypoxia. Assuming decreased ABCA1 function (Figures 4 and 6) occurs in vivo in hypoxic areas, the loss of ABCA1 protein would further compromise the capacity for cholesterol efflux from hypoxic plaque macrophages.
Figure 8. Decreased expression of ABCA1 protein in deeper regions of plaques and in macrophages maintained under prolonged hypoxia.
A, ApoE-/- mice were fed Western diet for 16 weeks and aortic root sections were prepared. Note that by immunostaining, ABCA1 protein was more abundant in the adlumenal plaque; B, Western blot analysis of ABCA1 protein in lysates of J774 macrophages incubated under hypoxic conditions for 24 h or 48 h. The GAPDH levels are shown for each lane. Results are representative of 3 separate experiments.
Discussion
Though hypoxic areas in atherosclerotic plaques have been found in humans and in animal models as small as the rabbit, the role of hypoxia in murine atherosclerosis has been controversial. On the one hand, the dimensions of mouse plaques are often within the diffusion distance for oxygen to penetrate from the lumen or the vasa vasorum. Nevertheless, plaque neovessels, presumably formed in response to hypoxia, have been demonstrated.39 In addition, a recent report showed increased uptake into large mouse plaques of a positron emission tomographic compound, [18F]EF5, a tracer designed for the detection of hypoxia in vivo.40 The present study supports the existence of hypoxia in murine plaques by demonstrating in them the presence of HIF-1α and an increase in the expression of HIF-1α targets. Furthermore, the results showed that hypoxia had a major effect on macrophage lipid metabolism and that HIF-1α was a participant in promoting changes that would favor foam cell formation.
The effects of hypoxia on macrophage lipid content were two-fold. First, there was an increased level of cellular triglyceride, a relatively neglected (compared to cholesteryl ester), but significant fraction of foam cell lipids in vivo (e.g., see 41). Triglyceride accumulation was previously noted in human macrophages rendered hypoxic.42 Second, under normoglycemic conditions, macrophages incubated in hypoxic conditions accumulated free endogenous sterol and exogenous cholesterol. Increased free cholesterol has been shown to promote ER stress and subsequent apoptosis in macrophages (e.g.,43). Both in J774 cells and BMDM, free cholesterol accumulated to higher levels when it was exogenously s (Figures 2 and 6). Whether endogenous desmosterol would also contribute to an ER-stress response remains to be determined. Under the conditions in vivo, however, in which macrophage cholesterol is predominately derived from apoB-lipoproteins, the greater accumulation of cholesterol from an exogenous source that we observed in hypoxia may not only contribute to foam cell formation, but may upplied also underlie the increase in apoptosis found in hypoxic murine44 and human42 macrophages in vitro and those deep in plaques, particularly near necrotic cores (reviewed in 45).
There were three components of sterol metabolism perturbed by hypoxia. The first was the dependence on HMGCoA reductase, with evidence of increased activity at the biosynthetic and mRNA levels. The latter finding is consistent with a recent report that the HMGCoA reductase gene is a HIF-1α target46 and that its promoter contains putative HIF-1 responsive elements. The second component was uncovered by the cholesterol loading of hypoxic and normoxic macrophages in vitro to simulate the environment of plaque macrophages and foam cells, which are chronically exposed to LDL and other atherogenic lipoproteins. As summarized in Results, cholesterol loading to ~3X of the level under normoxia, which would be expected to result in the formation of a large amount of cholesteryl esters (CE),47 instead resulted primarily in elevated free cholesterol (FC) in hypoxic macrophages. Initially surprising, these data are consistent with the findings reviewed by Tabas48, 49 in which lipid assays of various stages of human and animal atheromata revealed progressive increases in FC content as the plaques became more advanced, and presumably the macrophages in necrotic cores became more hypoxic. Whether the lack of the expected esterification of cholesterol (Figure 2D) represented an effect of hypoxia on Acetyl-Coenzyme A acetyltransferase (ACAT) (through, for example, ER-stress) or on the trafficking of free sterol to ACAT (as part of other trafficking defects- see below), remains to be determined, although the level of ACAT mRNA was not affected (data not shown).
The third component of sterol metabolism affected by hypoxia was cholesterol efflux to ABCA1. Indeed, hypoxia nearly abolished ABCA1 mediated cholesterol efflux, which would be expected to contribute to the increased cellular cholesterol content. Decreased ABCA1 function was associated, in vitro, with an acute sub-cellular redistribution of ABCA1 protein, and with prolonged hypoxia, decreased recovery of protein in vitro and in vivo. Whether macrophage ABCA1 undergoes both changes-first redistribution, then down regulationin a hypoxic plaque environment is not known, though with the prolonged period of hypoxia expected in vivo, this would certainly be possible.
There are a number of potential explanations for the effects of hypoxia on macrophage ABCA1. Regarding the redistribution finding, it has been shown that hypoxia modulates the vesicular trafficking of proteins from the trans-Golgi network to plasma membranes in a Rab-associated process (Rab 11;50). ABCA1 recycling between the plasma membrane and the endosomal compartment,51, 52 also involves at least one Rab (Rab 8; 53), whose function or level may be reduced under hypoxic conditions. ABCA1 may also misfold in hypoxia and be directed to degradation by the unfolded protein/ER-stress response, consistent with the increases observed in the mRNA for the ER-stress marker CHOP both in vivo and in vitro.
It should be noted that Bostrom et al.42 found, like we did, that in hypoxic macrophages triglycerides accumulated. In contrast, they did not observe cholesterol accumulation or enhanced cholesterol loading. The differences between this and the present study may be due to species variation, or more likely in the glucose concentrations of the media. The glucose concentration of the medium used by Bostrom et al. was 3.2 g/L (i.e., 18 mM), which is near the concentration of our hyperglycemic condition (25 mM). As presented in Results, hypoxic effects on macrophage sterol metabolism were lower in cells grown in high glucose, consistent with hyperglycemia blunting the hypoxic accumulation of HIF-1α.25, 26, 28
An important issue to consider is whether the hypoxic effects on macrophage lipid metabolism were mediated by HIF-1α. In support of a direct connection, as noted earlier, the promoter of HMGCoA reductase has putative HIF-1 binding sites. There is also the recent study54 in which siRNA for HIF-1α inhibited foam cell formation in response to loading human U937 macrophages with oxidized LDL. More to the point are the data from the HIF-1α over and under expression studies presented in Figure 5, which provide strong experimental support for an integral and direct role for HIF-1α in promoting many of the changes in lipid metabolism in hypoxic macrophages.
Although we have emphasized the effects of hypoxia on lipid metabolism, it is likely regulating additional processes in plaques. Findings to support this possibility include the co-over expression of markers of hypoxia and inflammation in human carotid plaques,55 as well as the association between stabilized HIF-1α and an inflammatory plaque phenotype,18 angiogenesis56, 57 and plaque hemorrhage.57 We have previously shown that under certain conditions, plaque macrophages can emigrate in a process requiring the chemokine receptor CCR7.58 Finding lower CCR7 mRNA levels in laser-captured cells from the plaque hypoxic regions (data not shown) suggests that hypoxia may promote the retention of macrophages in necrotic cores by regulating their chemotactic ability. It should be noted that despite the predominately pro-atherogenic effects of hypoxia on various aspects of arterial biology, enhancement by HIF-1α or its target genes of smooth muscle cell proliferation and migration59, 60 may, in fact, be protective by current concepts, thereby raising the interesting possibility that HIF-1α has the potential to ameliorate some of its deleterious effects in plaques.
In summary, hypoxia has been postulated as an important contributor to the progression of human and murine atherosclerosis, particularly based on studies of patients or mice experiencing intermittent hypoxia.6,7 The understanding the molecular effects of hypoxia on macrophage biology relevant to atherosclerosis, however, is relatively limited. To our knowledge the present report represents the first direct demonstration of stabilized HIF-1α and the increased expression of its downstream targets in murine atherosclerotic plaques. Additionally, we show that HIF-1α is an important mediator of the effects of hypoxia on murine macrophage lipid metabolism and promotes changes expected to be adverse.
Supplementary Material
Online Figure I: Hyperglycemia blunts HIF-1α expression. J774 cells were plated on chamber slides with normal (5.5 mM) or high (25 mM) glucose. These cells were then incubated under hypoxic conditions for 24h and immunostained for HIF-1α. The images were taken using confocal microscopy through a 63X oil emersion lens. Arrows indicate high levels of HIF-1α staining.
Online Figure II: Effect of CoCl2 and hyperglycemia on macrophage lipid accumulation. A, In normal glucose conditions (5.5 mM), J774 macrophages incubated with increasing concentrations of the hypoxia mimic CoCl2 showed significantly increased triglyceride content in untreated-vs-600μM treated cells. B, This effect was blunted by hyperglycemia (25 mM), however, at 600μM of CoCl2 it was still statistically significant vs untreated. C, Similarly, increases in total cholesterol/desmosterol (“Total sterol”) were observed under hypoxic, normal glucose conditions. D, In contrast, under hyperglycemia, no significant increase in total sterol content occurred with CoCl2 treatment. These experiments were performed 3 times in triplicate. *p<0.05
Online Figure III: Effects of CoCl2 and the statin mevinolin on sterol synthesis. J774 macrophages were treated with or without 600μM CoCl2 in serum-free conditions for 24h. A, Sterol (desmosterol) synthesis was measured at the end of 24h. Statins (5μM mevolin) were used to suppress sterol synthesis and showed 80% suppression of HMGCoA in cells treated with or without CoCl2. These experiments were done twice in duplicate.
Online Figure IV: Cholesterol efflux in Raw264 macrophages. To confirm that the reduction in cholesterol efflux by hypoxia was not restricted to J774 cells, cells of another murine macrophage line, Raw264, were incubated for 24 h under normoxic and hypoxic conditions, and efflux of radiolabeled free cholesterol to lipid-poor apoAI (an ABCA-1 acceptor). As with J774 cells (Fig 4a.), cholesterol efflux was diminished by hypoxia, with ABCA-1 mediated efflux significantly decreased. These are the results of two experiments done in quadruplicates, *** P<0.001.
Online Figure V: HIF-1α regulates HMGCoA reductase expression. J774 cells exhibited increased HMGCoA reductase gene expression under hypoxic conditions for 24 h as before (Fig 3b). When cells stably expressing HIF-1α shRNA or scrambled shRNA (“Control”) were subjected to hypoxic conditions, there was obvious inhibition of this increase. These experiments were performed twice in duplicate. * P<0.05
Online Figure IV: HIF-1α is a positive regulator of triglyceride content in macrophages . J774 cells were stably transfected either with control plasmid (pBabe-puro), scrambled shRNA (“Control”) , HIF-1α shRNA (“HIF Knockdown”) or a HIF-1α expression plasmid (“HIF-1α”). Cells are incubated in hypoxic conditions for 24 h and triglyceride levels were measured and compared to cells incubated in normoxia (control cells in normoxic conditions). These experiments were performed twice in duplicate; * p<0.05, ***p<0.001.
Novelty and Significance.
What is known?
Human atherosclerotic plaques have areas that are hypoxic.
Hypoxia in human macrophages increases storage of triglycerides.
A major regulator of the effects of hypoxia has been the transcription factor HIF1α
What new information does this article contribute?
Stabilization of HIF-1α protein and activation of its target genes occur in hypoxic areas of plaques of apolipoprotein E-deficient (apoE-/-) mice, a standard model of human athereosclerosis.
In primary mouse macrophages and macrophage cell lines, hypoxia promotes cholesterol storage by increasing its synthesis and reducing its efflux to HDL through ABCA1.
The changes in lipid metabolism in hypoxic macrophages are downstream of HIF-1α based on experiments in which its expression was directly manipulated.
Hypoxia is an important factor in human atherogenesis. Because mouse plaques are smaller, whether hypoxia develops within them is controversial. We demonstrated hypoxia in apoE-/- mouse plaques by showing stabilization of HIF-1α protein and activation of its target genes. In vitro, hypoxic macrophages accumulated cholesterol due to increased synthesis and decreased efflux to HDL, which accepts cellular cholesterol via the membrane protein ABCA1. The increased synthesis of cholesterol was associated with HMGCoA reductase regulation; the decreased efflux was associated with loss of ABCA1 from the cell surface. The effects of hypoxia on macrophage lipid metabolism in vitro were downstream of HIF1α, based on experiments in which its expression was altered. These studies reveal for the first time that hypoxia occurs in mouse plaques.. Furthermore, the actions of HIF1α are likely to be atherogenic based on its promotion of macrophage cholesterol accumulation. Stimulation of cholesterol synthesis likely reflects HIF1-cis-acting sequences in the HMGCoA reductase promoter, but the effects on ABCA1 cellular distribution are novel and their basis warrants further study. Therapeutic agents to combat adverse effects of hypoxia in other diseases, particularly cancer, are being developed. Our results suggest that such agents may have beneficial effects in preventing atherosclerosis.
Acknowledgments
SLM would like to thank Dr. Geoffrey Gurtner (Stanford University, formerly of NYU School of Medicine) for providing her with her initial training and education in hypoxia and for stimulating her interest and ideas in this area, which led to the inaugaration of the present studies. EAF thanks Prof. David Greaves (Oxford) for helpful discussions. SP thanks Liang (Charles) Guo of the Fisher lab for advice on some of the experimental protocols.
Sources of Funding These studies were supported by NIH grants R01-HL084312 (EAF), RO1-DK08164 (LBG), F32- HL087627 (SP), and F30-AG029748 (JEF).
Non-standard Abbreviations and Acronyms
- ABCA1
ATP binding cassette sub-family A member 1
- ABCG1
ATP-binding cassette sub-family G member 1
- ACAT
Acetyl-Coenzyme A acetyltransferase
- ApoA1
Apolipoprotein A-1
- ApoE
Apolipoprotein E
- BMDM
Bone marrow macrophage
- CCR7
Chemokine (C-C motif) receptor 7
- CD68
Cluster of Differentiation 68
- CE
Cholesteryl esters
- CHOP
C/EBP-homologous protein
- CVD
Cardiovascular disease (CVD)
- FC
Free cholesterol
- Glut-1
Glucose transporter-1
- HDL
High density lipoprotein
- HIF
Hypoxia-inducible transcription factor
- HMGCoA reductase
-3-hydroxy-3-methyl-glutaryl-CoA reductase
- LDL
Low density lipoprotein
- SRB1
Scavenger receptor class B type I
- SRE
Sterol response element
- VEGF
Vascular endothelial growth factor
Footnotes
Disclosures The authors have no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davidson MH. Overview of prevention and treatment of atherosclerosis with lipid-altering therapy for pharmacy directors. Am J Manag Care. 2007;13(Suppl 10):S260–269. [PubMed] [Google Scholar]
- 2.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the united states. Health Aff (Millwood) 2007;26:38–48. doi: 10.1377/hlthaff.26.1.38. [DOI] [PubMed] [Google Scholar]
- 3.Levin M, Leppanen O, Evaldsson M, Wiklund O, Bondjers G, Bjornheden T. Mapping of atp, glucose, glycogen, and lactate concentrations within the arterial wall. Arterioscler Thromb Vasc Biol. 2003;23:1801–1807. doi: 10.1161/01.ATV.0000092872.54026.8D. [DOI] [PubMed] [Google Scholar]
- 4.Bjornheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol. 1999;19:870–876. doi: 10.1161/01.atv.19.4.870. [DOI] [PubMed] [Google Scholar]
- 5.Leineweber C, Kecklund G, Janszky I, Akerstedt T, Orth-Gomer K. Snoring and progression of coronary artery disease: The stockholm female coronary angiography study. Sleep. 2004;27:1344–1349. doi: 10.1093/sleep/27.7.1344. [DOI] [PubMed] [Google Scholar]
- 6.Budhiraja R, Budhiraja P, Quan SF. Sleep-disordered breathing and cardiovascular disorders. Respir Care. 2010;55:1322–1332. discussion 1330-1322. [PMC free article] [PubMed] [Google Scholar]
- 7.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner LB, Corn PG. Hypoxic regulation of mrna expression. Cell Cycle. 2008;7:1916–1924. doi: 10.4161/cc.7.13.6203. [DOI] [PubMed] [Google Scholar]
- 9.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (hif-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 10.Elbarghati L, Murdoch C, Lewis CE. Effects of hypoxia on transcription factor expression in human monocytes and macrophages. Immunobiology. 2008;213:899–908. doi: 10.1016/j.imbio.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 12.Schoppmann SF, Fenzl A, Schindl M, Bachleitner-Hofmann T, Nagy K, Gnant M, Horvat R, Jakesz R, Birner P. Hypoxia inducible factor-1alpha correlates with vegf-c expression and lymphangiogenesis in breast cancer. Breast Cancer Res Treat. 2006;99:135–141. doi: 10.1007/s10549-006-9190-3. [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL. Targeting hif-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 14.Aiba RS Bel, Dimova EY, Gorlach A, Kietzmann T. The role of hypoxia inducible factor-1 in cell metabolism--a possible target in cancer therapy. Expert Opin Ther Targets. 2006;10:583–599. doi: 10.1517/14728222.10.4.583. [DOI] [PubMed] [Google Scholar]
- 15.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. Hif-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddad JJ. Oxygen sensing and oxidant/redox-related pathways. Biochem Biophys Res Commun. 2004;316:969–977. doi: 10.1016/j.bbrc.2004.02.162. [DOI] [PubMed] [Google Scholar]
- 17.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. Hif-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Vink A, Schoneveld AH, Lamers D, Houben AJ, van der Groep P, van Diest PJ, Pasterkamp G. Hif-1 alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages. Atherosclerosis. 2007;195:e69–75. doi: 10.1016/j.atherosclerosis.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Shatrov VA, Sumbayev VV, Zhou J, Brune B. Oxidized low-density lipoprotein (oxldl) triggers hypoxia-inducible factor-1alpha (hif-1alpha) accumulation via redox-dependent mechanisms. Blood. 2003;101:4847–4849. doi: 10.1182/blood-2002-09-2711. [DOI] [PubMed] [Google Scholar]
- 20.Nishi K, Oda T, Takabuchi S, Oda S, Fukuda K, Adachi T, Semenza GL, Shingu K, Hirota K. Lps induces hypoxia-inducible factor 1 activation in macrophage-differentiated cells in a reactive oxygen species-dependent manner. Antioxid Redox Signal. 2008;10:983–996. doi: 10.1089/ars.2007.1825. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto K, Ikeda U, Shimada K. Role of mechanical stress in monocytes/macrophages: Implications for atherosclerosis. Curr Vasc Pharmacol. 2003;1:315–319. doi: 10.2174/1570161033476565. [DOI] [PubMed] [Google Scholar]
- 22.Petersen W, Varoga D, Zantop T, Hassenpflug J, Mentlein R, Pufe T. Cyclic strain influences the expression of the vascular endothelial growth factor (vegf) and the hypoxia inducible factor 1 alpha (hif-1alpha) in tendon fibroblasts. J Orthop Res. 2004;22:847–853. doi: 10.1016/j.orthres.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Vink A, Schoneveld AH, Lamers D, Houben AJS, van der Groep P, van Diest PJ, Pasterkamp G. Hif-1alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages. Atherosclerosis. 2007;195:e69–e75. doi: 10.1016/j.atherosclerosis.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, Steele KE, Schweitzer MA, Patil SP, Bhanot S, Schwartz AR, Polotsky VY. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme a desaturase. Circ Res. 2008;103:1173–1180. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53:3226–3232. doi: 10.2337/diabetes.53.12.3226. [DOI] [PubMed] [Google Scholar]
- 26.Staab A, Loffler J, Said HM, Katzer A, Beyer M, Polat B, Einsele H, Flentje M, Vordermark D. Modulation of glucose metabolism inhibits hypoxic accumulation of hypoxia-inducible factor-1alpha (hif-1alpha) Strahlenther Onkol. 2007;183:366–373. doi: 10.1007/s00066-007-1649-6. [DOI] [PubMed] [Google Scholar]
- 27.Kanter JE, Johansson F, LeBoeuf RC, Bornfeldt KE. Do glucose and lipids exert independent effects on atherosclerotic lesion initiation or progression to advanced plaques? Circ Res. 2007;100:769–781. doi: 10.1161/01.RES.0000259589.34348.74. [DOI] [PubMed] [Google Scholar]
- 28.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008 doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho VT, Bunn HF. Effects of transition metals on the expression of the erythropoietin gene: Further evidence that the oxygen sensor is a heme protein. Biochem Biophys Res Commun. 1996;223:175–180. doi: 10.1006/bbrc.1996.0865. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Acebes S, de la Cueva P, Fernandez-Hernando C, Ferruelo AJ, Lasuncion MA, Rawson RB, Martinez-Botas J, Gomez-Coronado D. Desmosterol can replace cholesterol in sustaining cell proliferation and regulating the srebp pathway in a sterol-delta24-reductase-deficient cell line. Biochem J. 2009;420:305–315. doi: 10.1042/BJ20081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabas I, Feinmark SJ, Beatini N. The reactivity of desmosterol and other shellfish- and xanthomatosis-associated sterols in the macrophage sterol esterification reaction. J Clin Invest. 1989;84:1713–1721. doi: 10.1172/JCI114354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang N, Yvan-Charvet L, Lutjohann D, Mulder M, Vanmierlo T, Kim TW, Tall AR. Atp-binding cassette transporters g1 and g4 mediate cholesterol and desmosterol efflux to hdl and regulate sterol accumulation in the brain. FASEB J. 2008;22:1073–1082. doi: 10.1096/fj.07-9944com. [DOI] [PubMed] [Google Scholar]
- 33.Harms-Ringdahl M, Anderstam B, Vaca C. Heat-induced changes in the incorporation of [h3]acetate in membrane lipids. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;52:315–324. doi: 10.1080/09553008714551771. [DOI] [PubMed] [Google Scholar]
- 34.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Nemetski SM, Gardner LB. Hypoxic regulation of id-1 and activation of the unfolded protein response are aberrant in neuroblastoma. J Biol Chem. 2007;282:240–248. doi: 10.1074/jbc.M607275200. [DOI] [PubMed] [Google Scholar]
- 36.Yan Q, Bartz S, Mao M, Li L, Kaelin WG., Jr. The hypoxia-inducible factor 2alpha n-terminal and c-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol Cell Biol. 2007;27:2092–2102. doi: 10.1128/MCB.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, Koumenis C. Er stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. Embo J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geeraert B, De Keyzer D, Davey PC, Crombe F, Benhabiles N, Holvoet P. Oxidized low-density lipoprotein-induced expression of abca1 in blood monocytes precedes coronary atherosclerosis and is associated with plaque complexity in hypercholesterolemic pigs. J Thromb Haemost. 2007;5:2529–2536. doi: 10.1111/j.1538-7836.2007.02786.x. [DOI] [PubMed] [Google Scholar]
- 39.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silvola JM, Saraste A, Forsback S, Laine VJ, Saukko P, Heinonen SE, Yla-Herttuala S, Roivainen A, Knuuti J. Detection of hypoxia by [18f]ef5 in atherosclerotic plaques in mice. Arterioscler Thromb Vasc Biol. 2011;31:1011–1015. doi: 10.1161/ATVBAHA.110.221440. [DOI] [PubMed] [Google Scholar]
- 41.Meilin E, Aviram M, Hayek T. Insulin increases macrophage triglyceride accumulation under diabetic conditions through the down regulation of hormone sensitive lipase and adipose triglyceride lipase. Biofactors. 2011;37:95–103. doi: 10.1002/biof.144. [DOI] [PubMed] [Google Scholar]
- 42.Bostrom P, Magnusson B, Svensson PA, Wiklund O, Boren J, Carlsson LM, Stahlman M, Olofsson SO, Hulten LM. Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arterioscler Thromb Vasc Biol. 2006;26:1871–1876. doi: 10.1161/01.ATV.0000229665.78997.0b. [DOI] [PubMed] [Google Scholar]
- 43.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 44.Fong CC, Zhang Q, Shi YF, Wu RS, Fong WF, Yang M. Effect of hypoxia on raw264.7 macrophages apoptosis and signaling. Toxicology. 2007;235:52–61. doi: 10.1016/j.tox.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pallottini V, Guantario B, Martini C, Totta P, Filippi I, Carraro F, Trentalance A. Regulation of hmg-coa reductase expression by hypoxia. J Cell Biochem. 2008;104:701–709. doi: 10.1002/jcb.21757. [DOI] [PubMed] [Google Scholar]
- 47.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: Imlications for cholesterol deposition in atherosclerosis. Annu.Rev.Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 48.Tabas I. Consequences of cellular cholesterol accumulation: Basic concepts and physiological implications. J. Clin. Invest. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabas I. Free cholesterol-induced cytotoxicity. A possible contributing factor to macrophage foam cell necrosis in advanced atherosclerotic lesions. Trends Cardiovasc Med. 1997;7:256–263. doi: 10.1016/S1050-1738(97)00086-8. [DOI] [PubMed] [Google Scholar]
- 50.Yoon SO, Shin S, Mercurio AM. Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the rab11 trafficking of the alpha6beta4 integrin. Cancer Res. 2005;65:2761–2769. doi: 10.1158/0008-5472.CAN-04-4122. [DOI] [PubMed] [Google Scholar]
- 51.Oram JF. The ins and outs of abca. J Lipid Res. 2008;49:1150–1151. doi: 10.1194/jlr.E800006-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Neufeld EB, Remaley AT, Demosky SJ, Stonik JA, Cooney AM, Comly M, Dwyer NK, Zhang M, Blanchette-Mackie J, Santamarina-Fojo S, Brewer HB., Jr. Cellular localization and trafficking of the human abca1 transporter. J Biol Chem. 2001;276:27584–27590. doi: 10.1074/jbc.M103264200. [DOI] [PubMed] [Google Scholar]
- 53.Linder MD, Mayranpaa MI, Peranen J, Pietila TE, Pietiainen VM, Uronen RL, Olkkonen VM, Kovanen PT, Ikonen E. Rab8 regulates abca1 cell surface expression and facilitates cholesterol efflux in primary human macrophages. Arterioscler Thromb Vasc Biol. 2009;29:883–888. doi: 10.1161/ATVBAHA.108.179481. [DOI] [PubMed] [Google Scholar]
- 54.Jiang G, Li T, Qiu Y, Rui Y, Chen W, Lou Y. Rna interference for hif-1alpha inhibits foam cells formation in vitro. Eur J Pharmacol. 2007;562:183–190. doi: 10.1016/j.ejphar.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 55.Luque A, Turu M, Juan-Babot O, Cardona P, Font A, Carvajal A, Slevin M, Iborra E, Rubio F, Badimon L, Krupinski J. Overexpression of hypoxia/inflammatory markers in atherosclerotic carotid plaques. Front Biosci. 2008;13:6483–6490. doi: 10.2741/3168. [DOI] [PubMed] [Google Scholar]
- 56.Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Gelpke MD Sollewijn, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–1265. doi: 10.1016/j.jacc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 57.Higashida T, Kanno H, Nakano M, Funakoshi K, Yamamoto I. Expression of hypoxia-inducible angiogenic proteins (hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and e26 transformation-specific-1) and plaque hemorrhage in human carotid atherosclerosis. J Neurosurg. 2008;109:83–91. doi: 10.3171/JNS/2008/109/7/0083. [DOI] [PubMed] [Google Scholar]
- 58.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor ccr7 during atherosclerosis regression in apoe-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osada-Oka M, Ikeda T, Akiba S, Sato T. Hypoxia stimulates the autocrine regulation of migration of vascular smooth muscle cells via hif-1alpha-dependent expression of thrombospondin-1. J Cell Biochem. 2008;104:1918–1926. doi: 10.1002/jcb.21759. [DOI] [PubMed] [Google Scholar]
- 60.Osada-Oka M, Ikeda T, Imaoka S, Akiba S, Sato T. Vegf-enhanced proliferation under hypoxia by an autocrine mechanism in human vascular smooth muscle cells. J Atheroscler Thromb. 2008;15:26–33. doi: 10.5551/jat.e533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Figure I: Hyperglycemia blunts HIF-1α expression. J774 cells were plated on chamber slides with normal (5.5 mM) or high (25 mM) glucose. These cells were then incubated under hypoxic conditions for 24h and immunostained for HIF-1α. The images were taken using confocal microscopy through a 63X oil emersion lens. Arrows indicate high levels of HIF-1α staining.
Online Figure II: Effect of CoCl2 and hyperglycemia on macrophage lipid accumulation. A, In normal glucose conditions (5.5 mM), J774 macrophages incubated with increasing concentrations of the hypoxia mimic CoCl2 showed significantly increased triglyceride content in untreated-vs-600μM treated cells. B, This effect was blunted by hyperglycemia (25 mM), however, at 600μM of CoCl2 it was still statistically significant vs untreated. C, Similarly, increases in total cholesterol/desmosterol (“Total sterol”) were observed under hypoxic, normal glucose conditions. D, In contrast, under hyperglycemia, no significant increase in total sterol content occurred with CoCl2 treatment. These experiments were performed 3 times in triplicate. *p<0.05
Online Figure III: Effects of CoCl2 and the statin mevinolin on sterol synthesis. J774 macrophages were treated with or without 600μM CoCl2 in serum-free conditions for 24h. A, Sterol (desmosterol) synthesis was measured at the end of 24h. Statins (5μM mevolin) were used to suppress sterol synthesis and showed 80% suppression of HMGCoA in cells treated with or without CoCl2. These experiments were done twice in duplicate.
Online Figure IV: Cholesterol efflux in Raw264 macrophages. To confirm that the reduction in cholesterol efflux by hypoxia was not restricted to J774 cells, cells of another murine macrophage line, Raw264, were incubated for 24 h under normoxic and hypoxic conditions, and efflux of radiolabeled free cholesterol to lipid-poor apoAI (an ABCA-1 acceptor). As with J774 cells (Fig 4a.), cholesterol efflux was diminished by hypoxia, with ABCA-1 mediated efflux significantly decreased. These are the results of two experiments done in quadruplicates, *** P<0.001.
Online Figure V: HIF-1α regulates HMGCoA reductase expression. J774 cells exhibited increased HMGCoA reductase gene expression under hypoxic conditions for 24 h as before (Fig 3b). When cells stably expressing HIF-1α shRNA or scrambled shRNA (“Control”) were subjected to hypoxic conditions, there was obvious inhibition of this increase. These experiments were performed twice in duplicate. * P<0.05
Online Figure IV: HIF-1α is a positive regulator of triglyceride content in macrophages . J774 cells were stably transfected either with control plasmid (pBabe-puro), scrambled shRNA (“Control”) , HIF-1α shRNA (“HIF Knockdown”) or a HIF-1α expression plasmid (“HIF-1α”). Cells are incubated in hypoxic conditions for 24 h and triglyceride levels were measured and compared to cells incubated in normoxia (control cells in normoxic conditions). These experiments were performed twice in duplicate; * p<0.05, ***p<0.001.