Abstract
Brain tumours form the most common type of solid tumour in children and more that 50% of these are infratentorial. Cerebellar astrocytomas and brain stem gliomas are the commonest posterior fossa glial tumours in children. Cerebellar astrocytomas represent up to 10% of all primary brain tumours and up to 25% of posterior fossa tumors in children, with Low grade gliomas forming the commonest of the cerebellar gliomas. They commonly present with symptoms and signs of raised intracranial pressure due to obstructive hydrocephalus. Radiologically they may be solid or cystic with or without a mural nodule. Surgical excision is the mainstay of treatment and forms the most consistent factor influencing progression free and long term survival. While majority of the tumours are pilocytic astrocytomas, they may also be fibrillary astrocytomas or even high grade tumours. Tumour histology does not appear to be an independent factor in the prognosis of these children, and therefore no palliative treatment after surgery is advocated. Brain stem gliomas account for approximately 10% of all pediatric brain tumours. Cranial nerve signs, ataxia and cerebellar signs with or without symptoms and signs of raised intracranial pressure are classically described symptoms and signs. Radiographic findings and clinical correlates can be used to categorize brain stem tumours into four types: diffuse, focal, exophytic and cervicomedullary. Histologically most brain stem gliomas are fibrillary astrocytomas. Diffuse brain stem gliomas are the most commonly seen tumour in the brain stem. These lesions are malignant high grade fibrillary astrocytomas. Focal tumours of the brain stem are demarcated lesions generally less than 2 cms in size, without associated edema. Most commonly seen in the midbrain or medulla, they form a heterogeneous pathological group, showing indolent growth except when the lesion is a PNET. Dorsally exophytic tumours lie in the fourth ventricle, while cervicomedullary lesions are similar to spinal intramedullary tumours. Expanding lesions are the only lesions amenable for excision while infiltrative and ventral lesions are not.
Keywords: Brain Stem gliomas, Cerebellar gliomas, Pediatric, Pontine gliomas, Pilocytic astrocytoma
Introduction
The first successful treatment of a pediatric brain tumor was in 1879, when Sir William Macewen successfully removed a meningioma from a 14-year-old girl. Brain tumors are the most common form of solid tumors and the leading cause of death from solid tumors in children (SEER program 1975- 1999). Advances in technology in neurosurgery, radiology, radiotherapy and chemotherapy have led to a 73% 5-year survival rate for all pediatric brain tumors combined.[1] Pediatric brain tumors differ from adult tumors. For example, common adult tumors like meningioma, malignant gliomas, schwannomas and pituitary tumors are uncommon in children in whom low-grade gliomas and primitive neuro-ectodermal tumors are seen more commonly. Besides, more than 50% of brain tumors in children above 1 year of age are seen in the infratentorial compartment.[2,3] Gliomas form the largest fraction of pediatric primary brain tumors, representing between 45 and 50% of most series.[4,5] Majority of these tumors are low-grade astrocytomas, which form approximately 35-50% of all pediatric brain tumors. In contrast, high-grade gliomas and glioblastomas are the common tumors in adults. Looking at infratentorial tumors, low-grade cerebellar astrocytomas (15%), low-grade brain stem gliomas (3%) and malignant gliomas (3%) form the commonest glial tumors.[2,5]
Cerebellar astrocytomas
Cerebellar astrocytomas are common tumors in children, representing up to 10% of all primary brain tumors and up to 25% of posterior fossa tumors in children.[6] Low-grade gliomas form the commonest of the cerebellar gliomas. They occur mostly between the ages of 5 and 19 years with a peak incidence in the 5-9 year age range. In their study of 102 children below the age of 12 years with cerebellar astrocytomas, Desai et al., found the mean age of presentation to be 7 years and 11 months, with only 2.9% presenting below the age of 1 year.[6] The male to female ratio was 2.4:1. Cerebellar astrocytomas commonly present with symptoms and signs of raised intracranial pressure due to obstruction of the fourth ventricle. Cerebellar signs with ataxia and dysmetria may also be seen. These tumors may occasionally present acutely due to intratumoral hemorrhage.[7–9] Other rare presentations include precocious puberty,[10] epilepsy,[11] and hearing difficulty.[12]
Radiologically, these tumors are seen as one of the following: (i) cystic tumor with non-enhancing wall and only the mural nodule enhancing [Figure 1]; (ii)cystic tumor with enhancing cyst wall (both the cyst wall and the mural nodule enhancing); (iii) tumor with a false cystic appearance (largely necrotic tumor) and (iv) solid or mainly solid tumor with irregular contrast enhancement [Figure 2].[13] Tumors that arose primarily in the cerebellum and infiltrated the brain stem were called “transitional.”[13] Studies show that (1)H magnetic resonance spectroscopy detects key differences in the metabolites of the main types of cerebellar tumors and yields potential non-invasive biomarkers of prognosis.[14,15] In the series of Desai et al., 74.6% of the tumors were in the cerebellar hemispheres, while only 25.4% involved the vermis.[6] Brain stem involvement was seen in 19.6% of patients. In the 12 patients in whom the tumor involved the medulla, the lesion lay mainly in the vermis. In their series, 22 of 26 vermian lesions were solid enhancing lesions, while 60 of the 76 cerebellar tumors were cystic.[6] In contrast, Bilginer et al., reporting from Turkey found 45% of tumors in the cerebellar hemispheres and 55% in the vermis.[16]
Figure 1.
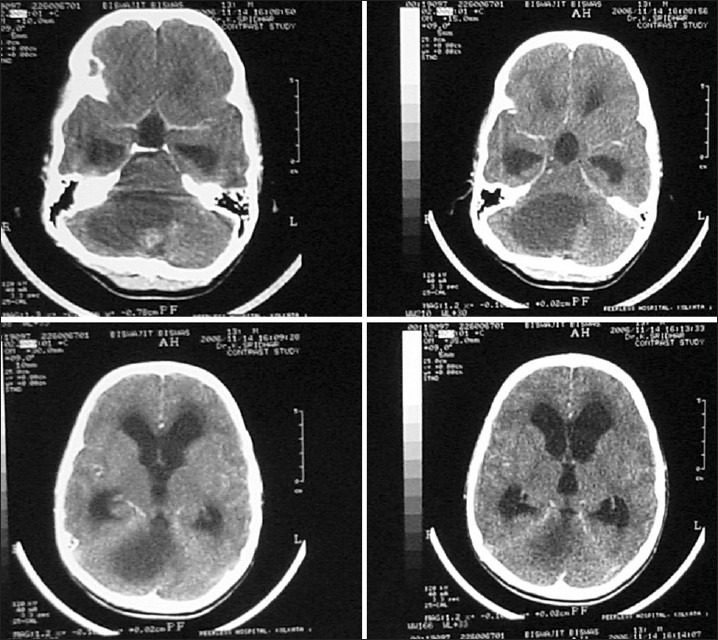
CT scan showing a large cerebellar cystic lesion with a small enhancing nodule. At surgery, the nodule was excised. The cyst was not removed. Histologically, the lesion was a pilocytic astrocytoma
Figure 2.
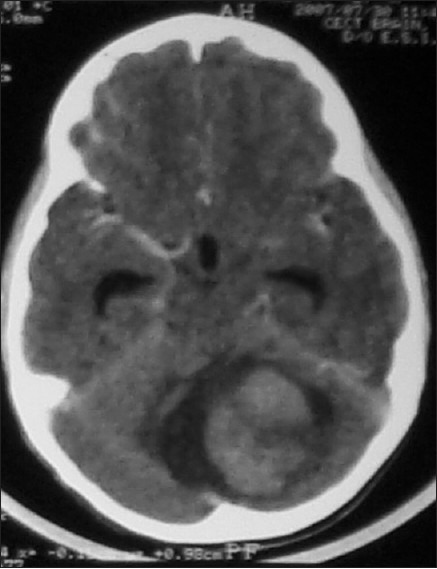
CT scan showing a large solid enhancing cerebellar lesion with areas of hypodensity within. There is a small cystic area around the lesion. At surgery, the solid tumor was radically excised. Histologically, it was a fibrillary astrocytoma
Surgery is the mainstay of treatment in children with cerebellar astrocytomas. The aim of surgery is radical excision of the lesion. This forms the most consistent factor influencing progression-free and long-term survival.[17] When there is a cyst with a mural nodule, it is sufficient to excise the nodule completely. Cyst wall excision in these cases is not required. When the cyst wall enhances with contrast, the entire wall must be excised. Pancalet et al., believe that contrast enhancement of the cyst wall is not a good indicator of whether it has tumor cells or not.[13] They believe that because of the significant role that radical excision plays in the prognosis of these children, any macroscopically abnormal tissue in the cyst wall must be excised, whether contrast enhancing or not. Most lesions in the cerebellar hemisphere are easily excised. Vermian lesions are more difficult to excise radically, especially if they infiltrate the brain stem. Desai et al., achieved total excision in 80.3% of their tumors.[6] Total excision could be achieved in 96.8% of cystic and 52.6% of solid tumors.
Cerebellar astrocytomas are histologically most often pilocytic astrocytomas (PA). Low-grade fibrillary astrocytomas (LGFA) and high-grade tumors are also seen. Cystic tumors are mostly PA, while solid tumors are more likely to be high-grade lesions.[6] Bernhardtsen et al., are of the opinion that histological subclassification of low-grade cerebellar astrocytomas (pilocytic and fibrillary astrocytomas) has no clinical significance and no predictive value.[18] Rare tumors include pleomorphic xanthoastrocytoma, lipidized glioblastoma multiformis and pilomyxoid astrocytomas of cerebellum.[19,20] Posterior fossa low-grade astrocytomas have the best prognosis with 10-year survival rates exceeding 90% if total excision has been performed.[2,5] Radiation and chemotherapy is not recommended for these children. Tumor histology does not appear to be an independent factor in the prognosis of these children, and therefore no further treatment is advocated. Those with partial resections may be observed before any adjuvant therapy is planned. Depending on the surgeon's assessment, re-surgery may be performed with the aim of removing the residual tumor.[17,21] If the lesion is not amenable to total excision, an observant policy may be followed as these lesions often show an indolent growth pattern. Gunny et al., have reported spontaneous regression of tumor in 5 of 11 children with cerebellar low-grade pilocytic astrocytomas who were followed with imaging alone for a mean of 6.83 years.[22] There were no differences in age, gender, histology or Ki-67 fractions between those with spontaneous tumor regression and those with progression. The value of postoperative adjuvant radiotherapy for residual low-grade gliomas is not clear. Presently, chemotherapy and/or radiation therapy is reserved for children whose residual tumors show growth and are not amenable for surgical excision.[1,5,17,23] The genetics of juvenile pilocytic astrocytoma (JPA) to date supports its biologic behavior as a slow-growing, non-infiltrative and relatively benign tumor that can be cured with surgical resection. However, currently there are no reliable molecular prognostic criteria that can be used to predict the natural course or patient response to chemotherapy.[1] In literature, various factors have been shown by different authors to play a role in the prognosis of these tumors. These include the age at the time of diagnosis, histological grading of the tumor, extent of tumor resection, tumor location and characteristics, and radiology. Desai et al., found location of tumor, histological grade and completeness of tumor excision were the factors that affected survival rates.[6] Vilarejo et al., concluded from their study that completeness of excision and tumor characteristics affected prognosis.[24] Pencalet et al., found that brain stem involvement was a very significant factor, as it had a direct bearing on neurological outcome and length of survival as well as an indirect bearing on recurrence as these children were more likely to have an incomplete excision of tumor.[13] Kurwale et al., studying recurrences in PA, found that radiology, histology and proliferative indices did not offer any prognostic information. They also concluded that angiogenesis markers did not predict early recurrence.[25] Paixao Becker et al., concluded similarly that other than gross total removal, no clinical, histopathologic or immunohistochemical features were related to prognosis in children with PA.[26] Looking at cognitive deficits and outcomes in children, 3 years after treatment for PA, Aarsen et al., found that almost 60% had problems with academic achievement, for which risk factors were relapse and a younger age at diagnosis. Predictors for lower cognitive functioning were hydrocephalus, radiotherapy, residual tumor size, and a younger age, while predictors for better cognition were treatment of hydrocephalus and chemotherapy.[27] Following up 104 children with cerebellar juvenile pilocytic astrocytomas, over a mean period of 8.3 years, Daszkiewicz et al., found that 60/104 had permanent neurological deficits while 47 had significant behavioral disorders. However, these problems did not preclude independent functioning and high school education in the majority.[28]
Brain stem gliomas
Brain stem gliomas account for approximately 10% of all pediatric brain tumors. The peak age of onset is between 5 and 9 years of age. Sixty percent of 111 patients with brain stem glioma, reviewed by Kansal et al., were below the age of 16 years.[29] Suspicion of a brain stem glioma is often based on clinical history and findings. Cranial nerve signs, ataxia and cerebellar signs with or without symptoms and signs of raised intracranial pressure are classically described. The symptomatology of children with these lesions can be grouped into one of seven clinical associations.[30]
The radiological diagnosis of brain stem gliomas has vastly improved with the MRI scan. While CT scans provided a reasonably accurate diagnosis in a significant proportion of cases, the true extent of infiltration and involvement could not be made out [Figure 3]. A combination of T1- and T2-weighted images, along with contrast-enhanced images, is necessary for a proper radiological assessment and diagnosis. Radiographic findings and clinical correlates can be used to categorize brain stem tumors into four types: diffuse, focal, exophytic and cervicomedullary.[31] Histologically, most brain stem gliomas are fibrillary astrocytomas (80%), compared to cerebellar astrocytomas which are predominantly pilocytic. Uncommon tumors include subependymomas, lymphomas, gangliogliomas, and oligodendroglioma. Roonprapunt and Abbot have suggested a treatment algorithm for the initial surgical workup of brain stem gliomas.[31]
Figure 3.
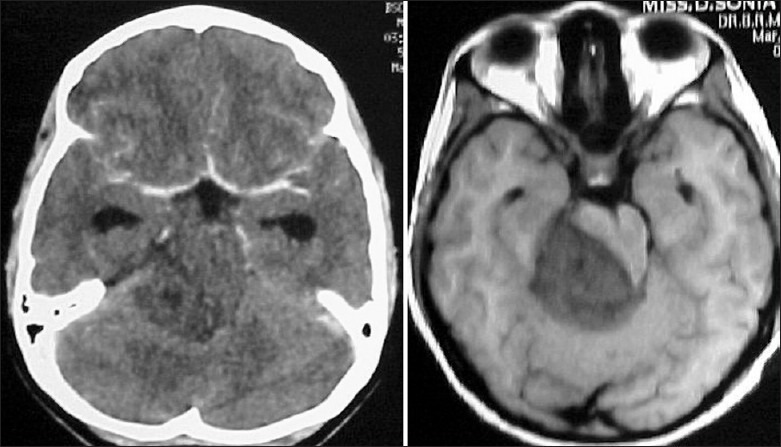
A seemingly diffuse infiltrative non-enhancing mid brain lesion on the CT scan is seen as a focal lesion on MRI scan
Diffuse brain stem gliomas are the most commonly seen tumor in the brain stem, accounting for between 58% and 75% of all gliomas in this area.[31] Seen as hypointense lesions in T1-weighted images, generally greater than 2 cm in size, centered around and expanding the pons, they are associated with significant edema seen in the corresponding T2-weighted images [Figure 4]. Gadolinium enhancement may be variable and has no prognostic implication.[32] These lesions are malignant high-grade fibrillary astrocytomas. Cytoanalysis has revealed molecular mutations unlike that normally seen in childhood tumors and similar to that seen in adults.[33]
Figure 4.
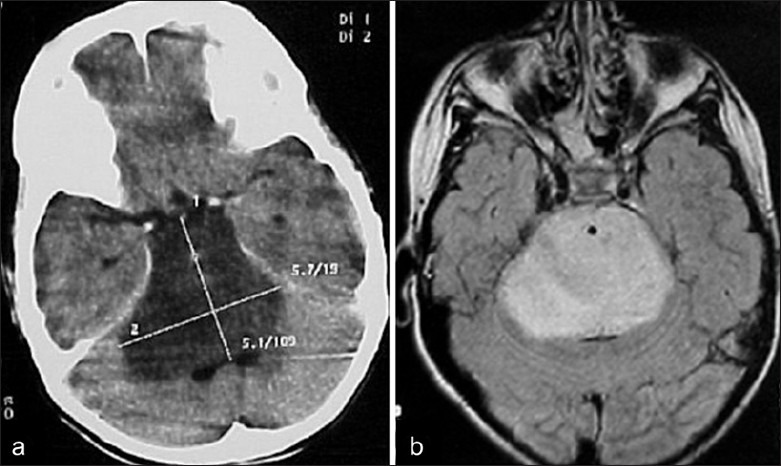
Expansile pontine lesion typical of a diffuse brain stem glioma on (a) CT and (b) MRI
Focal tumors of the brain stem are demarcated lesions generally less than 2 cm in size, without associated edema.[31] Most commonly seen in the midbrain or medulla, they may occur anywhere in the brainstem [Figure 5]. They have distinct borders on the MRI, whether solid or cystic. These lesions are mostly histologically benign (grade I-II). However, they form a heterogeneous group including LGFA, PA, gangliocytomas, anaplastic gangliogliomas and primitive neuro-ectodermal tumour (PNETs).[34] Regardless of grade and type, their biological behavior seems to be the same-indolent and slow growth-with PNETs being the exception.[31]
Figure 5.
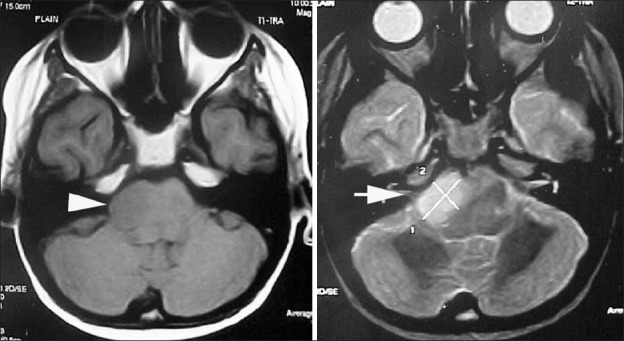
Focal pontine lesion (block arrows) seen on T1- and T2-weighted MR scans
Dorsally exophytic tumors arise from the subependymal glial tissue, with the bulk of the tumor lying within the fourth ventricle. This explains the relatively late onset of symptoms. Seen as a well-demarcated lesion, they are always low-grade gliomas which tend to grow along paths of least resistance, rather than infiltrate the brain stem. Most enhance with contrast, making them difficult to distinguish from choroid plexus papillomas and ependymomas.
Cervicomedullary tumors are similar to the intramedullary spinal cord gliomas. Most are benign low-grade astrocytomas with no infiltrative capability.[31] They are therefore limited rostrally by the decussating white matter tracts [Figure 6]. Thus, they appear exophytic displacing the medulla rostrally and expanding the upper cervical cord. Caudal extension follows an even cylindrical growth without an exophytic component.
Figure 6.
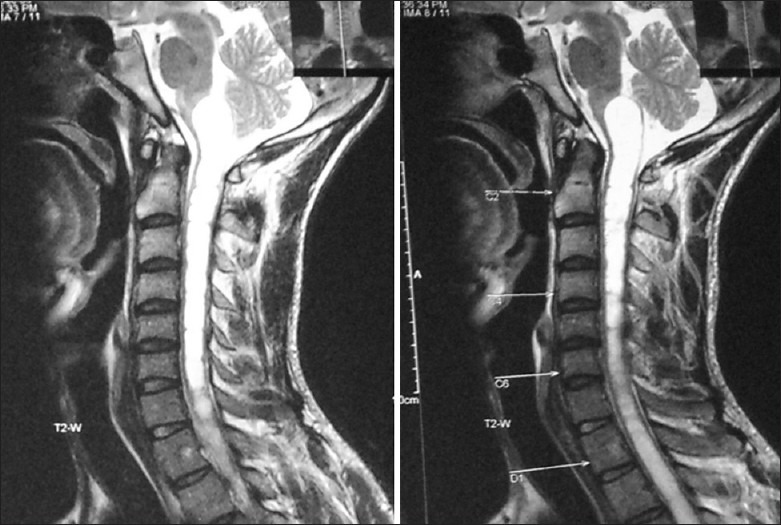
A predominantly cystic cervicomedullary lesion seen on sagittal MR scan, expanding the upper medulla
Children with diffuse brain stem gliomas present acutely with a short history. Prognosis is poor with most children dying within 2 years of diagnosis and a 3-year survival of 5-10%.[1] The radiology is typical, and thanks to their highly malignant and infiltrative nature, there is no role for surgery of these lesions.[35] Stereotactic biopsy (SB) has shown to change management decisions only rarely, and therefore when the radiology is characteristic, even a biopsy is now not indicated.[5,35–38] Radiation and chemotherapy have been the mainstay of treatment of these lesions, though they have not changed the prognosis.[5,35] Jalali et al., have not found any benefit with the use of Temozolamide with radiotherapy compared to radiotherapy alone.[39]
Focal lesions present with a longer history compared to diffuse tumors. Upper brain stem focal lesions present with hydrocephalus, oculomotor dysfunction and cerebellar signs, while lower brain stem lesions present with lower cranial nerve and long tract deficits. Conservative treatment or management of the hydrocephalus with CSF diversionary techniques is advocated for tectal and tegmental lesions. Pontine lesions carry a poorer prognosis and surgical excision is performed to remove the lesion as well as procure an accurate histological diagnosis. Focal medullary lesions tend to grow in spurts. Due to the location of the lesion, lending itself to a high surgical morbidity, surgery is advocated when the tumor and symptoms progress.
Dorsally exophytic lesions present with a long clinical course with symptoms of hydrocephalus or compression of the brain stem. They can be successfully managed by a subtotal resection and CSF diversion when required. Cervicomedullary lesions also carry a good prognosis and are amenable to radical excision.
Mehta et al., reporting on 40 patients with intrinsic brain stem lesions that they operated, felt that the above method of classifying the intrinsic tumors led to a confusion in surgical planning, especially in differentiating between diffuse gliomas and large focal lesions.[40] They have proposed a classification based on their experience, where the intrinsic brain stem lesions are divided into three types: (1) expanding variety-the lesion is well delineated on the contrast-enhanced MRI, is located posteriorly, posterolaterally or ventrolaterally, has a slow evolution of clinical symptoms and the patient is well preserved; (2) infiltrating diffuse variety-the margin between the tumor and the surrounding parenchyma is not well delineated and there is a short duration of symptoms with rapid progression; (3) ventral lesions-the tumor is located ventrally in the brain stem without any lateral or posterior extension. In their opinion, only the expanding variety is amenable for surgical excision, regardless of the size of the lesion [Figure 7].
Figure 7.
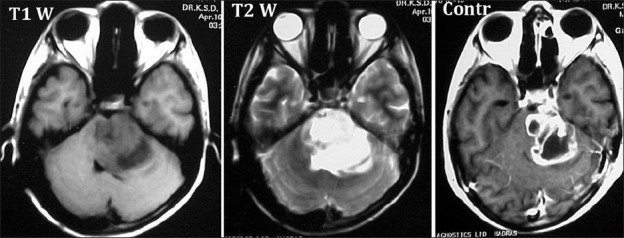
A large lesion is seen on contrast MR to have well-demarcated border. On histology, this was a Glioblastoma Multiforme
The role of stereotactic biopsy (SB) of brain stem lesions has been debated.[36–38,41,42] It is now clear that SB done by an experienced team with modern imaging equipment can be as safe as in any other location in the brain. The procedure is offered when unresectable lesions do not exhibit the classical characteristics of a diffuse glioma on clinical and radiological examination. This is also true of focal lesions with atypical findings.[41]
Conclusion
Gliomas of the posterior fossa are a mixed group of lesions, the commonest being the relatively benign pilocytic astrocytomas of the cerebellum and the malignant diffuse pontine glioma. Management and surgical strategies vary for each type of lesion as does the outcome. Radical surgery is possible in many lesions, including brain stem lesions, with a good outcome. There is still a lot that needs to be understood about many of these lesions.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Karajannis M, Allen JC, Newcomb EW. Treatment of pediatric brain tumours. J Cell Physiol. 2008;217:584–9. doi: 10.1002/jcp.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollack IF. The role of surgery in pediatric gliomas. J Neurooncol. 1999;42:271–88. doi: 10.1023/a:1006107227856. [DOI] [PubMed] [Google Scholar]
- 3.Pollack IF. Brain tumours in children. N Engl J Med. 1994;331:1500–7. doi: 10.1056/NEJM199412013312207. [DOI] [PubMed] [Google Scholar]
- 4.Duffner PK, Cohen ME, Myers MH, Heise HW. Survival of children with brain tumors: SEER program. 1973-1980. Neurology. 1986;36:597–601. doi: 10.1212/wnl.36.5.597. [DOI] [PubMed] [Google Scholar]
- 5.Robertson PL. Advances in treatment of pediatric brain tumours. NeuroRx. 2006;3:276–91. doi: 10.1016/j.nurx.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai KI, Nadkarni TD, Muzumdar DP, Goel A. Prognostic factors for cerebellar astrocytomas in children. A study of 102cases. Pediatr Neurosurg. 2001;35:311–7. doi: 10.1159/000050443. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Deopujari CE, Biyani N, Mhatre MV. Pediatric Cerebellar Pilocytic astrocytoma presenting with hemorrhage. Neurol India. 2010;58:972–4. doi: 10.4103/0028-3886.73775. [DOI] [PubMed] [Google Scholar]
- 8.Lee CS, Huh JS, Sim KB, Kim YW. Cerebellar pilocytic astrocytoma presenting with intratunour bleeding, subarachnoid hemorrhage and subdural hematoma. Childs Nerv Syst. 2009;25:125–8. doi: 10.1007/s00381-008-0678-5. [DOI] [PubMed] [Google Scholar]
- 9.White JB, Piepgras DG, Scheithauer BW, Parisi JE. Rate of spontaneous hemorrhage in histologically proven cases of pilocytic astrocytoma. J Neurosurg. 2008;108:223–6. doi: 10.3171/JNS/2008/108/2/0223. [DOI] [PubMed] [Google Scholar]
- 10.Josan VA, Timms CD, Rickert C, Wallace D. Cerebellar astrocytoma presenting with precocious puberty in a girl. Case Report. J Neurosurg. 2007;107(Suppl 1):6–68. doi: 10.3171/PED-07/07/066. [DOI] [PubMed] [Google Scholar]
- 11.Strazzer S, Zucca C, Fiocchi I, Genitori L, Castelli E. Epilepsy and neuropsychological deficit in a child with cerebellar astrocytoma. J Child Neurol. 2006;21:817–20. doi: 10.1177/08830738060210091701. [DOI] [PubMed] [Google Scholar]
- 12.Berg AL, Olson TJ, Feldstein NA. Cerebellar pilocytic astrocytoma with auditory presentation: Case study. J Child Neurol. 2005;20:914–5. doi: 10.1177/08830738050200111001. [DOI] [PubMed] [Google Scholar]
- 13.Pencalet P, Maixner W, Sainte-Rose C, Lellouch-Tubiana A, Cinalli G, Zerah M, et al. Benign cerebellar astrocytomas in childhood. J Neurosurg. 1999;90:265–73. doi: 10.3171/jns.1999.90.2.0265. [DOI] [PubMed] [Google Scholar]
- 14.Harris LM, Davies NP, Macpherson L, Lateef S, Natarajan K, Brundler MA, et al. Magnetic resonance spectroscopy in the assessment of pilocytic astrocytomas. Eur J Cancer. 2008;44:2640–7. doi: 10.1016/j.ejca.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Davies NP, Wilson M, Harris LM, Natarajan K, Lateef S, Macpherson L, et al. Identification and characterization of childhood cerebellar tumours by in vivo proton MRS. NMR Biomed. 2008;21:908–18. doi: 10.1002/nbm.1283. [DOI] [PubMed] [Google Scholar]
- 16.Bilginer B, Narin F, Oguz KK, Uzun S, Soylemezoglu F, Akalan N. Benign cerebellar pilocytic astrocytomas in children. Turk Neurosurg. 2011;21:22–6. [PubMed] [Google Scholar]
- 17.Sievert AJ, Fisher MJ. Pediatric low grade gliomas. J Child Neurol. 2009;24:1397–408. doi: 10.1177/0883073809342005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernhardtsen T, Laursen H, Bojsen-Moller M, Gjerris F. Sub-classification of low grade cerebellar astrocytomas: Is it clinically meaningful? Childs Nerv Syst. 2003;19:729–35. doi: 10.1007/s00381-003-0825-y. [DOI] [PubMed] [Google Scholar]
- 19.Ajani OA, Sulaiti GA, Bozom IA. Pilomyxoid astrocytomas of the cerebellum. J Neurosurg Pediatr. 2011;7:539–42. doi: 10.3171/2011.2.PEDS10314. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Retnam TM, Menon G, Nair S, Bhattacharya RN, Radhakrishnan VV. Cerebellar hemisphere an uncommon location for pleomorphic xanthoastrocytoma and lipidized glioblastoma multiformis. Neurol India. 2003;51:246–7. [PubMed] [Google Scholar]
- 21.Benesch M, Eder HG, Sovinz P, Raith J, Lackner H, Moser A, et al. Residual or recurrent cerebellar low grade glioma in children after tumour resection: Is retreatment needed. A single center experience from 1983 to 2003? Pediatr Neurosurg. 2006;42:159–64. doi: 10.1159/000091859. [DOI] [PubMed] [Google Scholar]
- 22.Gunny RS, Hayward RD, Phipps KP, Harding BN, Saunders DE. Spontaneous regression of residual low grade cerebellar pilocytic astrocytomas in children. Pediatr Radiol. 2005;35:1086–91. doi: 10.1007/s00247-005-1546-z. [DOI] [PubMed] [Google Scholar]
- 23.Cohen KJ, Broniscer A, Glod J. Pediatric glial tumours. Curr Treat Options Oncol. 2001;2:529–36. doi: 10.1007/s11864-001-0074-9. [DOI] [PubMed] [Google Scholar]
- 24.Villarejo F, de Diego JM, de la Riva AG. Prognosis of cerebellar astrocytomas in children. Childs Nerv Syst. 2008;24:203–10. doi: 10.1007/s00381-007-0449-8. [DOI] [PubMed] [Google Scholar]
- 25.Kurwale NS, Suri V, Suri A, Sarkar C, Gupta DK, Sharma BS, et al. Predictive factors for early symptomatic recurrence in pilocytic astrocytomas: Does angiogenesis have a role to play? J Clin Neurosci. 2011;18:472–7. doi: 10.1016/j.jocn.2010.04.055. [DOI] [PubMed] [Google Scholar]
- 26.Paixao Becker A, de Oliveira RS, Saggioro FP, Neder L, Chimelli LM, Machado HR. In pursuit of prognostic factors in children with pilocytic astrocytomas. Childs Nerv Syst. 2010;26:19–28. doi: 10.1007/s00381-009-0990-8. [DOI] [PubMed] [Google Scholar]
- 27.Aarsen FK, Paquier PF, Arts WF, Van Veelen ML, Michiels E, Lequin M, et al. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytomas in childhood. J Clin Oncol. 2009;27:3526–32. doi: 10.1200/JCO.2008.19.6303. [DOI] [PubMed] [Google Scholar]
- 28.Daszkiewicz P, Maryniak A, Roszkowski M, Barszcz S. Long term functional outcome of surgical treatment of juvenile pilocytic astrocytomas of the cerebellum in children. Childs Nerv Syst. 2009;25:855–60. doi: 10.1007/s00381-009-0855-1. [DOI] [PubMed] [Google Scholar]
- 29.Kansal S, Jindal A, Mahapatra AK. Brain stem glioma- a study of 111 patients. Indian J Cancer. 1999;36:99–108. [PubMed] [Google Scholar]
- 30.Maria BL, Rehder K, Eskin TA, Hamed LM, Fennel EB, Quisling RG, et al. Brain stem gliomas Pathology, clinical features, and therapy. J Child Neurol. 1993;8:112–28. doi: 10.1177/088307389300800203. [DOI] [PubMed] [Google Scholar]
- 31.Roonprapunt C, Abbot R. Surgical treatment of brain stem gliomas in children. Neurosurg Q. 2002;12:160–70. [Google Scholar]
- 32.Fischbein NJ, Prados MD, Wara W, Russo C, Edwards MS, Barkovich AJ. Radiological classification of brain stem tumours: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996;24:9–23. doi: 10.1159/000121010. [DOI] [PubMed] [Google Scholar]
- 33.Raffel C. Molecular biology of pediatric gliomas. J Neurooncol. 1996;28:121–8. doi: 10.1007/BF00250194. [DOI] [PubMed] [Google Scholar]
- 34.Abbot R. Tumours of the medulla. Neurosurg Clin N Am. 1993;4:519–27. [PubMed] [Google Scholar]
- 35.Fangusaro J. Pediatric high grade gliomas and diffuse intrinsic pontine gliomas. J Child Neurol. 2009;24:1409–17. doi: 10.1177/0883073809338960. [DOI] [PubMed] [Google Scholar]
- 36.Albright AL, Packer RJ, Zimmerman R, Rorke LB, Boyett J, Hammond GD. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: A report from the children's Cancer group. Neurosurgery. 1993;33:1026–30. doi: 10.1227/00006123-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Rajshekhar V, Chandy MJ. Computerised tomography guided stereotactic surgery for brainstem masses: A risk benefit analysis in 71 patients. J Neurosurg. 1995;82:976–81. doi: 10.3171/jns.1995.82.6.0976. [DOI] [PubMed] [Google Scholar]
- 38.Selvapandian S, Rajshekhar V, Chandy MJ. Brain stem glioma: Comparative study of clinoco-radiological presentation, pathology and outcome in children and adults. Acta Neurochir (Wein) 1999;141:721–7. doi: 10.1007/s007010050367. [DOI] [PubMed] [Google Scholar]
- 39.Jalali R, Raut N, Arora B, Gupta T, Dutta D, Munshi A, et al. Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2011;77:113–8. doi: 10.1016/j.ijrobp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 40.Mehta VS, Chandra PS, Singh PK, Garg A, Rath GK. Surgical considerations for intrinsic brain stem gliomas. Neurol India. 2009;57:274–81. doi: 10.4103/0028-3886.53272. [DOI] [PubMed] [Google Scholar]
- 41.Perez-Gomez JL, Rodriguez-Alvarez CA, Marhx-Bracho A, Rueda-Franco F. Stereotactic biopsy for brainstem tumours in pediatric patients. Childs Nerv Syst. 2010;26:29–34. doi: 10.1007/s00381-009-1000-x. [DOI] [PubMed] [Google Scholar]
- 42.Patel P, Balamurugan M. Transcerebellar stereotactic biopsy for brain stem lesions in children. J Pediatr Neurosci. 2009;4:17–9. doi: 10.4103/1817-1745.49101. [DOI] [PMC free article] [PubMed] [Google Scholar]


