Abstract
Intramedullary tumors of the spinal cord account for 35-40% of intraspinal tumors in children. The biological behavior of these tumors is of slow progression, and hence aggressive surgery has been advocated. Surgical adjuncts include use of intraoperative neurophysiological monitoring, preoperative ultrasound, microsurgical techniques and ultrasonic suction devices. Osteoplastic laminoplasty approaches avoid post-laminectomy deformities in younger children. Postoperative radiotherapy and more recently chemotherapy regimes have been proposed for incompletely resected tumors.
Keywords: Intramedullary tumours, intra-operative monitoring, laminoplasty, astrocytomas, ependymomas
Introduction
Intramedullary spinal cord tumors are rare neoplasms, accounting for 4–10% of central nervous system tumors. Unlike in adults where intramedullary tumors form 20% of intraspinal neoplasms, in children about 35–40% of all intraspinal tumors are found in this location. Originally, a two-stage strategy was described[1] for operation on these tumors where a surgeon would perform only a myelotomy on the first day and return after 1 week to remove the extruded portion in the second sitting. Thereafter, neurosurgeons began to rely on more aggressive surgical approaches, but were faced with neurological morbidity including infection, paralysis and even death in a number of patients. This led to the era where simple biopsy and dural decompression followed by radiotherapy began to gain vogue.[2] Modern neurosurgical instruments, techniques and intraoperative monitoring techniques have again tilted the balance in favor of a more aggressive surgical philosophy.
Moreover, the biological behavior of most intramedullary tumors of the spinal cord is of slow progression, and often may not be recognized till they have attained a significant size.[3] This is of particular significance in India as often children present after they have severe neurological deficits which generally occur fairly late in the course of the disease.
A review of the database of pediatric intramedullary tumors at the Park Clinic Kolkata from 1998 to 2010 showed a total of 44 such tumors whose breakup is given in Table 1. The single most common tumor type in this series was low-grade astrocytoma.
Table 1.
Histological types of pediatric intramedullary tumors at author's institution (1998–2010)

Clinical Presentation
The intramedullary tumors may remain asymptomatic for several months to even years before the diagnosis is made in these children. In our own modest series, the median time of presentation was 7.5 months after the onset of symptoms.
Pain is without doubt the commonest symptom of presentation. Local pain along the spinal axis is frequent,[4] and is often described over the spinal segments overlying the tumor. Often a trivial injury is quoted as the start of the symptoms. Nocturnal pain that awakens the children from sleep has been described. Young infants may present with abdominal pain.[5] Other symptoms include motor deficits, motor regression in infants, or frequent falls. Sensory symptoms or sphincter dysfunctions are rare and indeed only 8% of the children in our series presented with sphincter problems quite in contradistinction to the adult counterparts. One-third of the children in our series presented with scoliosis. The scoliotic curve associated with intramedullary tumors in children is not specific to either direction.[5] Torticollis is common with cervical tumors and can precede the development of neurological problems.[6]
Hydrocephalus is reported to occur in about 15% of children with intramedullary tumors.[7–9] In high-grade tumors, the subarachnoid spread of tumor creates an increase in outflow resistance at the level of the basal cisterns, explaining the genesis of the hydrocephalus. In low-grade tumors, the causative mechanism of hydrocephalus is much more controversial,[8] where an anatomical isolation of the spinal subarachnoid space from the intracranial compartment caused by the presence of the tumor has been held responsible for the hydrocephalus.
On examination, most patients had mild to moderate motor deficits, with findings like spasticity, hyperreflexia and ankle clonus. Dorsal column dysfunction is rare, and sphincter dysfunction presents late in the course of the disease. The only exception has been in tumors at the conus medullaris which generally present with bladder disturbances.[10]
Diagnostic Studies
Magnetic Resonance Imaging (MRI) is the study of choice to identify and delineate intramedullary spinal cord tumors. MR studies should be performed with intravenous contrast to demonstrate the solid component, and to separately show the cysts, edema and syrinx cavity if present. Astrocytomas and gangliogliomas show heterogeneous enhancement patterns [Figure 1]. Both these tumors are generally located slightly eccentrically and produce asymmetric dilatation of the cord. Ependymomas, on the other hand, demonstrate uniform enhancement,[11] and are located centrally in the cord. They are often capped by caudal and rostral low signal intensity areas (the “cap sign”) on either side of the tumor mass, and this imaging finding is a result of hemosiderin deposits caused by chronic hemorrhage[12] [Figure 2].
Figure 1.
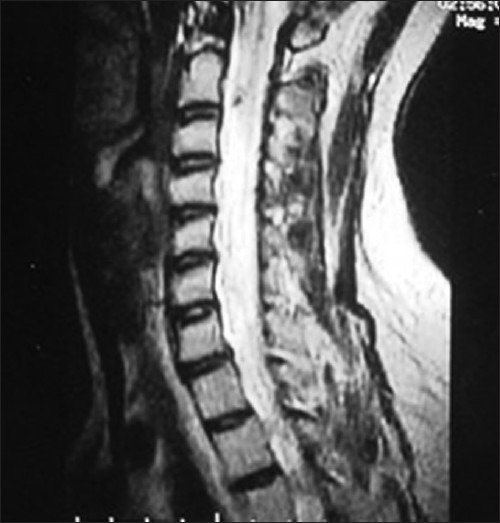
MR scan T1-weighted image with gadolinium, showing astrocytoma
Figure 2.
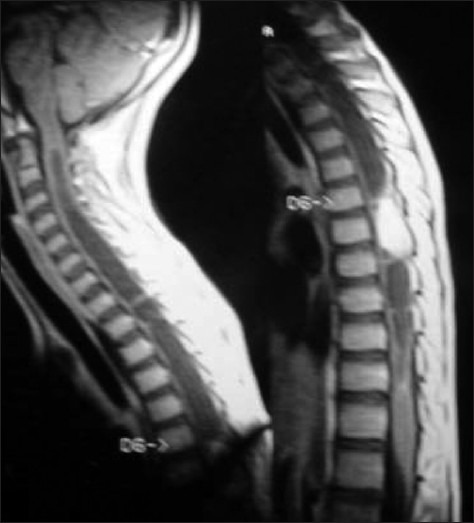
T1-weighted MR image of dorsal intramedullary ependymoma
In children with scoliosis, digital X-rays in erect position and with bending views are necessary to provide baseline studies to compare later on for correction of spinal deformity.
CT scans of the brain may be necessary in children who present with hydrocephalus.
Pathology
Ependymomas presumably arise from ependymal cell remnants of the central canal. This is the possible reason why their growth is vertical along the spinal canal. Ependymomas are graded according to the World Health Organization (WHO) classification of tumors. The subependymoma (WHO grade I) is a slowly growing tumor with a very good prognosis. Myxopapillary ependymomas are also WHO grade I tumors which arise in the filum terminale or conus medullaris and are usually associated with a very good prognosis. The most common intramedullary ependymoma is a WHO grade II tumor. Anaplastic (WHO gradeIII) ependymomas probably arise from malignant transformation of low-grade ependymomas.[11]
The classical WHO grade II ependymoma is a highly cellular tumor with rare mitotic activity. It stains positive for Glial fibrillary acidic protein (GFAP) immunostains, has perivascular pseudorosettes, and occasional ependymal rosettes. The myxopapillary type is characterized by abundant mucin production, GFAP immunoreactivity and rare mitosis. Anaplastic variants have clear features of malignancy like multiple mitotic figures, necrosis and neovascularization.
The pilocytic astrocytomas and the WHO grade II fibrillary astrocytomas are the two commonest types of astrocytomas in children. Astrocytomas are located eccentrically in the spinal cord, have ill-defined margins, often span five to six vertebral bodies, and demonstrate predilection to occur in the cervicothoracic region in 75% cases.[13] Astrocytomas often extend through the whole or multiple segments of the spinal cord, the so-called holocord astrocytoma.[14] In our series, 6 of the 23 astrocytomas were holocord in nature.
Gangliogliomas are graded from I to III depending on cellular atypia, mitosis, necrosis and neovascularization of the glial component of the tumor. They are characterized by binucleate ganglion cells with eosinophilic granular bodies and prominent nucleoli.[15]
Surgical Treatment
Surgical resection is the treatment of choice for pediatric intramedullary tumors. The extent of resection depends on each tumor type because some tumors like ependymomas, gangliogliomas and pilocytic astrocytomas have a good plane of cleavage and radical resection has low neurological morbidity; others like high-grade astrocytomas are difficult to resect with acceptable morbidity because of poorly defined tumor-cord interface. The latter policy has been contested in a number of recent publications,[16] as it is felt that even the majority of astrocytomas, being low grade in nature, are amenable to radical resection. Hence, the role of conservative resection[17] has been questioned. In cases of holocord astrocytomas [Figure 3], staged surgery has been advocated and laminoplasty needs to be performed only overlying the solid portion of the tumor.
Figure 3.
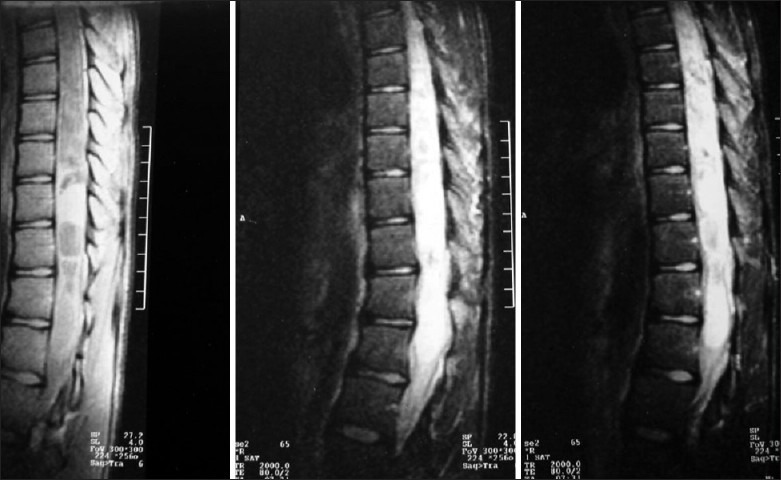
MR imaging of holocord astrocytomas
In a series from India,[18] radical resection has been quoted as achieved in 59% cases, with subtotal resection done in 32%. Another Indian publication on intramedullary tumors in pediatric and adult population has advocated a similar approach and philosophy.[19]
Recent advances in microsurgical techniques, combined with availability of ultrasonic aspirator, Laser, intraoperative ultrasound, and intraoperative neurophysiological monitoring have led to a much more aggressive approach to these tumors as a whole.[20–22] Gross total resection is attempted now in most cases. A conservative approach has been, however, proposed for tumors of the conus medullaris. Gross total removal has been defined as at least 95% tumor removal, evidenced by a clean surgical field under the microscope at the end of the procedure and a clear postoperative MR scan obtained within 48 hours of the surgery.
Certain special modalities deserve special mention:
Neurophysiological monitoring
The use of neurophysiological monitoring has not been demonstrated to be a definite predictor of outcome.[23] Initially, the monitoring was restricted to obtaining somatosensory evoked potentials (SSEP) and this was dependant on the integrity of the dorsal columns. Hence, as has been in our experience, these SSEPs disappear immediately after a myelotomy, and therefore have a limited value during surgery for intramedullary tumors. Currently, however, and in our practice, the use of SSEPs is combined with the use of motor evoked potentials (MEPs). Two different recording techniques are available for measuring MEPs. The transcranial stimulus technique allows recording of the D-wave by an epidural catheter electrode placed epidurally or subdurally (eMEPs), whereas the multipulse technique is suitable for eliciting MEPs from limb muscles (mMEP). The advantage of the latter is that the entire motor system from the cortex down to the neuromuscular junction is monitored. The problem of mMEPs is that they are more easily blocked by muscle relaxants and anesthesia.[24]
It has been our own experience that if MEPs are maintained at more than 50% of their original amplitude, tumor removal can be continued safely. If MEPs drop beyond 50%, waiting for them to return is necessary before proceeding with further cord manipulation. It is always necessary to find a balance between removing too much tumor and causing unacceptable neurodeficits.
Equipments used
Ultrasonic aspirator
This is a very useful tool for rapidly reducing tumor volume by intratumoral decompression. Neurophysiological function and blood flow in the adjacent neural tissue is well maintained beyond 1 mm from the vibrating tip.[25] This instrument minimizes the traction on adjacent white matter while resecting a tumor. The problem is that with firm to hard tumors, it is often not possible to use.
Laser
Lasers minimize thermal and mechanical trauma to surrounding structures. After an intramedullary tumor has been debulked by an ultrasonic aspirator, lasers may be used to remove residual fragments. There have been descriptions of use of lasers to perform the initial myelotomy. However, due to costs and charring of tissue, their use has not gained much popularity.
Peroperative ultrasound
Use of preoperative ultrasonography to locate the level of tumor and study the solid versus cystic components of the intramedullary tumor has been described.[3] In holocord astrocytomas, ultrasound may be used to site the laminectomy/laminotomy directly over the solid portion of the tumor. Blood within the operative field is a major problem for its use.[26]
Dural substitutes
Initially, we used to leave the dura widely open after surgery for intramedullary tumors, but there was clearly a problem with formation of pseudomeningoceles. Hence, it became our practice to routinely perform duroplasty. It has been suggested that polytetrafluoroethylene (Gore Tex; WL Gore and Associates, USA) produces no adhesions,[27] but a recent study failed to demonstrate any advantage of one dural substitute over another.[28] Tissue glue has also been used by us for the duroplasty.
Prevention of deformity
Post-laminectomy deformities like kyphosis, swan-neck deformities, and scoliosis are common after surgery for intramedullary tumors in the pediatric age group, although they become less frequent with increasing age.[29,30] It has been shown that 46% of patients younger than 15 years of age developed deformity after multilevel laminectomies, whereas after the age of 1 year the incidence dropped to 6%.[31] Many surgeons prefer to use osteoplastic laminotomy for all children undergoing surgery for spinal tumors with a view to preserving the posterior tension band and restoring normal anatomy.[32,33] An aggressive approach to spinal stabilization and fusion should be considered for pediatric patients with spinal cord tumors who present with significant spinal deformity.[34]
Radiation and Chemotherapy
Many reports have suggested[35] that radiation therapy is effective in malignant spinal cord tumors where a considerable residual mass remains. However, benefits in young children are controversial because of local recurrence, radiation myelopathy, spinal deformity and secondary tumors.[36] Also, unlike in the brain, low-grade spinal intramedullary tumors have little potential to transform into high-grade tumors in the absence of radiotherapy.[37] Tumoricidal doses can be given to astrocytomas (4500–5000 cGy in 180–200 cGy fractions) and for those with ependymomas (4000–4500 cGy in 180–200 cGy fractions), and the chances of radiation myelopathy developing at these doses are small.
Radiation therapy may be withheld in ependymomas and low-grade astrocytomas that have undergone gross total or near-total resection. Radiation therapy is thus reserved for patients with incompletely resected low-grade tumors where repeat surgery is not feasible for some reason or other or for tumors which have rapid recurrence or for high-grade astrocytomas.
Chemotherapy is used for malignant intramedullary tumors in combination with radiation therapy,[38] but the results are conflicting. Variable responses to chemotherapy regimes have been reported in low-grade astrocytomas in children.[39,40] The French Society of Paediatric Oncology (SFOP) designed a study[41] where chemotherapy with carboplatin, procarbazine, vincristine, cyclophosphamide, etoposide, and cisplatin was used in all grades of intramedullary spinal cord gliomas and reported good progression-free survival in majority of their patients. The most significant result of this study was a marked improvement in the functional status of the patients.
Outcome
The two most significant predictors of outcome in children who have surgery for intramedullary spinal cord tumors are 1) histological grade of the tumor and 2) the pre-operative neurological status.[42,43] Children who have a significant pre-operative deficit are more likely to deteriorate after surgery. Children who have ependymomas (WHO grade II) fare better than those who have WHO grade II astrocytomas. Those with a pilocytic astrocytoma have a better prognosis than those with ependymomas.
Outcome also depends on the extent of resection, and whether the tumor has a solid component only or is mainly cystic in nature.[44]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Elsberg CA, Beer E. The operation of intramedullary tumours of the spinal cord. A report of two operations with remarks upon the extrusion of intraspinal tumours. Am J Med Sci. 1911;142:636–47. [Google Scholar]
- 2.Wood EH, Berne AS, Taveras JM. The value of radiation therapy in the management of intrinsic tumours of the spinal cord. Radiology. 1954;63:11–24. doi: 10.1148/63.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Nadkarni TD, Rekate HL. Paediatric intramedullary spinal cord tumours: Critical review of the literature. Childs Nerv Syst. 1999;15:17–28. doi: 10.1007/s003810050321. [DOI] [PubMed] [Google Scholar]
- 4.Keating RF, Goodrich JT, Packer RJ. Tumours of the paediatric central nervous system. New York, NY: Thieme Medical Publishers Inc; 2000. p. 416. [Google Scholar]
- 5.Jallo GI, Freed D, Epstein F. Intramedullary spinal cord tumours in children. Childs Nerv Syst. 2003;19:641–9. doi: 10.1007/s00381-003-0820-3. [DOI] [PubMed] [Google Scholar]
- 6.Epstein F, Epstein N. Surgical treatment of spinal cord astrocytomas of childhood. A series of 19 patients. J Neurosurg. 1982;57:685–9. doi: 10.3171/jns.1982.57.5.0685. [DOI] [PubMed] [Google Scholar]
- 7.Rifkinson-Mann S, Wisoff JH, Epstein F. The association of hydrocephalus with intramedullary spinal cord tumours: A series of 25 patients. Neurosurgery. 1990;27:749–54. doi: 10.1097/00006123-199011000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Cinalli G, Sainte-Rose C, Lellouch-Tubiana A, Sebag G, Renier D, Pierre-Kahn A. Hydrocephalus associated with intramedullary low grade glioma: Illustrative cases and review of literature. J Neurosurg. 1995;83:480–5. doi: 10.3171/jns.1995.83.3.0480. [DOI] [PubMed] [Google Scholar]
- 9.Prasad VS, Basha A, Prasad BC, Raja Reddy D. Intraspinal tumour presenting as hydrocephalus in childhood. Childs Nerv Syst. 1994;10:156–7. doi: 10.1007/BF00301081. [DOI] [PubMed] [Google Scholar]
- 10.Houten JK, Weiner HL. Paediatric intramedullary spinal cord tumours: Special considerations. J Neurooncol. 2000;47:225–30. doi: 10.1023/a:1006418506213. [DOI] [PubMed] [Google Scholar]
- 11.Auguste KI, Gupta N. Paediatric intramedullary spinal cord tumours. Neurosurg Clin N Am. 2006;17:51–61. doi: 10.1016/j.nec.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Baleriaux DL. Spinal cord tumours. Eur Radiol. 1999;9:1252–8. doi: 10.1007/s003300050831. [DOI] [PubMed] [Google Scholar]
- 13.Stein BM. Intramedullary spinal cord tumours. Clin Neurosurg. 1983;30:717–41. doi: 10.1093/neurosurgery/30.cn_suppl_1.717. [DOI] [PubMed] [Google Scholar]
- 14.Mauser HW, Dokkum TA. Astrocytomas involving the whole spinal cord. Two case reports. Clin Neurol Neurosurg. 1981;83:239–45. doi: 10.1016/0303-8467(81)90046-9. [DOI] [PubMed] [Google Scholar]
- 15.Miller DC, Lang FF, Epstein FJ. Central nervous system gangliogliomas – Part I: Pathology. J Neurosurg. 1993;79:859–66. doi: 10.3171/jns.1993.79.6.0859. [DOI] [PubMed] [Google Scholar]
- 16.Constantini S, Miller D, Allen J, Rorke LB, Freed D, Epstein F. Radical excision of intramedullary spinal cord tumours: Surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg (Spine 2) 2000;93:183–93. doi: 10.3171/spi.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 17.Garcia DM. Primary spinal cord tumours treated with surgery and postoperative irradiation. Int J Radiat Oncol Biol Phys. 1985;11:1933–9. doi: 10.1016/0360-3016(85)90274-3. [DOI] [PubMed] [Google Scholar]
- 18.Kumar Raj, Singh V. Intramedullary mass lesion of the spinal cord in children of a developing milieu. Paediatr Neurosurg. 2004;40:16–22. doi: 10.1159/000076572. [DOI] [PubMed] [Google Scholar]
- 19.Nair S, Menon G, Rao BR, Boyini JR, Thiagrajan M, Abraham M, et al. Intramedullary spinal cord glial tumours: Management philosophy and surgical outcome. Minim Invasive Neurosurg Multidiscip traumatol. 2006;1:36–46. [Google Scholar]
- 20.Brotchi J, Dewitte O, Levivier M, Balériaux D, Vandesteene A, Raftopoulos C, et al. A survey of 65 tumours within the spinal cord: Surgical results and importance of preoperative magnetic resonance imaging. Neurosurgery. 1991;29:651–7. [PubMed] [Google Scholar]
- 21.Bouffet E, Pierre-Kahn A, Marchal JC, Jouvet A, Kalifa C, Choux M, et al. Prognostic factors in paediatric spinal cord astrocytoma. Cancer. 1998;83:2391–9. doi: 10.1002/(sici)1097-0142(19981201)83:11<2391::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Wilson P, Oleszek JL, Clayton GH. Paediatric spinal cord tumours and masses. 2007;30 Supp. J Spinal Cord Med. 2007;30(Suppl. 1):S15–20. doi: 10.1080/10790268.2007.11753963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sala F, Bricolo A, Facioli F, Lanteri P, Gerosa M. Surgery for intramedullary spinal cord tumours: The role of intraoperative (neurophysiological) monitoring. 2007;16 Supp. Eur Spine J. 2007;16(Suppl. 2):130–9. doi: 10.1007/s00586-007-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi M, Cedzich C, Schramm J. Modifications of cortical stimulation for motor evoked potentials under general anaesthesia: Technical description. Neurosurgery. 1993;32:219–26. doi: 10.1227/00006123-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Crista L, Herrmann HD. Surgical management of intramedullary spinal cord tumours: Functional outcome and sources of morbidity. Neurosurgery. 1994;35:69–76. doi: 10.1227/00006123-199407000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami N, Mimatsu K, Kato F. Intraoperative sonography of intramedullary spinal cord tumour. Neuroradiology. 1992;34:436–9. doi: 10.1007/BF00596511. [DOI] [PubMed] [Google Scholar]
- 27.Aliredjo RP, de Vries J, Menovsky T, Grotenhuis JA, Merx J. The use of Gore-Tex membrane for adhesion prevention in tethered spinal cord surgery: Technical case reports. Neurosurgery. 1999;44:674–7. doi: 10.1097/00006123-199903000-00139. [DOI] [PubMed] [Google Scholar]
- 28.Abla AA, Link T, Wilson DA, Sonntag VK. Comparison of dural grafts in Chiari decompression surgery: Review of the literature. J Craniovertebr Junction Spine. 2010;1:29–37. doi: 10.4103/0974-8237.65479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fassett DR, Clark R, Brockmeyer DL, Schmidt MH. Cervical spine deformity associated with resection of spinal cord tumours. Neurosurg Focus. 2006;20:E2. [PubMed] [Google Scholar]
- 30.Winter RB, Hall JE. Kyphosis in childhood and adolescence. Spine (Phila Pa 1976) 1978;3:285–308. doi: 10.1097/00007632-197812000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Yasuoka S, Peterson HA, MacCarty CS. Incidence of spinal column deformity after multilevel laminectomy in children and adults. J Neurosurg. 1982;57:441–5. doi: 10.3171/jns.1982.57.4.0441. [DOI] [PubMed] [Google Scholar]
- 32.Abbott R, Feldstein N, Wisoff JH, Epstein FJ. Osteoplastic laminotomy in children. Pediatr Neurosurg. 1992;18:153–6. doi: 10.1159/000120656. [DOI] [PubMed] [Google Scholar]
- 33.McGirt MJ, Chaichana KL, Atiba A, Bydon A, Witham TF, Yao KC, et al. Incidence of spinal deformity after resection of intramedullary spinal cord tumours in children who underwent laminectomy compared with laminoplasty. J Neurosurg Pediatr. 2008;1:57–62. doi: 10.3171/PED-08/01/057. [DOI] [PubMed] [Google Scholar]
- 34.Parikh SN, Crawford AH. Orthopaedic implications in the management of pediatric vertebral and spinal cord tumours: A retrospective review. Spine (Phila Pa 1976) 2003;28:2390–6. doi: 10.1097/01.BRS.0000085323.71376.42. [DOI] [PubMed] [Google Scholar]
- 35.Sandalcioglu IE, Gasser T, Asgari S, Lazorisak A, Engelhorn T, Egelhof T, et al. Functional outcome after surgical treatment of intramedullary spinal cord tumour: Experience with 78 patients. Spinal Cord. 2005;43:34–41. doi: 10.1038/sj.sc.3101668. [DOI] [PubMed] [Google Scholar]
- 36.Clayton PE, Shalot SM. The evolution of spinal growth after irradiation. Clin Oncol (R Coll Radiol) 1991;3:220–2. doi: 10.1016/s0936-6555(05)80744-7. [DOI] [PubMed] [Google Scholar]
- 37.Dirks PB, Jay V, Becker LE. Development of anaplastic changes in low grade astrocytomas of childhood. Neurosurgery. 1994;34:68–78. [PubMed] [Google Scholar]
- 38.Allen JC, Aviner S, Yates AJ, Boyett JM, Cherlow JM, Turski PA, et al. Treatment of high-grade spinal cord astrocytoma of childhood with ‘8 in 1” chemotherapy and radiotherapy: A pilot study of CCG-945. Children's Cancer Group. J Neurosurg. 1998;88:215–20. doi: 10.3171/jns.1998.88.2.0215. [DOI] [PubMed] [Google Scholar]
- 39.Chamberlain MC. A review of leptomeningeal metastasis in pediatrics. J Child Neurol. 1995;10:191–9. doi: 10.1177/088307389501000304. [DOI] [PubMed] [Google Scholar]
- 40.Goh KY, Velasquez L, Epstein FJ. Paediatric intramedullary spinal cord tumours: Is surgery alone enough? Pediatr Neurosurg. 1997;27:34–9. doi: 10.1159/000121222. [DOI] [PubMed] [Google Scholar]
- 41.Doireau V, Grill J, Zerah M, Lellouch-Tubiana A, Couanet D, Chastagner P, et al. Chemotherapy for unresectable and recurrent intramedullary glial tumours in children. Brain Tumours Subcommittee of the French Society of Paediatric Oncology (SFOP) Br J Cancer. 1999;81:835–40. doi: 10.1038/sj.bjc.6690772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Innocenzi G, Salvati M, Cervoni L, Delfini R, Cantore G. Prognostic factors in intramedullary astrocytomas. Clin Neurol Neurosurg. 1997;99:1–5. doi: 10.1016/s0303-8467(96)00555-0. [DOI] [PubMed] [Google Scholar]
- 43.Prybylski GJ, Albright AL, Martinez AJ. Spinal cord astrocytomas: Long-term results comparing treatments in children. Childs Nerv Syst. 1997;13:375–82. doi: 10.1007/s003810050103. [DOI] [PubMed] [Google Scholar]
- 44.Lunardi P, Licastro G, Missori P, Ferrante L, Fortuna A. Management of intramedullary tumours in children. Acta Neurochir (Wien) 1993;120:59–65. doi: 10.1007/BF02001471. [DOI] [PubMed] [Google Scholar]


