Abstract
Hydrocephalus is a common clinical problem seen in pediatric neurosurgical practice. Hydrocephalus involves dilatation of the cerebral ventricular system with corresponding, compressive effects on the parenchyma. It can be communicative or obstructive types. Congenital, acquired, infective, and secondary hydrocephalus have different clinical features with different modality of treatments. Ventriculoperitoneal shunt is the gold standard of treatment. Endoscopic 3rd ventriculostomy is rapidly gaining prominence as an alternative. Various kinds of hydrocephalus, their pathophysiology, treatment and complications are reviewed.
Keywords: Complications, hydrocephalus, outcome, shunts
Introduction
Hydrocephalus is a condition wherein excess of cerebrospinal fluid (CSF) accumulates within the ventricular system and cisterns of the brain leading to increased intracranial pressure (ICP) and related consequences. This can apparently result from various causes that can affect a fetus, infant, child or adult (Rekate). Numerous definitions of hydrocephalus have been proposed. Summarily, it can be described as an imbalance between production and absorption of CSF.[1] Over production of CSF can also be a cause of hydrocephalus due to choroid plexus tumors, but these are rare (tumors) in clinical practice.
History
Hydrocephalus has been recognized for centuries. Accumulation of fluid in various intracranial compartments was recognized by Hippocrates (BC 460–377) and Claudies Galen (130–200 AD). The studies of Thomas Willis (1621–1675) facilitated the understanding of ventricular system and CSF pathways. Franciscus Sylvius (1614–1672), Alexander Monroe (1733–1817) and Francois Magendie (1783–1855) have made important anatomical contributions for the CSF pathway. Finally, Key and Retzeus (1876) established the modern concept of CSF circulation. At that stage, the diagnosis and management was not clear, resulting in high mortality. Dandy and Blackfan (1913) further contributed by creating experimental models of hydrocephalus which led to the classification and differentiation between the non-communicating (obstructive) and communicating forms with distinct possible treatment strategies. The treatment options were extirpation of choroid plexus, removing obstructive pathologies or creation of conduits to drain CSF from the intracranial compartment. The history of the treatment of hydrocephalus has been described in detail by John Scarff in 1963. Walter Dandy proposed 3rd ventriculostomy in 1922 for obstructive variety. Other surgical procedures include Torkildson′s procedure which involves draining the lateral ventricle into the cisterna magna, and ventriculocisternostomy in case of aqueductal obstruction. A flood of operative techniques for the diversion of CSF have come into vogue since 1939. In the 1950s, synthetic, biologically tolerant polymers, particularly silicone elastomers, became available and thus heralded an era of shunts. Meanwhile, advances in optics and endoscopes repopularized the endoscopic 3rd ventriculostomy as originally described by Mixter.
Indian scenario
Large heads were generally ignored in the past. Proving the fact that excessive fluid inside the head was the cause of hydrocephalus and its effects used to be a challenge. Though plain X-rays were indicative, they were not necessarily confirmatory. Air and contrast ventriculography was the key diagnostic investigation. The introduction of computed tomography (CT) in 1980s has rapidly advanced the detection and treatment. The number of ventriculoperitoneal (VP) shunts has risen in many centers across the country. Indian economy had put several restraints in the usage of western shunts, hence surgeons resorted to valve less infant feeding tubes as shunts. However, they were found to be useful particularly in post-infective hydrocephalus. They were not only economical but also effective in situations where CSF protein was high. Regional research and development and industrial association paved the way to the development of economical Indian shunts which have now became popular even outside India. Though etiological factors have changed over the years, the incidence of hydrocephalus has remained more or less similar across the country. Both neurosurgeons and pediatric surgeons were treating hydrocephalus. In the last decade, endoscopic third ventriculostomy (ETV) has become a popular surgical procedure for hydrocephalus and the technique is being practiced all over the country. An Indian study group of neuroendoscopy was founded creating a platform for scientific discussion and sharing the experience. Over the years, improvement in social status, awareness, better nutrition and better perinatal care have resulted in reduction in major anomalies and associated hydrocephalus. Prenatal diagnosis has become a reality in most centers. The post-infective hydrocephalus has seen a decline. However, recently, there has been a resurgence of tuberculosis leading to increase in the incidence of TB meningitis with hydrocephalus. Programmable shunts are now available but sparingly used.
Embryology
Ventricular system develops from the corresponding vesicles of the developing neural tube. The cavity of each telencephalic vesicle becomes the lateral ventricle and that of the diencephalic becomes the 3rd ventricle. The cavity of rhombencephalon forms the 4th ventricle. Its continuation into the spinal cord is the central canal. During development, each lateral ventricle is a spherical space within the telencephalic vesicle. With the forward and backward growth, the ventricle gets elongated anteroposteriorly. The posterior end of the telencephalic vesicle now grows downward and forward to form the temporal horns, making the ventricles “C” shaped. Finally, the occipital horns grow backward. The approximation of the two growing telencephalic vesicles makes the medial walls of the lateral ventricles appose each other forming a septum. The floor of this becomes the roof of 3rd ventricle and its lateral invagination forms the choroidal fissure. A fold of piameter extends into this fissure, forming the tela choroidea. A bunch of capillaries develops within this fold, leading to the formation of choroid plexus.[2–4]
Cerebrospinal fluid production and absorption
Majority of the CSF production is by the choroid plexus which contributes 70 – 80% of the total daily volume. A small proportion of CSF may be produced from ventricular ependyma and brain parenchyma. CSF production occurs by a combination of filtration across the endothelium and active transport of sodium by the choroidal epithelia. Cerebral perfusion pressure and ICP appear to have only minimal effect on CSF production under physiological conditions. CSF, which is largely formed in the lateral ventricles, passes through foramen of Monroe to the 3rd ventricle and reaches 4th ventricle through the aqueduct of Sylvius. Then, it presumably exits through median foramen of Magendi and lateral foramen of Luschka. The CSF dissects into intercellular space of meninx primitive to form the subarachnoid space. From the subarachnoid space, the CSF reaches the parasaggital arachnoid granulations.[5]
CSF is produced at a rate of 0.33 ml/min, which is approximately 500 ml/day. The total volume of CSF varies with age and in the adults is 100–150 ml of which 15–25 ml is contained within the ventricles. The mechanisms of absorptions of CSF have been extensively investigated. Direct absorption from the brain parenchyma, choroid plexus or by the lymphatic channels in the region of the cribriform plate has been postulated. The arachnoidal villi and granulations contribute to the maximum absorption. The villi are the herniations of arachnoidal tissue into the dural venous sinuses. Two mechanisms have been proposed. The “closed” mechanism is where the villi are a blind diverticulation and absorption occurs by a process of seepage across the endothelial covering. The open mechanism indicates the presence of channels across the villi, opening and closing in a valve-like manner and permitting unidirectional flow of CSF. Tripathi and Tripathi have proposed transmembrane transport mechanism consisting of vacuoles carrying CSF across the endothelial layer. Recently, the role of CNS microcirculation in the absorption of CSF has contributed to the understanding of the pathogenesis of hydrocephalus. As these mechanisms are not still clear, we are forced to follow our understanding and classification based on traditional concepts of CSF circulation.[6]
Etiology and pathophysiology of hydrocephalus
The incidence of congenital hydrocephalus is about 0.2–0.5/1000 live births. A higher incidence has been reported in elderly primiparous mothers. It can be associated with a variety of physiological and pathological conditions.[7,8] Obstruction at any point along the CSF pathway may result in hydrocephalus. Traditionally, the obstruction may be within the ventricular system, resulting in non-communicating hydrocephalus or the impairment of circulation is through the subarachnoid space or defective absorption in the venous system, resulting in communicating hydrocephalus. Whatever be the etiology, hydrocephalus is further divided into congenital and acquired forms. The etiology of congenital hydrocephalus remains obscure. An inheritable form of aqueductal stenosis has been described in males (X-linked hydrocephalus). The other mechanism of hydrocephalus is over production of CSF, seen in choroid plexus papillomas.[9]
Rekate has classified hydrocephalus based on the CSF flow obstruction. Impaired absorption is another mechanism wherein venous sinus occlusions, vein of Galen malformations and developmental anomalies like craniostenosis with malformation of the skull base can lead to formation of hydrocephalus. Diseases of the arachnoidal villi can also result in hydrocephalus due to impaired absorption.[10]
Classification
Based on the results of their neutral phenosulfonaphthalein tests, Dandy and Blackfan subdivided hydrocephalus into two groups.[11] If the chemical injected into the lateral ventricle was recovered within 20 min from the spinal subarachnoid space, the hydrocephalus was termed “communicating,” implying a patent communication between the ventricles and the subarachnoid space. If no recovery was possible, the hydrocephalus was termed “non-communicating” or obstructive. With the present CT and magnetic resonance imaging (MRI) techniques, it is possible to localize with accuracy the exact site of blockage of flow to CSF. Hence, a more helpful classification is as follows: The hydrocephalus may be due to 1) overproduction of CSF (a rare entity); 2) obstructive, wherein the obstruction to the flow of CSF in the a) lateral ventricles, b) foramen of Monroe, c) 3rd ventricle, d) aqueduct of Sylvius, e) 4th ventricle or f) subarachnoid spaces; or 3) absorption defect. Based on the site of blockage to the CSF flow, the hydrocephalus may be 1) monoventricular or unilateral, 2) biventricular (both lateral ventricles), 3) triventricular (3rd and both lateral ventricles), or 4) pan ventricular (4th, 3rd and both lateral ventricles). Depending on the exact etiology, a secondary classification could be added under the following headings: 1) congenital, 2) traumatic, 3) inflammatory, 4) neoplastic, and 5) degenerative.[12]
Clinical features
The presentation of hydrocephalus differs in the case of the neonate and infant compared with the older child or adult. Prior to closure of the cranial sutures and obliteration of the fontanella, hydrocephalus results in disproportionate head growth. Thus, over the first 2–3 years of life, measurement of the occipito-frontal circumference and plotting this on a centile chart provides a simple and sensitive test. Wherever possible, sequential measurements (corrected for gestational age) should be obtained in order that the trend of head growth in relation to the centile lines can be demonstrated. Clinical symptoms are often subtle and include general irritability, poor feeding and slow attainment of milestones. In addition to head size, clinical signs include bulging of the fontanellae, wide separation of the cranial sutures, prominence of scalp veins, and “setting sun” of the eyes [Figure 1]. This later clinical sign is attributed to pressure on the mid-brain tectum by CSF in the suprapineal recess. Papilledema can be difficult to diagnose in an infant and indeed is commonly absent in infantile hydrocephalus and so is an unreliable sign.
Figure 1.
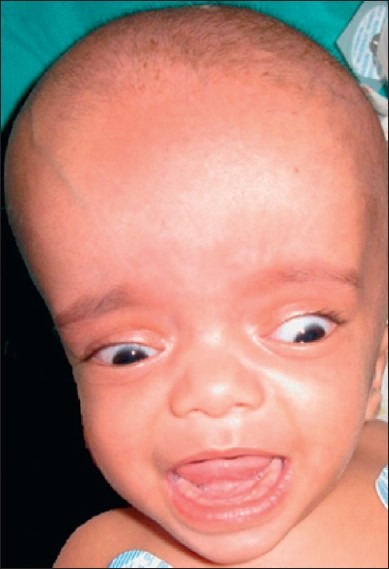
Setting sun sign
In older children and adults, the classical symptom complex consisting of raised ICP, headache, vomiting and drowsiness is more likely to herald an underlying diagnosis of hydrocephalus. When hydrocephalus has developed insidiously, cognitive impairment, poor concentration and behavioral changes occur. Visual obscurations and papilledema are more common in adults than in the younger age group. In both groups of patients, the presence of bradycardia, hypertension and irregularities in breathing pattern implies critical elevation of ICP and should be treated promptly.
Investigations
In the neonate, the supratentorial ventricular system can be reliably evaluated using ultrasound. This is the imaging modality of choice in the investigation and monitoring of the infant with an open fontanella. Hematomas or other ventricular masses responsible for hydrocephalus can also be identified. Ultrasound provides a non-invasive and readily available tool for both diagnostic purposes and for measuring ventricular size in serial studies.
CT and MRI
In order to fully evaluate the entire ventricular system and investigate the underlying etiology of hydrocephalus, CT or MRI imaging is required. There is a range for normal ventricular size, but ventricular size changes with age, rendering absolute measurements of ventricular dimensions of little use. No single radiological parameter can be relied upon to distinguish hydrocephalus from the other causes of ventricular enlargement mentioned above. Some features, however, are strongly suggestive, particularly if present in combination. Enlargement of the temporal horns of the lateral ventricles and enlargement of the 3rd ventricle, commensurate with the enlargement of the rest of the ventricular system, are in favor of hydrocephalus. Obliteration of the basal cisterns and effacement of the cortical sulci further support a diagnosis of hydrocephalus with increased ICP. When the ventricles are under pressure, there may be transependymal flux of CSF into the periventricular parenchyma, particularly at the tips of the frontal, occipital and temporal horns. This appears as low density on CT scan or a rim of high signal intensity on the T2-weighted MRI scans.
Plain X-rays of skull give an indication about large size skull with different shapes of vault, sutural separation, cranio lacunae, flat anterior cranial fossa, thinning of vault bones, sellar changes and “beaten silver” appearance may be seen as a sign of raised ICP. Small posterior fossa is often associated aqueductal stenosis [Figure 2]. On the contrary, a large one might suggest Dandy walker cyst. Multiple calcifications may be an indication of infectious etiology. Ventriculography, which was the “gold standard” during the yesteryears, can demonstrate the large size of the ventricles and also the site of the obstruction. It is still valuable in evaluating CSF dynamics. Cerebral angiography is not a usual investigation except in vein of Galen malformations and in assessing major venous anomalies. However, hyperplasia, occlusion and elongation of cerebral arteries are usually seen. Venous angle during the venous phase is an indicator of degree of hydrocephalus.[13,14]
Figure 2.
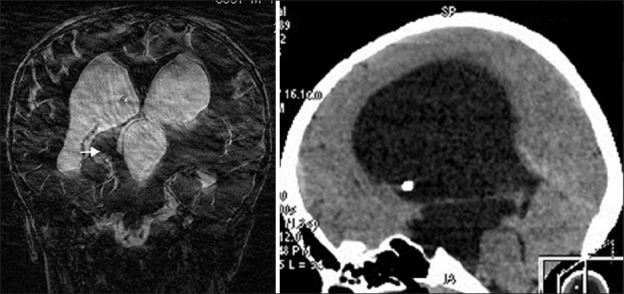
Aqueductal stenosis
Figure 3.
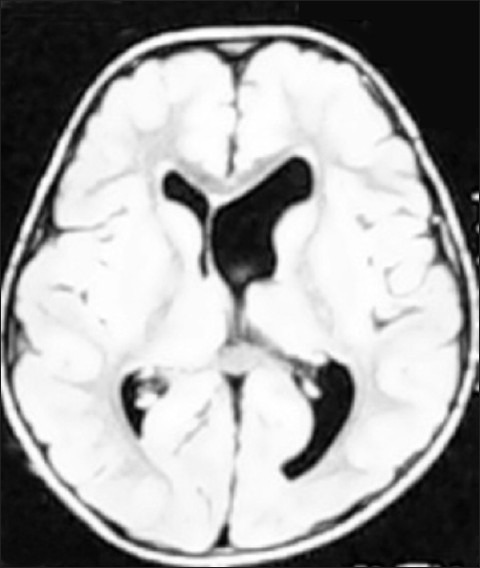
Asymmetrical ventricles
Figure 4.
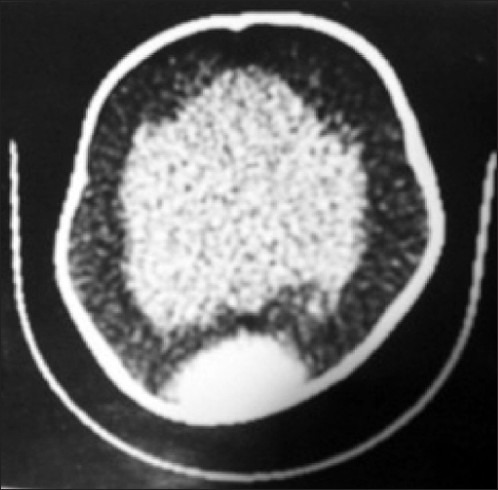
CT ventriculography
Figure 5.
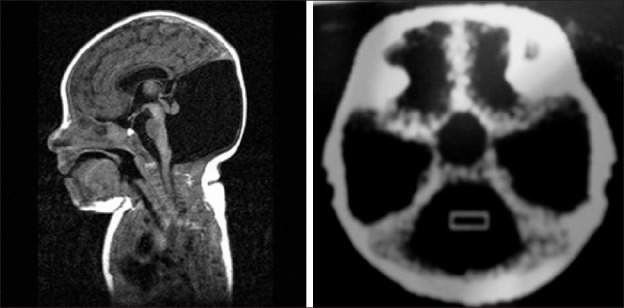
Dandy Walker malformation
Figure 6.
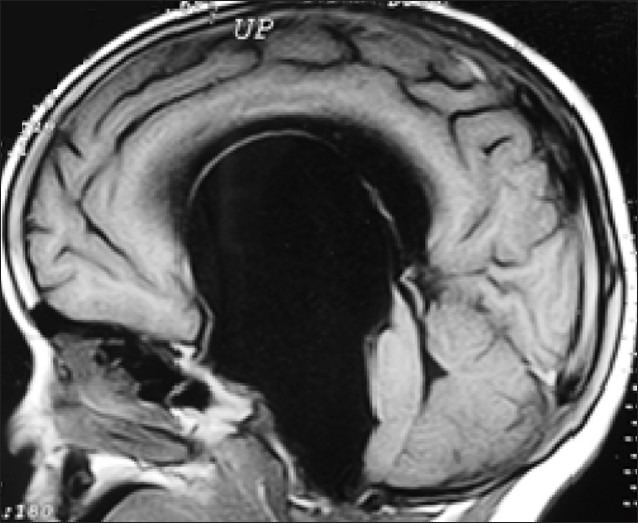
Pre-op. Arachnoid cyst with hydrocephalus
Figure 7.
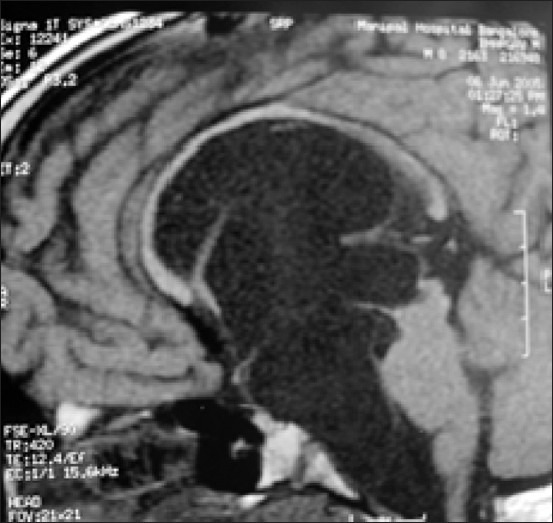
Post-op. Following endoscopic ventriculocystocisternostomy
Figure 8.
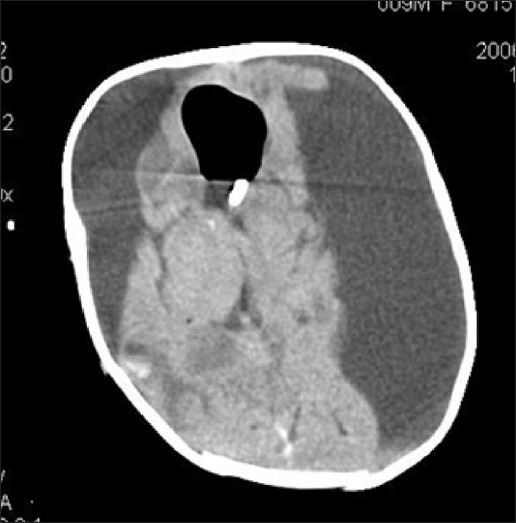
Bilateral SDH following shunt
Figure 9.
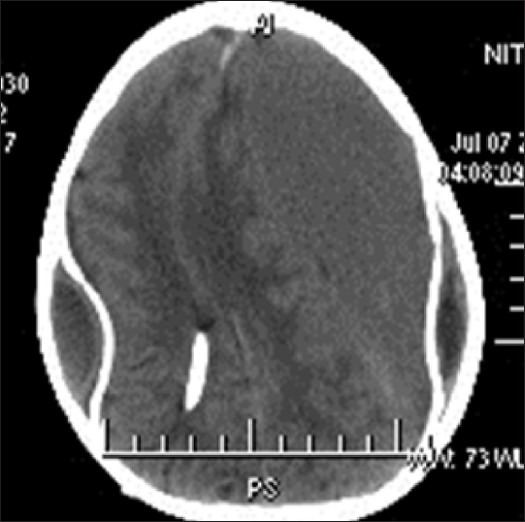
Shunt complication extradural and subdural hematoma
Figure 10.
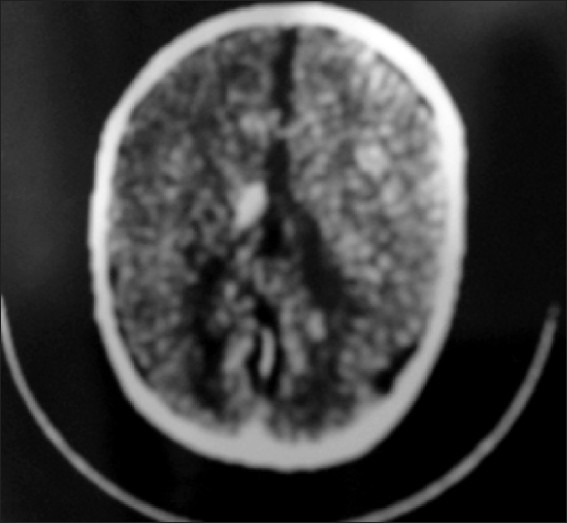
Slit ventricle
Figure 11.
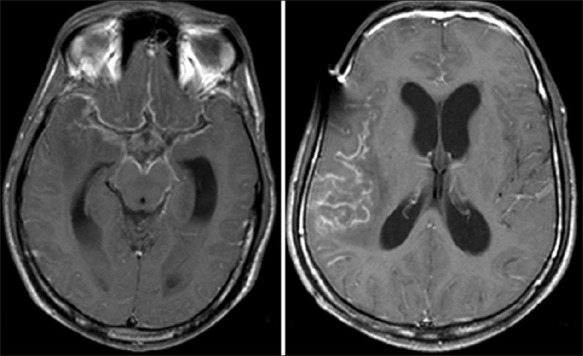
Meningitis with hydrocephalus
Figure 12.
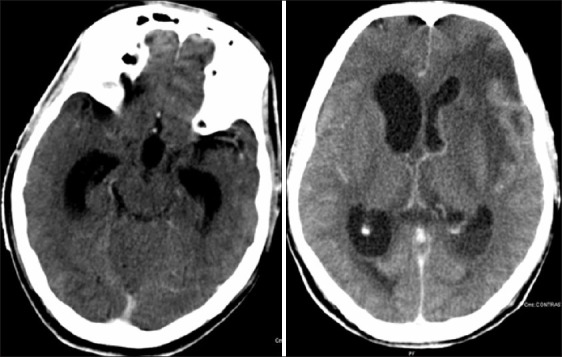
Meningitis with ventriculitis
Figure 13.
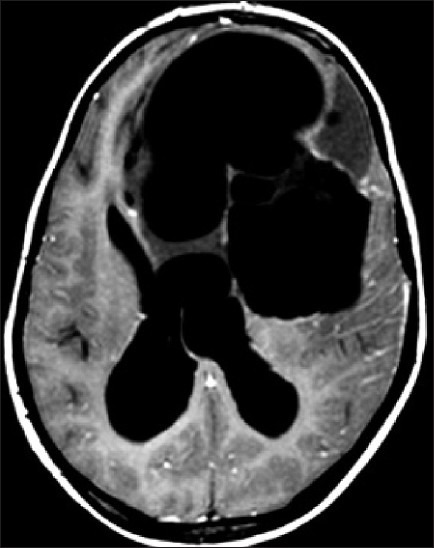
Hydatid cyst with hydrocephalus
Figure 14.
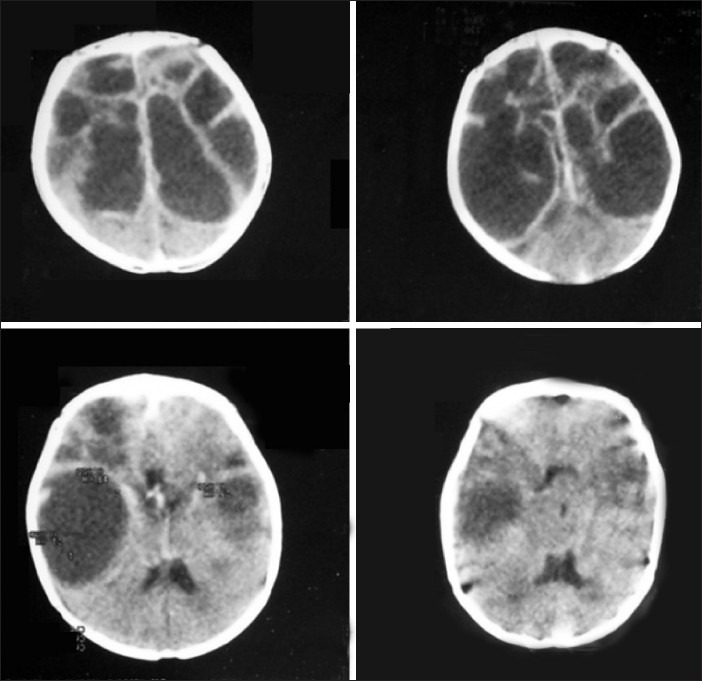
Multiloculated hydrocephalus
Electrophysiology
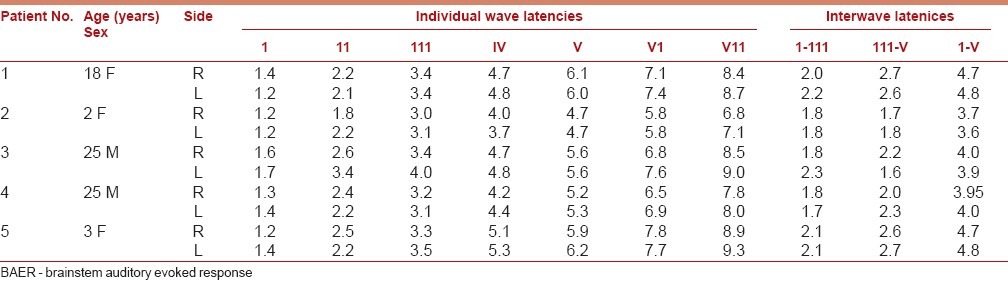
EEG and evoked potentials, though not of diagnostic value, have been found to be useful in evaluating clinical outcomes in hydrocephalus. The electrical activity has been correlated to the functional integrity of the cortical mantle. The EEG abnormalities could be focal or diffuse, and useful in detecting seizure discharges. The incidence of seizures in hydrocephalus can vary from 18.2 to 65%. The other abnormalities include focal slowing, focal attenuation, and multifocal paroxysmal discharges and generalized discharges.[15,16] Because of the close anatomical relationship of posterior visual pathway, enlarged ventricles might contribute to visual evoked potential (VEP) abnormalities. Similar abnormalities were reported in brainstem auditory evoked response (BAER). These could be due to increased ICP, decreased cerebral flow, herniation of upper brainstem or congenital anomalies affecting the auditory or visual system and technically due to alteration of the median through the electrical signals called volume conduction.[17,18] Brainstem auditory evoked potentials, when serially performed, help in identifying the structural abnormalities of brainstem and can also be an earlier indicator of shunt malfunction [Tables 1 and 2].[19–21]
Table 1.
Pre-operative BAER in congenital Hydrocephalus
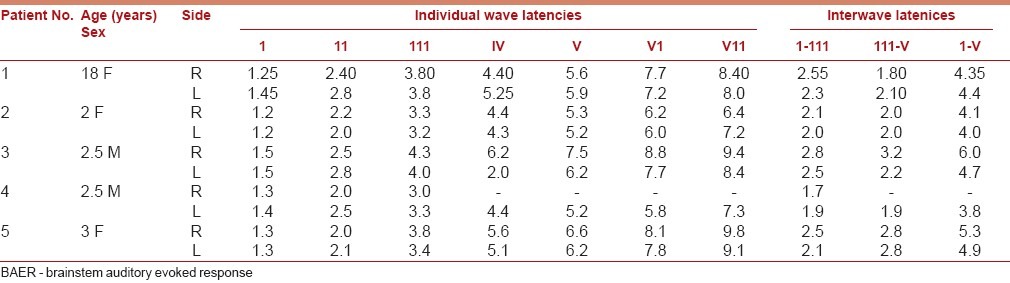
Table 2.
Post-operative BAER after shunt
Natural history
Laurence suggested that outlook in hydrocephalus was not without hope and survival to adult life was between 20% and 30%. Among the survivors, 73% were educable.[22,23]
Treatment
Treatment of hydrocephalus is indicated wherever the hydrocephalus is progressive and associated with increased ICP. A variety of treatments have been tried for hydrocephalus.
Medical management
Medical measures may be appropriate under certain circumstances. Osmotic diuretics and acetazolamide (inhibitor of carbonic anhydrase) have been used. Carbonic anhydrase is an enzyme present in the choroid plexus and is necessary for the formation of CSF. However, the effects are not sustained. Hence, it is useful in only as a temporary measure in post-hemorrhagic hydrocephalus. Historically, compression bandage of the head had been advocated in neonatal hydrocephalus.
Bypassing the site of obstruction to CSF flow by diverting the CSF from ventricular cavity to a site where it is readily absorbed is the basic principle underlying the treatment of hydrocephalus.[24] Based on this, shunt procedures have become the mainstay of surgical treatment even in severe hydrocephalus. Shunts can alter the process dramatically in infantile hydrocephalus. Endoscopic 3rd ventriculostomy is an important alternative in select situations beyond 1 year. Numerous shunt systems have been devised and marketed
The shunt assembly comprises a proximal catheter located in the cerebral ventricles and a distal catheter draining into selected site of CSF absorption, connected by a valve and reservoir incorporated into the shunt system. Numerous shunt systems are available in the market, though all of them have their shortcomings and are prone to similar complications. The proximal catheter has blind-ended tube with multiple side holes to facilitate CSF drainage. A number of devices which are available, such as stylet, endoscope, and neuronavigation, assist to facilitate the placement of the catheter into the ventricle. The valve designs are based on differential pressures, and may be fixed or programmable. Four types of differential pressure valves are commonly encountered: Slit valves, miter valves, ball and spring, and diaphragm valves. The fixed valves have low-, medium- or high-pressure alternatives. The setting defines the opening or more commonly the closing pressure of the valve. In order to overcome the limitations of the fixed-resistance valves, programmable valves were developed whose operating pressure can be varied by an externally applied magnetic field. They alter the position of an internal rotor, and thus vary the pressure setting. The pressure gradient across the valve is the difference between the intraventricular pressure and the intra-abdominal pressure in the supine position. In addition, posture gravity and the added hydrostatic pressure can alter the differential pressures. Anti-siphon device is a siphon-controlled device that can be incorporated with the valves. This device houses a mobile membrane which moves in response to the pressure change. When the intrashunt pressure falls, the membrane will occlude the shunt lumen, thereby preventing overdrainage.[25] The alternative sites for placing the distal end include the right atrium, pleural cavity and the gall bladder. Peritoneal cavity is the commonest site of placement of distal catheter.[26] In communicating hydrocephalus, lumboperitoneal shunt is another good option.[27]
Indian shunt systems
The cost of most internationally available shunt systems is relatively high for people in most developing countries. To make matters worse, periodic revisions of shunt systems and associated infections add to the cost. To overcome this problem, many innovative shunt systems have been designed in India. These include Upadhyaya Shunt System, Chhabra Shunt System, and Sri Chitra Shunt System.[28] Among these, Chhabra Shunt systems have become more versatile and popular. Several studies have confirmed their efficacy and functionality on a par with the standard shunt systems.[29] They have “Slit N Spring”, “Z” flow, low-pressure, medium-pressure, and high-pressure valves. These valves were evaluated and compared with the western systems and found to be equally effective.[30] Low cost of these shunts is of major advantage. These shunt systems are becoming popular now in countries outside India. These shunts are economical and effective.
Endoscopic treatment
With the improvement in the optics and advent of modern neuroendoscopes, a variety of treatments are now possible through endoscopic techniques. Popular among them is the endoscopic 3rd ventriculostomy for aqueductal stenosis. In addition, septostomy, extirpation of choroid plexus, removal of parasitic cysts, removal of migrated shunts and arachnoid cysts can be treated effectively through neuroendoscopy.
Endoscopic 3rd ventriculostomy is effective in most obstructive hydrocephalic children. The efficacy in infective hydrocephalus is debatable. Recently, there have been several publications indicating that ETV is effective in some children suggesting that ETV can be attempted as a primary procedure before a VP shunt. Similarly, the effectiveness of ETV in children under the age of 1 year is variable.[31,32]
Intellectual outcome
The determinants of intelligence levels and the pattern of intelligence in hydrocephalic children are not fully established.[33] Many studies have been published concerning the intelligence in both treated and untreated groups. But the criteria like diagnostic studies, time of intervention and IQ assessment methodologies are different. Hagberg and Sgogrin defined an IQ of 90 as normal. But Lorbar and Zachary used IQ greater than 70 as normal.[34] Foltz and Shurtlef described the children who have an IQ more than 75 as “functional”. Age at which the shunt is placed, type of hydrocephalus, status of shunt function, associated anomalies and consequent complications, have all been incriminated as the responsible factors. In addition, genetic, social, educational and economic backgrounds also seem to influence the issue. However, reviews suggest there is a decline in mortality and achievement of 50–70% incidence of normal IQ with an effective functional shunt. Nelson and Rekate correlated IQ with the width of frontal cortical mantle and concluded that IQ was found to be normal with cortex of more than 3 cm.[35–37] Type of hydrocephalus, degree of ventricular dilatation and number of revisions do not seem to affect IQ, as was suggested by Dennis and Raimondi. Children with myelomeningocele pose additional problems due to added physical disability. Untreated children have a mortality of over 80%. Gilllian, Soare and Raimondi reported an IQ of above 80 in 63% of their children with meningomyelocele with hydrocephalus. IQ seems to have an inverse relationship with location of the sac and sensory level, as reported by Lorbar. The perceptual motor deficit in children with myelomeningocele could be part of the disease entity or related to decreased stimulation due to disability or due to a combination.[38,39] Upadhyaya in his Indian series did not find any correlation between age at operation and nature of hydrocephalus. But severity of hydrocephalus seems to correlate with decline in the IQ. The prognostic factors reported are a) clinica – etiology, degree of motor and sensory deficits, level of meningocele, severity of hydrocephalus, seizures, ventriculitis and sex of the child; b) radiologica – nature of the hydrocephalus, degree of ventricular dilatation and topography of cerebral vasculature; c) perioperativ – age of surgery, continuing shunt function and complications. Venkataraman et al., conducted a prospective study on neuropsychological development of 40 children with hydrocephalus. Fifteen of them had associated myelomeningocele and only six of them had age – appropriate neuropsychological development before surgery. Following shunt surgery, improvement in neuropsychological function was observed in all children. However, the extent of improvement in relation to mental age was significantly higher when CSF diversion was done prior to 6 months of age. Recovery of neuropsychological function seems to have a particular pattern and suggested some form of hemispheric functional lateralization in their series.[40] The children with head circumference more than 50 cm and shunt intervention done beyond the age of 18 months had developed higher incidence of subdural hematomas and their intellectual outcomes were uniformly poor, suggesting that beyond a stage, cortical mantle loses its ability to reconstitute, thereby leading to poorer outcomes as well as complications.[41–44]
Complications of shunts
Extensive range of complications has been reported in the literature. They could be classified as mechanical, like shunt blockage, disconnection, migration and relative shortening of length. The flow-related complications are CSF overdrainage leading to subdural hematoma, subdural collections, low-pressure headaches, secondary craniostenosis, cranial deformity, and asymmetrical drainage can lead to trapping or isolation of a part of a ventricular system. The slit ventricular syndrome is a complication related to absorption. Besides, ascites, loculations, hydrocele, perforation of the stomach, large and small bowel, gall bladder and vagina are also described.[45–48]
Shunt infection is the common complication accounting for significant morbidity and mortality. Reported series have shown the incidence ranging from 5 to 15%. Some centers have reported an incidence as low as 1%. Although many factors appear to contribute to shunt infection, it is likely that the contamination of the shunt system at the time of surgery is the primary cause. Approximately 70% of shunt infections present within 2 months and the remaining by 6 months of the surgical procedure.[49,50] A high index of suspicion is maintained for symptoms and signs such as pyrexia, meningismus, irritability and even a general lack of well-being. CSF examination is needed to confirm the diagnosis and may be obtained by the aspiration of reservoir or from the ventricle. Appropriate antimicrobial therapy and management of the shunt system is necessary for a good outcome. The commonest organism is coagulase-negative staphylococci, but Staphylococcus epidermidis and Staphylococcus aureus are also often recognized. Enterococci, micrococci and coryneforms also account for a significant proportion.[3] Controversy exists as to the retaining or immediate removal of shunt system. The most common strategy is removal of the shunt with placement of an external drain with appropriate antibiotics. A fresh shunt may be placed once the CSF is sterile. There are several reports indicating that antibiotics given at the time of surgery reduce the incidence of infection. The role of prophylactic antibiotic, its type, duration and route of administration are still controversial. The more recent technique of antibiotic delivery has been to incorporate antibiotics into the silicon tubing which gradually leaches out and provides protection in the early postoperative period. Antibiotic-impregnated shunts are now available; however, the long-term results need to be evaluated.[51–56]
Miscellaneous – Seizures, metastasis, hemorrhage related to catheters and silicone allergy.[57–60]
Special types of hydrocephalus
Post-hemorrhagic
During the embryonic development, germinal matrix is the site of intense cellular perforation. The germinal matrix is a large structure, which begins to involute by the end of 2nd trimester and completes by 34 weeks. The blood vessels of the germinal matrix have immature connective tissue and lack autoregulatory capacity. Due to these factors, premature infants born before 34 weeks have a high incidence of hemorrhage. Hemorrhage is detected in 40–45% of immature infants whose birth weight is less than 500 g, and 20% of infants who suffer intraventricular hemorrhage will develop hydrocephalus requiring a shunt. Majority occurs within the first few days of birth. Clinical symptoms can be misleading due to prematurely. Serial ultrasound examinations are helpful especially the head circumference is increasing. Ventricular catheter with a subcutaneous reservoir is much safer in avoiding repeated cerebral punctures. Recently, intraventricular fibrinolytic therapy instituted soon after the hemorrhage has been shown to prevent chemical arachnoiditis and reduce a shunt dependency.[61–65]
Meningomyelocele
Hydrocephalus complicates open spina bifida in 85–90% of patients. It may manifest after closure of the meningomyelocele as the sac acts as a CSF sump. This is usually associated with Chiari malformation; it is preferable to treat hydrocephalus simultaneously to facilitate wound healing after the repair of myelomeningocele.[66]
Aqueductal stenosis
The growth of the tectum and tegmentum makes the lumen of the neural tube narrow in the region of mesencephalon, leading to narrowing of aqueduct of Sylvius. Aqueductal stenosis occurs in approximately 10% of children. Several theories exist regarding primary versus secondary forms of aqueductal stenosis. External pressure on mesencephalon has been proposed to obliterate aqueduct secondarily. Scarring and gliosis following infection or hemorrhage can cause acquired aqueductal stenosis. Tumors from the surrounding structures have potential chance to block the aqueduct. Imaging is confirmatory in such situations.[67]
Dandy Walker syndrome
This anomaly comprises agenesis of the cerebellar vermis with cystic dilation of the 4th ventricle, enlargement of the posterior fossa and hydrocephalus. The hydrocephalus manifests in the postnatal period. Additional brain malformations leading to neural developmental delay are reported in 70% of cases. Treatment with placement of proximal catheter in the lateral ventricle, 4th ventricle and both the ventricles with a wide connecter has been described. Ideal situation is to treat 4th ventricular hydrocephalus and subsequently supratentorial hydrocephalus by shunt or endoscopic method.
Post-meningitic
Hydrocephalus can occur following a range of infectious or inflammatory diseases. Organization of the inflammatory exudates, along with scarring or gliosis can produce obstruction to CSF flow both in the ventricular system and in the subarachnoid spaces, leading to either obstructive or communicating hydrocephalus. Bacterial, parasitic and granulomatous infections like tuberculosis and fungal infections can also lead to hydrocephalus [Tables 3 and 4]. Hydatidosis and toxocara and viral infections are rare causes. Hydrocephalus in the presence of infection poses several management challenges. Hydrocephalus can be acute causing a large increase in the ICP and rapid deterioration of clinical condition. The goals of treatment are early diagnosis, effective relief of raised ICP, CSF diversion and treatment of primary infection. Clinical evaluation, radiological imaging and CSF examination including culture are essential in establishing the diagnosis. In granulomatous infections, at times it is very difficult to differentiate the type of infection warranting tissue diagnosis or stereotactic biopsy. The outcome depends on effective management of infection, hydrocephalus and the associated complications. External ventricular drainage may be used as a temporary measure till the infection is resolved before implanting a shunt or 3rd ventriculostomy. The incidence of shunt infections and malfunction is very high, needing multiple revisions.[66] Endoscopic 3rd ventriculostomy is gaining popularity with 50–60% success rates in select cases.[32,68,69]
Table 3.
Imaging characteristics in infections

Table 4.
Special sequences in infections

Subarachnoid hemorrhage
Hydrocephalus can follow 10–15% of patients suffering from subarachnoid hemorrhage. The incidence increases with the presence of intraventricular hemorrhage. The mechanism is impaired absorption due to blockade at multiple sites.
Normal pressure hydrocephalus
Usually seen in adulthood, this is classically characterized by gait deterioration, dementia and urinary incontinence. Imaging usually shows enlarged ventricles. In some cases, there may be an attributable cause like infection or hemorrhage. The ICP related symptoms may not be evident. A number of investigations have been advocated in selection of patient for shunt therapy, like isotope cisternography, infusion tests to detect increased CSF resistance in flow, ICP monitoring and therapeutic lumbar drainage. CSF diversion in carefully selected patients gives favorable clinical outcomes.[70]
Hydrocephalus and venous hypertension
The role of raised venous pressure as a cause of hydrocephalus has been described long ago as otitic hydrocephalus. Similar clinical situations have been described in achondroplasia and syndromiccranio stenosis. The deformed skull base resulting in narrowing of the jugular foramen leading to impaired intracranial venous drainage has been described. The raised pressure within the cranial venous sinuses reduces the pressure gradient across the arachnoid villi, resulting in impaired absorption. Hydrocephalus also accompanies vein of Galen aneurysms.[71]
Arrested hydrocephalus
Hydrocephalus may evolve into a chronic state in which persistent ventricular enlargement with normal CSF pressure exists. Though it is controversial, this entity appears more of a compensated ventriculomegaly. Treatment strategies should be weighed between the benefits versus risks in individual situations. The exact criteria on which hydrocephalus is labeled as arrested are not clear. Many do not believe in such a concept. However, they need to be monitored clinically and by intellectual development. Disproportionate head growth or progression of ventricular size or an intellectual decline is an indication for intervention.
Multiloculated hydrocephalus
Multiloculated hydrocephalus is still a challenge to treat. It usually occurs after an initial episode of neonatal meningitis or a germinal matrix hemorrhage. CT scan or MRI is usually diagnostic. CT ventriculography has been advocated to know the communication between the cysts. This condition needs to be differentiated from the cysts associated with hydrocephalus, including dorsal cyst malformations. Among the various treatment options available, placement of multiple shunts, single shunt with multiple fenestrations of all the loculations or craniotomy with lysis of intraventricular septations, stereotactic cyst aspiration, or endoscopic cyst fenestrations with shunt insertion have also been described. The endoscopic option appears the most ideal and can be performed through a single burr hole with the aid of a steerable endoscope. Large fenestrations are usually recommended to prevent a recurrent cyst formation. Identifying the loculated cysts is usually a problem as most often the differentiation between a normal parenchyma and the septum is difficult, as well as it is easier to get disoriented during the scopy. Intraoperative ultrasound or navigational systems can be of considerable help. After the perforations are made, the multiple cavities are communicated with each other and a single shunt can be placed into one of the major cavities. Repeat procedures are also advised in case the initial one fails to relieve the symptoms. However, the intellectual outcome in these children has been poor. Only 20% of them can reach normal expected levels of IQ with the best possible treatment. The number, extent of loculations, degree of parenchymal damage, etiology and effectiveness of treatment seem to influence the ultimate outcome. If the loculations are unilateral, they can be communicated to the opposite ventricle through a septum pellucidotomy.
Hydrocephalus ex vacuo
This condition generally indicates enlargement of ventricles, secondary to cerebral parenchymal damage. The ex vacuo of cerebral atrophy can be associated with aging, head trauma, severe infection, hypoxia or ischemic insults. Of late, ventriculomegaly following radiotherapy and chemotherapy has been noted. They are usually associated with white matter loss, concomitant enlargement of the cortical subarachnoid spaces and basal cisterns. The periventricular lucency is absent. A number of structural abnormalities of the brain such as calpocephali, holoprocencephali and agenesis of the corpus callosum may also be associated with ventricular enlargement and do not necessarily require any intervention.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Thompson D. Hydrocephalus and Shunts. In: Moore AJ, Newell DW, editors. Neurosurgery Principles and practice. Springer: Specialist Surgery Series Neurosurgery; 2005. pp. 425–42. [Google Scholar]
- 2.Hamilton, Boyd, Mossman′s . Human Embryiology. 4th ed. The Mac Millan Press Ltd. The Williams and Wilkin Company; 1972. [Google Scholar]
- 3.Rekate HL. Chap 215, Youmans Neurological surgery. 5th edition. Hydrocephalus in Children; pp. 3387–404. [Google Scholar]
- 4.Singh I. An Introduction to “Human Embryology”. Mc Mullar India: Macmillan India Ltd; 1976. pp. 278–310. [Google Scholar]
- 5.Weed LH. a. Studies on Cerebro Spinal Fluid III. The pathways of escape from the subarachnoid spaces with particular reference to the arachnoid villi. J Med Res. 1914;31:51–93. b. Meninges and cerebrospinal fluid. J Anat 1938;72:181-215. [PMC free article] [PubMed] [Google Scholar]
- 6.Tripathi BJ, Tripathi RC. Vacuolar transcellular channels as a drainage pathway for the cerebrospinal fluid. J Physiol. 1974;239:195–206. doi: 10.1113/jphysiol.1974.sp010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davson H, Welch K, Segal MB. The physiology and pathophysiology of the cerebrospinal fluid. Edinburgh: Churchill Livingstone; 1987. [Google Scholar]
- 8.De Lange SA. Progressive Hydrocephalus. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. Vol. 30. Amsterdam: North Holland Publ. Co; 1977. pp. 525–63. Congenital Malformations of the Brain and Skull, Part 1. [Google Scholar]
- 9.Menon G, Nair SN, Baldawa SS, Rao RB, Krishnakumar KP, Gopalakrishnan CV. Choroid plexus tumors: An institutional series of 25 patients. Neurol India. 2010;58:429–35. doi: 10.4103/0028-3886.66455. [DOI] [PubMed] [Google Scholar]
- 10.Venkataramana NK, Kolluri VR, Swamy KS, Arya BY, Das BS, Reddy GN. Progressive unilateral hydrocephalus in adults. Neurosurgery. 1989;24:282–4. doi: 10.1227/00006123-198902000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Drake JM, Sainte-Rose C. The shunt book. Cambridge: Blackwell Science; 1995. [Google Scholar]
- 12.Brassow F, Baumann K. Volume of brain ventricles in man determined by computer tomography. Neuroradiology. 1978;16:187–9. doi: 10.1007/BF00395246. [DOI] [PubMed] [Google Scholar]
- 13.Abstracts of paper from Nervous system in children. Vol. 22. Benda: Childs Nervous system; 1954. Benda-Angiographic findings in dysgenic hydrocephaly; p. 12. (543-1568, 1973). [Google Scholar]
- 14.Raimondi AJ. Angiographic diagnosis of hydrocephalus in the New born. J Neurosurg. 1969;31:550–60. doi: 10.3171/jns.1969.31.5.0550. [DOI] [PubMed] [Google Scholar]
- 15.Dan NG, Wade MJ. The incidence of epilepsy after ventricular shunting procedures. J Neurosurg. 1986;65:19–21. doi: 10.3171/jns.1986.65.1.0019. [DOI] [PubMed] [Google Scholar]
- 16.Graebner RW, Celesia GG. EEG findings in Hydrocephalus and their relation to shunting procedures. Electroencephalogr Clin Neurophysiol. 1973;35:517–21. doi: 10.1016/0013-4694(73)90028-x. [DOI] [PubMed] [Google Scholar]
- 17.Venkataramana NK, Satishchandra P, Hegde AS, Reddy GN, Das BS. Evaluation of brainstem auditory evoked responses in congenital hydrocephalus. Childs Nerv Syst. 1988;4:334–8. doi: 10.1007/BF00270606. [DOI] [PubMed] [Google Scholar]
- 18.Venkataramana NK, Satishchandra P, Hegde AS, Reddy GN. Brainstem auditory evoked response in infantcy (Normative Data) NIMHANS J. 1990;8:33–5. [Google Scholar]
- 19.Ines DF, Markand ON. Epileptic Seizures and abnormal electro encephalographic findings in hydrocephalus and their relation to the shunting procedures. Electroencephalogr Clin Neurophysiol. 1977;42:761–8. doi: 10.1016/0013-4694(77)90229-2. [DOI] [PubMed] [Google Scholar]
- 20.Piatt JH, Carlson CV, Wyler AB, Engel J, De Giorgio CM. Hyrdrocephalus and epilepsy: An actuarial analysis. Neurosurgery. 1996;39:722–8. doi: 10.1097/00006123-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Varfis G, Berney J, Beaumanoir A. Electro-clinical follow-up of shunted hydrocephalic children. Childs Brain. 1977;3:129–39. doi: 10.1159/000119661. [DOI] [PubMed] [Google Scholar]
- 22.Casey A, Kimmings EJ, Kleinlugtebeld AD, Taylor WA, Harkness W, Hayward R. The longterm outlook for hydrocephalus in childhood: A ten year cohort study of 155 patients. Pediatr Neurosurg. 1998;27:63–70. doi: 10.1159/000121229. [DOI] [PubMed] [Google Scholar]
- 23.Fernell E, Hagberg G, Hagberg B. Infantile hydrocephalus epidemiology: An indicator of enhanced survival. Arch Dis Child. 1994;70(Suppl 2):F123–8. doi: 10.1136/fn.70.2.f123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balasubramanium V, Ramamurthi B, Kanaka TS. Treatment of hydrocephalus. Indian J Surg. 1967;29:619. [Google Scholar]
- 25.Khan RA, Narasimhan KL, Tewari MK, Saxena AK. Role of shunts with antisiphon device in treatment of pediatric hydrocephalus. Clin Neurol Neurosurg. 2010;112:687–90. doi: 10.1016/j.clineuro.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Chitale VR, Kasaliwal GT. Our experience of ventriculoatrial shunt suing Upadhyaya valve in hydrocephalus associated with tuberculous meningitis. Prog Pediatr Surg. 1982;15:223–36. [PubMed] [Google Scholar]
- 27.Yadav YR, Parihar V, Sinha M. Lumbar peritoneal shunt. Neurol India. 2010;58:179–84. doi: 10.4103/0028-3886.63778. [DOI] [PubMed] [Google Scholar]
- 28.Upadhyaya P, Bhargava S, Dube S, Sundaram KR, Ochaney M. Results of ventriculoatrial shunt surgery for hydrocephalus using Indian shunt valve evaluation of intellectual performance with particular reference to computerized axial tomography. Prog Pediatr Surg. 1982;15:209–22. [PubMed] [Google Scholar]
- 29.Chhabra DK, Agarwal GD, Mittal P. “Z” flow hydrocephalus shunt, a new approach to the problem of hydrocephalus, the rationale behind its design and the initial results of pressure monitoring after “Z” flow implantation. Acta Neurochir (Wien) 1993;121:43–7. doi: 10.1007/BF01405181. [DOI] [PubMed] [Google Scholar]
- 30.Warf BC. Comparison of 1-year outcomes for the Chhabra and Codman-Hakim Micro Precision shunt systems in Uganda: A prospective study in 195 children. J Neurosurg. 2005;102(Suppl 4):358–62. doi: 10.3171/ped.2005.102.4.0358. [DOI] [PubMed] [Google Scholar]
- 31.Rajshekhar V. Management of hydrocephalus in patients with tuberculous meningitis. Neurol India. 2009;57:368–74. doi: 10.4103/0028-3886.55572. [DOI] [PubMed] [Google Scholar]
- 32.Yadav YR, Jaiswal S, Adam N, Basoor A, Jain G. Endoscopic third ventriculostomy in infants. Neurol India. 2006;54:161–3. [PubMed] [Google Scholar]
- 33.Ahmed A, Sandlas G, Kothari P, Sarda D, Gupta A, Karkera P, et al. Outcome analysis of shunt surgery in hydrocephalus. J Indian Assoc Pediatr Surg. 2009;14:98–101. doi: 10.4103/0971-9261.57700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorber J, Zachary RB. Primary congenital hydrocephalus.Long-term results of controlled therapeutic trial. Arch Dis Child. 1968;43:516–27. doi: 10.1136/adc.43.231.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin RC, Hochwald GM, Tiell M, Epstein F, Ghatak N, Wisniewski H. Hydrocephalus: III.Reconstitution of the cerebral cortical mantle following ventricular shunting. Surg Neurol. 1976;5:179–83. [PubMed] [Google Scholar]
- 36.Rubin RC, Hochwald G, Tiell M, Liwnicz B, Epstein F. Reconstitution of the cerebral cotical mantle in shunt –corrected hydrocephalus. Dev M Ned Child Neurol Suppl. 1975;35:151–6. doi: 10.1111/j.1469-8749.1975.tb03595.x. [DOI] [PubMed] [Google Scholar]
- 37.Rubin RC, Hochwald G, Tiell M, Liwnicz B, Epstein F. Reconstitution of the cerebral cortical mantle in shunt-corrected hydrocephalus. Dev Med Child Neurol Suppl. 1975:151–6. doi: 10.1111/j.1469-8749.1975.tb03595.x. [DOI] [PubMed] [Google Scholar]
- 38.Soare PL, Raimondi AJ. Intellectual and perceptual motor characteristics of treated meningomyelocele children. Am J Dis Child. 1979;131:199–204. doi: 10.1001/archpedi.1977.02120150081017. [DOI] [PubMed] [Google Scholar]
- 39.Thompson DN, Hayward RD, Harkness WJ, Bingham RM, Jones BM. Lessons from a case of Kleeblattschadel: Case report. J Neurosurg. 1995;82:1071–4. doi: 10.3171/jns.1995.82.6.1071. [DOI] [PubMed] [Google Scholar]
- 40.Venkataramana N K, Mukundan CR. Evaluation of functional outcomes in congenital hydrocephalus. J Pediatr Neurosurg. 2011;6:4–12. doi: 10.4103/1817-1745.84399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donders J, Canady AI, Rourke BP. Psychometric intelligence after infantile hydrocephalus. Childs Nerv Syst. 1990;6:148–54. doi: 10.1007/BF00308492. [DOI] [PubMed] [Google Scholar]
- 42.Gessell, Aornold, editors. The first five years of life – A guide to the study of the Preschool Child. London: Methaen; 1971. [Google Scholar]
- 43.Noetzel MJ, Blake JN. Seizures in children with congenital hydrocephalus: Long-term outcome. Neurology. 1992;42:1277–81. doi: 10.1212/wnl.42.7.1277. [DOI] [PubMed] [Google Scholar]
- 44.Raimondi AJ, Soare P. Intellectual development in shunted hydrocephalic children. Am J Dis Child. 1974;127:664–71. doi: 10.1001/archpedi.1974.02110240050005. [DOI] [PubMed] [Google Scholar]
- 45.Sridhar K, Karmarkar V. Peroral extrusion of ventriculoperitoneal shunt: Case report and review of literature. Neurol India. 2009;57:334–6. doi: 10.4103/0028-3886.53283. [DOI] [PubMed] [Google Scholar]
- 46.Vuyyuru S, Ravuri SR, Tandra VR, Panigrahi MK. Anal extrusion of a ventriculo peritoneal shunt tube: Endoscopic removal. J Pediatr Neurosci. 2009;4:124–6. doi: 10.4103/1817-1745.57342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bharnagar V, George J, Mitra DK, Upadhyaya P. Complications of cerebrospinal fluid shunts. Indian J Pediatr. 1983;50:133–8. doi: 10.1007/BF02821431. [DOI] [PubMed] [Google Scholar]
- 48.Kumar R, Singh V, Kumar MV. Shunt revision in hydrocephalus. Indian J Pediatr. 2005;72:843–7. doi: 10.1007/BF02731111. [DOI] [PubMed] [Google Scholar]
- 49.Bierbrauer KS, Storrs BB, McLone DG, Tomita T, Dauser R. A prospective, randomized study of shunt function and infections as a function of shunt placement. Pediatr Neurosurg. 1990;16:287–91. doi: 10.1159/000120544. [DOI] [PubMed] [Google Scholar]
- 50.Choux M, Genitori L, Lang D, Lena G. Shunt implantation: Reducing the incidence of shunt infection. J Neurosurg. 1992;77:875–80. doi: 10.3171/jns.1992.77.6.0875. [DOI] [PubMed] [Google Scholar]
- 51.Bayston R, Rogers J. Production of extracellular slimes by staphylococcus epidermidis in the stationary phase of growth and its association with adherence to implantable devices. J Clin Pathol. 1990;43:866–70. doi: 10.1136/jcp.43.10.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bayston R, Lambert E. Duration of protective activity of cerebrospinal fluid shunt catheters impregnated with antimicrobial agents to prevent bacterial catheter - related infection. J Neurosurg. 1997;87:247–51. doi: 10.3171/jns.1997.87.2.0247. [DOI] [PubMed] [Google Scholar]
- 53.George R, Leibrock L, Epstein M. Long term analysis of cerebrospinal fluid shunt infections. J Neurosurg. 1979;51:804–11. doi: 10.3171/jns.1979.51.6.0804. [DOI] [PubMed] [Google Scholar]
- 54.Haines SJ, Walters BC, McComb JG. Antibiotic prophylaxis for cerebrospinal fluid shunts: A metanalysis. Neurosurgery. 1994;34:87–93. [PubMed] [Google Scholar]
- 55.James HE, Walsh JW, Wilson HD, Connor JD. The management of cerebrospinal fluid shunts infections: A clinical experience. Acta Neurochir (Wien) 1981;59:157–66. doi: 10.1007/BF01406345. [DOI] [PubMed] [Google Scholar]
- 56.Langley JM, LeBlanc JC, Drake J, Milner R. Efficacy of antimicrobial prophylaxis in placement of cerebrospinal fluid shunts: Meta-analysis. Clin Infect Dis. 1993;17:98–103. doi: 10.1093/clinids/17.1.98. [DOI] [PubMed] [Google Scholar]
- 57.Pople IK, Bayston R, Hayward RD. Infection of cerebrospinal fluid shunts in infants: A study of etiological factors. J Neurosurg. 1992;77:29–36. doi: 10.3171/jns.1992.77.1.0029. [DOI] [PubMed] [Google Scholar]
- 58.Berger MS, Baumeister B, Geyer JR, Milstein J, Kanev PM, LeRoux PD. The risks of metastases from shunting in children with primary central nervous system tumors. J Neurosurg. 1991;74:872–7. doi: 10.3171/jns.1991.74.6.0872. [DOI] [PubMed] [Google Scholar]
- 59.Jamjoom ZA, Jamjoom AB, Sulaiman AH, Naim UR, Al Rabiaa A. Systemic metastasis of medulloblastoma through ventriculoperitoneal shunt: Report of a case and critical analysis of the literature. Surg Neurol. 1993;40:403–10. doi: 10.1016/0090-3019(93)90221-l. [DOI] [PubMed] [Google Scholar]
- 60.Jimenez DF, Keating R, Goodrich JT. Silicone allergy in ventriculoperitoneal shunts. Childs Nerv Syst. 1994;10:59–63. doi: 10.1007/BF00313586. [DOI] [PubMed] [Google Scholar]
- 61.Kennedy CR, Campbell M, Elbourne D, Hope P, Johnson A. International randomized controlled trial of acetazolamide and furosemide in post haemorrhagic ventricular dilatation in infancy. Lancet. 1998;352:433–40. [Google Scholar]
- 62.Till K. Hydrocephalus in Paediatric Neurosurgery for pediatricians and Neurosurgeons. Oxford, London Edinburgh, Melbourne: Blackwell Scientific Publications; 1975. pp. 115–42. [Google Scholar]
- 63.Luciano M, Patisapu JV, Wickremesekera A. Infantile Post hemorrhagic Hydrocephalus. Ch. 216. Childs Nervous system. 2007;23:623–6. [Google Scholar]
- 64.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 65.Whitelaw A. Repeated lumbar or ventricular punctures in new born with intraventricular hemorrhage. Cochrane Database Syst Rev. 2001:CD000216. doi: 10.1002/14651858.CD000216. [DOI] [PubMed] [Google Scholar]
- 66.McLone DG, Knepper PA. The cause of Chiari II malformation: A unified theory. Pediatr Neurosurg. 1989;14:1–12. doi: 10.1159/000120432. [DOI] [PubMed] [Google Scholar]
- 67.Williams B. Is aqueduct Stenosis a result of hydrocephalus? Brain. 1973;96:399–412. doi: 10.1093/brain/96.2.399. [DOI] [PubMed] [Google Scholar]
- 68.Sil K, Chatterjee S. Shunting in tuberculous meningitis: A neurosurgeon′s nightmare. Childs Nerv Syst. 2008;24:1029–32. doi: 10.1007/s00381-008-0620-x. [DOI] [PubMed] [Google Scholar]
- 69.Murthy JM. Tuberculous meningitis: The challenges. Neurol India. 2010;58:716–22. doi: 10.4103/0028-3886.72178. [DOI] [PubMed] [Google Scholar]
- 70.Fouyas IP, Casey AH, Thompson DN, Hayward D, Harkness WF, Hayward RD, et al. Use of intracranial pressure monitoring in the management of childhood hydrocephalus and shunt-related problems. Neurosurgery. 1996;38:726–32. [PubMed] [Google Scholar]
- 71.Portnoy HD, Branch C, Castro ME. The relationship of intracranial venous pressure to hydrocephalus. Childs Nerv Syst. 1994;10:29–35. doi: 10.1007/BF00313582. [DOI] [PubMed] [Google Scholar]


