Abstract
To review the clinical features and current understanding of spina bifida with an emphasis on the Indian Scenario. Selected articles and current English language texts were reviewed. The authors experience was also reviewed and analysed. Spina bifida is a common congenital anomaly encompassing a wide spectrum of neural tube defects.It is broadly classified as spina bifida aperta and occulta. With the prenatal screening, the incidence of aperta is gradually declining, whereas the detection of occulta has increased with the advent of magnetic resonance imaging. Over the years, the understanding of pathophysiology has made a significant changein the management of these anomalies. Early detection and complete correction can significantly reduce the neurological disability. This article is an overview of spina bifida with a special emphasis on Indian scenario.
Keywords: Aperta, Indian scenario, occulta, spina bifida, tethered cord syndrome
Introduction
Spina bifida literally means “spine in two parts” or “open spine”.[1] Spinal dysraphism involves a spectrum of congenital anomalies resulting in a defective neural arch through which meninges or neural elements are herniated, leading to a variety of clinical manifestations.[1,2] They are divided into aperta (visible lesion) and occulta (with no external lesion).[1,2] Meningocele, myelomeningocele, lipomeningomyelocele, myeloschisis and rachischisis are the usual names associated depending on the pathological findings. Meningocele by definition involves only the meninges with no neural involvement; others have variable extent of neural involvement. The spina bifida aperta is usually associated with skin defect with an impending risk of CSF leak constituting “open defects,” whereas the occult forms have normal skin cover. Both forms demand different approaches in their management. The clinical importance of occult lesion has grown tremendously in the recent years.
Indian scenario
The number of children suffering from spina bifida and hydrocephalus was large though the detection was sporadic by the pediatricians in the past. With the awareness and growth of neurosurgery in the country, few referral centers established special clinics with an ability to address these problems. Gradually, with the availability of magnetic resonance imaging (MRI), several such anomalies were identified, especially of the occult variety. This also helped to visualize a variety of underlying anomalies and their clinical consequences. After pediatric neurosurgery became a subspeciality, several neurosurgeons took interest in this field and contributed in early detection and treatment and also with several good publications. The number of centers has increased across the country. Several unusual anomalies and rare anomalies have been detected and published. Meanwhile, there has been a steady decline in these anomalies over the last decade. Prenatal diagnosis and the medical termination of pregnancy as well as folic acid fortification have contributed. As a result, the anomalies which are not compatible with life and severe anomalies with major defects have reduced, leading to social comfort. Prenatal diagnosis and genetic analysis has become a routine across the country. Few specialized centers have involved in clinical genetics and metabolic workup of these children, contributing to the etiology of many developmental and metabolic diseases. Hence, there has been a shift from open lesion to greater detection and treatment of occult lesions. Considering the size and numbers in the country, a lot needs to be done to provide uniform standards of care in all centers. But it is a happy moment to all of us that several neurosurgeons are practicing pediatrics as their special interest and many are becoming dedicated pediatric neurosurgeons, thanks to ISPN CME Programs for educating neurosurgeons across the country. This phenomenal change and specialization has become a reality by such educational programs.
Prevalence
The estimated incidence of spinal dysraphism is about 1–3/1000 live births.[2] The prevalence of spinal dysraphism has been in decline the world over in the last few decades due to the better nutrition for women, folic acid supplementation, improved antenatal care and high-resolution ultrasound for prenatal screening and biochemical markers.
Embryology
Embryogenesis in the first 2 months of gestation can be divided into 23 stages. Neural plate is formed in stage 8 around the 18th day, followed by neural folds and their fusion. The expansion of the neural tube and subsequent closure is completed by day 28. Open defects occur when the caudal neuropore fails to close. The secondary neurulation sets the spinal cord formation. Defects at this stage result in occult dysraphism connecting the epidermis and the mesenchymal tissues, leading to variety of anomalies and tethered cord.[2,3]
Failure of primary neurulation leads to open dysraphism posing the risks of CSF leakage and exposure of neural placode. Extent and severity of neurological deficit depends on the degree of malformation of the neural placode and also the level of the defect. The higher the level, usually worse is the prognosis. A spectrum of neurological abnormalities like hydrocephalus, Chiari malformation, syrinx, gyral malformations, skeletal malformations and uro-vesical defects can be associated. In occult dysraphism, the overlying skin is intact but the spinal cord is anchored to various tissues starting from skin, subcutaneous tissue, adipose tissue or cartilage.
Etiology
Spina bifida has a multifactorial causation, involving both genetic and environmental. Recent information stressed on the importance of maternal nutrition and folic acid supplementation which has contributed to its reduction.[3,4] In India, certain regional factors and consanguinity seem to play a role [hospital-based epidemiological study from NIMHANS (unpublished)].
Symptomatology
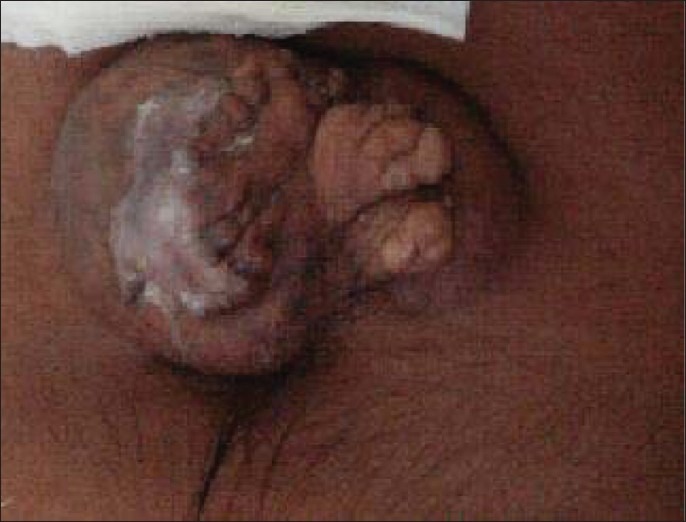
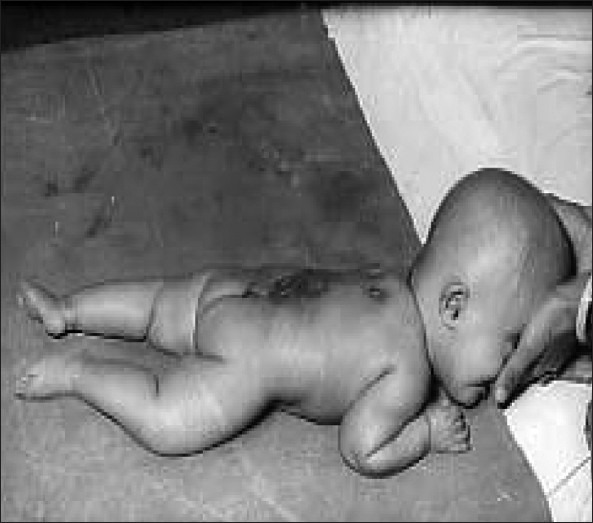
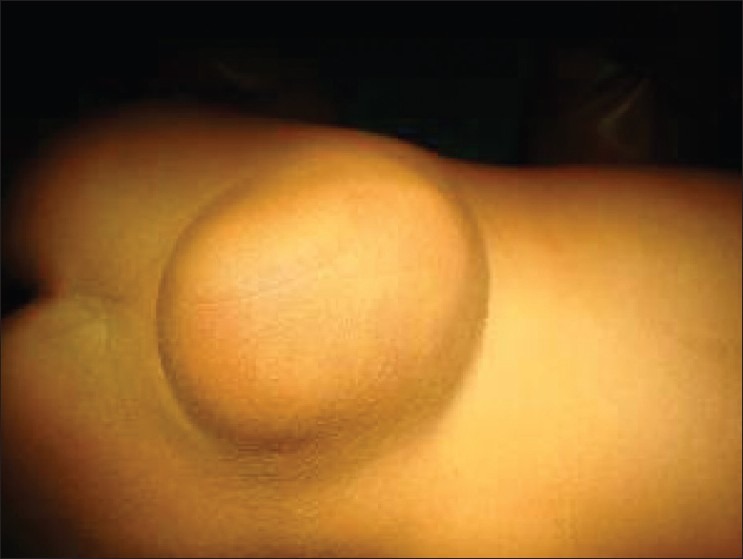
Open dysraphism presents with a swelling over the back which is noticed at birth [Figure 1]. Symptoms are primarily referable to CSF leak or the exposed spinal cord. Since the skin over the swelling is poorly developed, it usually gives way during labor, resulting in CSF leak, contamination and meningitis. Defects predominantly involve thoracolumbar, lumbosacral, lumbar, thoracic, sacral and cervical in the order of frequency of occurrence. Incidence of high cervical lesions is about 3.9%.[5] Neurological deficits include motor, sensory and sphincter dysfunction, depending upon the severity and level. In severe cases, hypotonic areflexic limbs, sphincter atonia with rectal prolapse may be associated. Chiari malformation presents with lower brainstem and lower cranial nerve dysfunction. The presence of large head usually indicates hydrocephalus. The associated skeletal abnormalities are kyphosis, scoliosis, and deformities of the long bones and feet, hemivertebrae, defective ribs, etc.
Figure 1.
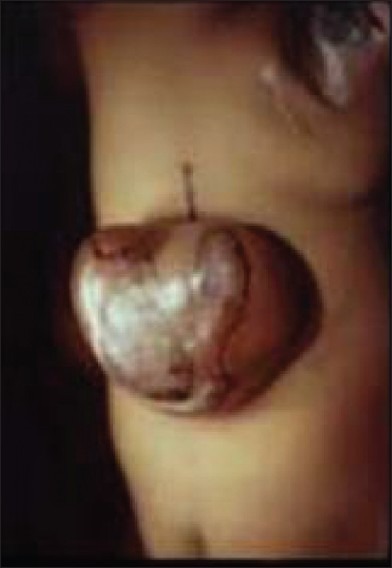
Myelomeningocele
Assessment
Initial assessment of the newborn is extremely important, preferably both by a pediatrician and a neurosurgeon. Examination of head and neck involves the assessment of head size, shape, skull bones, and openness of fontanelles, lacunar skull defects and the size of the posterior fossa. Examination of the back needs assessment of the neural placode, level of the lesion, condition of the skin, extent of skin defect and associated deformities. Examination of the lower limbs for detection of the deformities of the foot and abnormalities of long bones is important. A detailed neurological examination is necessary to assess the level of motor weakness, sensory level and sphincter dysfunction.
Prenatal diagnosis
Prenatal screening for neurological abnormalities is based on ultrasound performed routinely or oriented by maternal Alpha Feto Protein (AFP) screening. It should be performed around 12, 22 and 32 weeks. Maternal serum screening can detect up to 80% cases of spina bifida and 90% cases of anencephaly.[6,7] Sonography may identify up to 90% cases of myelomeningoceles. Over the last few decades, the diffusion of routine ultrasound has changed the spectrum of the neonatal neurological malformations. Gross lethal abnormalities nearly always result in termination of pregnancy, depending on the regional law system. A growing number of more subtle abnormalities including midline or posterior fossa abnormalities are being discovered. But their postnatal outcome cannot always be predicted accurately, despite the use of fetal MRI. Maternal serum screening for chromosomal abnormalities is also increasingly used. Only in select situations, amniocentesis is contributory.[6,8–13]
Radiological assessment
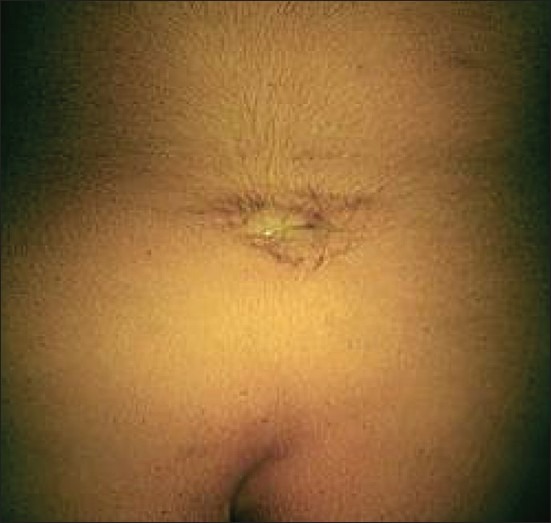
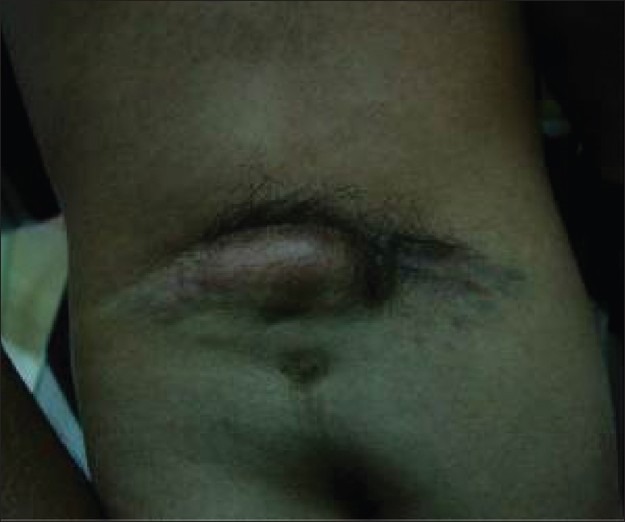
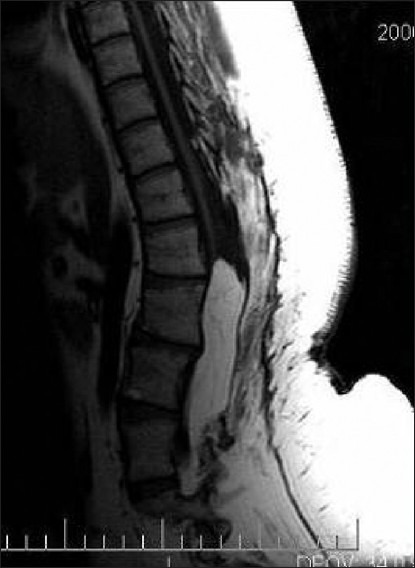
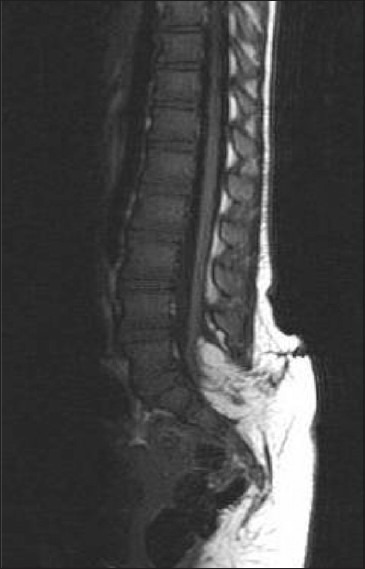
Radiological assessment [Figures 2–4] should be done at the earliest depending on the clinical condition. Plain X-rays reveal the skull defects, spine deformities and bony anomalies. MRI is the investigation of choice to study the neural tissue abnormalities and also to assess the severity of hydrocephalus and Chiari malformation. However, ultrasonography can be used to obtain quick information regarding hydrocephalus.[1,14,15]
Figure 2.
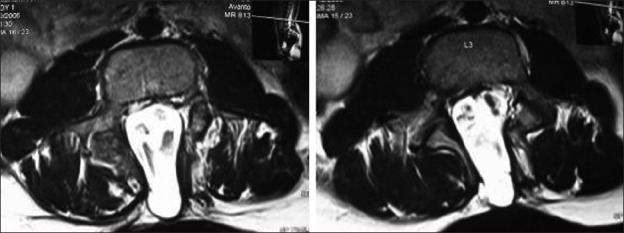
Bifid spine
Figure 4.
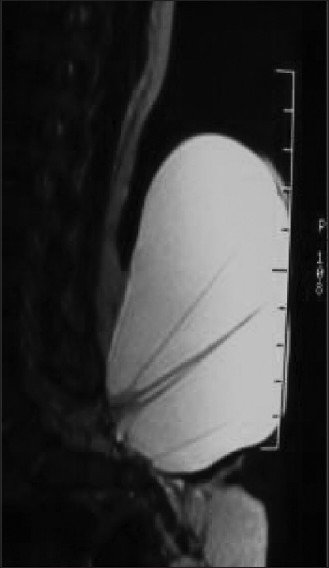
Meningocele
Figure 3.
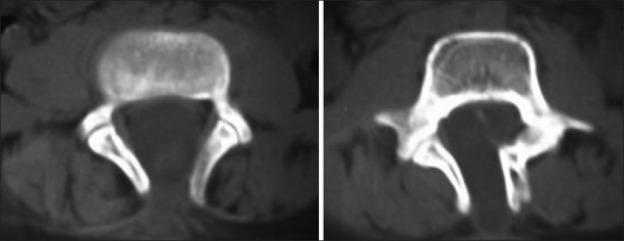
Bifida spine
Management
Management of these children needs multidisciplinary approaches. Complete clinical evaluation and appropriate investigations are necessary. Parents need to be counseled and informed regarding the immediate as well as long-term management strategy.[16]
Surgical treatment
The aim of surgery is to free the placode from the surrounding abnormal skin and reposition into the spinal canal with reconstruction of the dura and coverings to prevent CSF leak and infection. The surgical technique depends on the size and the level of the lesion. The help of pediatric, orthopedic and plastic surgeons may be necessary. Several attempts for maternal fetal surgeries to improve their outcomes have been made. The role of fetal surgery for myelomeningocele is yet to be proven.[17]
Timing of intervention primarily depends on the clinical condition of the child and the impending risks. Surgery need not be done as a compelling emergency but should be undertaken as soon as it is practical.[18] In case of suspected meningitis or CSF infection or colonization of the wound, prophylactic antibiotics and anticonvulsants form the initial treatment. Child is nursed in an incubator; routine blood counts and serum electrolytes are monitored. Blood grouping and cross matching is done for possible transfusion. Careful assessment of body weight is essential for intraoperative management. The newborn child with myelomeningocele should have saline dressings. It is essential not to use corrosive agents, spirit or antiseptics indiscriminately over the open defects to avoid damage to the underlying exposed neural tissue.
Surgical technique
To obtain successful repair, it is essential to study the surface anatomy and its relationships to the surrounding structures. At the apex of the myelomeningocele usually, the flat neural placode is located and from its edge the remnants of the arachnoid membrane get attached at the nerve root entry zone. From this junction, the nerve roots emerge and exit through neural foramina located ventrally. They are seen through the transparent arachnoid membrane which is fused with the skin at the lateral edges of the lesion. The dura matter which is defective posteriorly is loosely adherent to the underlying soft tissue of the back and densely adherent to the bony structures underneath. Rostrally, the dura forms tube and the neural placode continues into it, which leads to functional spinal cord.
The child is operated under general anesthesia with endotracheal intubation in prone position, with the head and neck placed comfortably. The first step is to isolate the neural placode, while salvaging the nerve roots as much as possible irrespective of their functional status. It is essential that no skin element becomes buried in the repair to prevent the possibility of an implantation dermoid. Vertical midline incision is preferable. In a circumferential fashion, arachnoid is lifted and the nerve roots are identified. This technique is continued all around till the neural placode is completely free. The neural placode can then be inverted and sutured. The dura is dissected from the underlying soft tissue. The dural closure needs to be made water tight with a graft, if necessary. The overlying skin is dissected from underlying fascia and musculature, mobilized and approximated. If necessary, relaxing incisions or flaps may be used to close larger defects. Kyphotic deformity or gibbus can pose special problems. Reigel had advocated resection of the kyphus with primary spinal fusion at the time of surgical repair.[19] When the dysplastic skin is large causing larger skin defects, balloon tissue expanders can be used to enlarge the normal skin area which can then cover the defect.[20]
Postoperative care is equally important. To nurse in prone position, protect the wound from fecal and urinary contamination. Symptomatic hydrocephalus or arnold chiari malformation (ACM) needs to be treated simultaneously. Supportive nutrition and antibiotics are required. Adequate urological treatment can prevent future complications of the upper urinary tract.[21–23] Early management, maintaining a low intravesical pressure and continuing intermittent catheterization with or without pharmacotherapy, is beneficial. But urological management should ideally be based on and modified by the urodynamic studies.[21–28]
Tethered cord syndrome
Tethered cord syndrome has been defined as progressive neurological deficits from the restraint of the spinal cord movement and traction due to either anatomical or physiological reasons. Advent of MRI and the present understanding of embryogenesis have made this syndrome popular in pediatric neurosurgical practice. The neurological deficits associated are insidious, progressive, and may be motor, sensory, urorectal, pain and/or scoliosis.[29–31]
Tethered cord can result from a variety of conditions. This can be broadly classified into the following:
Anatomical in which the conus is placed at a lower level than the normal.
Physiological due to tight or thick filum. In this variety, the conus may be at normal level, but the movement of spinal cord is restrained resulting in neurological deficits and vesicle dysfunction called “neurogenic nonneurogenic bladder.”
Developmental in which it could be secondary to a variety of conditions like congenital anomalies, terminal lipoma, intraspinal lipoma, lipomyelomeningocele, split cord malformations, sacral agenesis and other occult dysraphic states.
Postoperative tethering that occurs after surgery for myelomeningocele or dermoid or recurrent adhesions from previous surgery often termed retethering.[32,33]
Symptoms
The predominant symptoms can be motor in the form of insidiously progressive distal weakness and spasticity, and sensory ones like sensory loss, paresthesias and trophic ulcers. Neurovesical dysfunction is one of the commonest presentations, ranging from a minor bladder disturbance to frank urinary incontinence, occasionally associated with bowel movement dysfunction. At times, pain can be the major symptom. Pain may be radicular or funicular, low back or calf muscle pain. The scoliosis due to tethering is a much debated issue, but with enough evidence to suggest its role.
Neurological examination
A detailed neurological assessment is mandatory. Neurocutaneous markers, like tuft of hair, nevous, dysplastic skin, angiomatous patches, lipoma, dermal sinus, presence of human tail and absence of gluteal folds, can be the strong indicators [Figures 5–9].[34] Neurovesical dysfunction, leg pain, scoliosis, and motor or sensory deficits should prompt the clinician to look for possible tethering even in the absence of cutaneous markers.
Figure 5.
Dysplastic skin
Figure 9.
Dysplastic skin
Figure 6.
Lipoma with tuft of Hair
Figure 7.
Tail
Figure 8.
Tuft of hair
With tethering being a congenital phenomenon, these children often present to their pediatricians with vague leg pains, to orthopedicians with gait disturbance and deformities, to urologists with bladder symptoms like frequent persistent bed wetting, and finally to neurosurgeons when the neurological deficits become overt. Hence, awareness about the recent developments among the medical fraternity is necessary.
Pathophysiology
The name tethered cord implies that the spinal cord is attached tightly to a congenitally abnormal structure in the lumbosacral area, as well as at the craniovertebral junction. With growth, the spinal cord is stretched between these two points, resulting in insidious and progressive neurological deterioration. The popular belief that the deterioration occurs mostly during the phases of rapid growth is not confounded. During embryonic life, the spinal cord lies way down at sacral segments, and gradually the conus ascends and reaches the adult level at the time of birth, as confirmed by ultrasonographic studies. In this pathological situation, the spinal cord is tethered to anatomical structures lower down, preventing the ascent of the conus in parallel to the vertebral growth. On the other hand, there is disproportionate growth between the spine and the spinal cord, resulting in progressive traction. Normally, when a child bends forward, there is an ascent of the spinal cord by one to two segments. The filum can be short and thick, with anatomically variant fibroelastic fibrous tissue, making it tight and preventing such movement.
Several theories have been proposed to explain the neurological deterioration in tethered cord syndrome. With ongoing stretching, central fibers are subjected to traction injury, resulting in structural damage. Yamada et al., have shown that the mechanical stretching of the cord during physical activity leads to changes in intracellular respiration, reduction in cytochrome oxidase and shift in redox curves.[31] The third possible mechanism is by ischemic injury. Traction and elongation of the spinal cord can compress the radial perforating vessels, resulting in ischemic damage. Once the neurological deficit occurs, it is rarely reversible. Therefore, it is important to treat them prophylactically.[35–38]
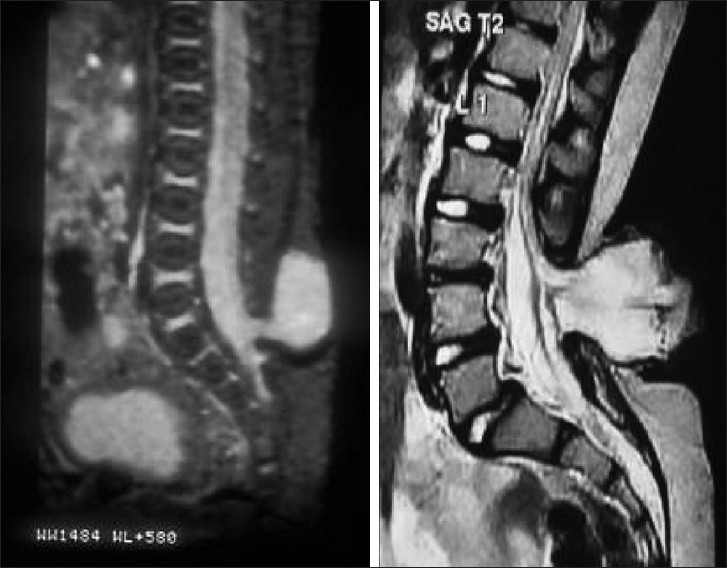
The diagnosis should be based on the high index of clinical suspicion, a detailed neurological examination and investigation for confirmation. MRI is the choice of investigation to identify the anatomical location of the conus and the associated abnormalities. The classical dorsal displacement of the conus with a large, ventral CSF space is often suggestive [Figures 10 and 11]. Early appearance of lumbar potentials in somato sensory evoked potentials (SSEP) has been found valuable, though often difficult to record in children.[39,40]
Figure 10.
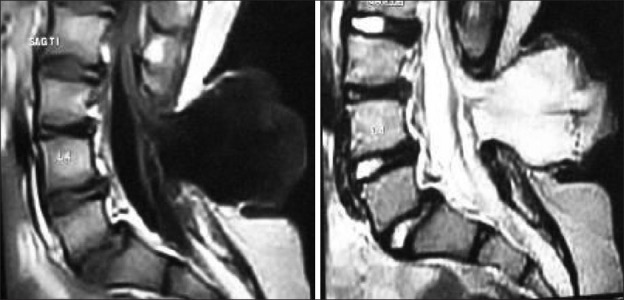
Imaging of tethering
Figure 11.
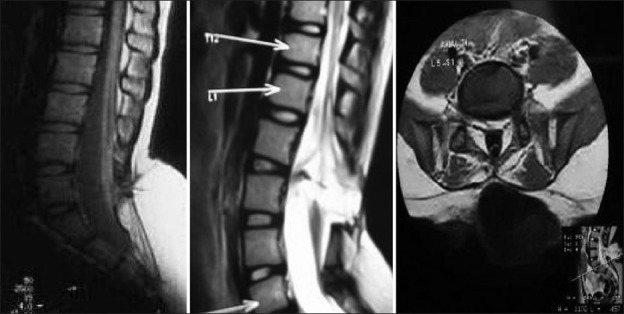
MRI of tethered cord
Indication for treatment
Progressive neurological deterioration in terms of motor, sensory or urinary dysfunction in a child is a clear indication. Pain, spasticity, gait abnormality, and persistent or progressive scoliosis are the other indications. Knowing the mechanisms of neurological deterioration, prophylactic surgery is logical. If the neurological deficit has already occurred, surgery should be done as soon as the diagnosis is established.
The principles of surgery
Complete untethering of the spinal cord
Proper dural reconstruction with adequate CSF space around the spinal cord to prevent retethering.
Retethering
A theoretical risk of retethering exists after the treatment of any tethered cord or some of these complex anomalies. Every known precaution should be adopted to prevent such a possibility. This includes complete untethering of all the contributory structures, proper concealing of rough and possible adhesive surfaces by pial sutures, laminectomy to accommodate thickened cord and to facilitate upward movement, duraplasty to create a reservoir of CSF around the repaired structures and epidural fat grafts to prevent fibrosis. Some studies reported advantage of nursing the child in prone position postoperatively. Avoid intradural lumbar drains as far as possible. During follow-up, subtle neurological worsening or progression of deformities should be looked for. MRI should be repeated at the earliest suspicion. At present, there is no foolproof investigation to demonstrate retethering. MRI in prone position to demonstrate CSF dorsal to the conus has been suggested. Serial electrophysiological and urodynamic studies seem to be indicative. Till such time, clinical diagnosis remains the gold standard. All symptomatic children due to suspected retethering should be reoperated to prevent further deterioration. But surgery on pure radiological suspicion for retethering is controversial [Figure 12].
Figure 12.
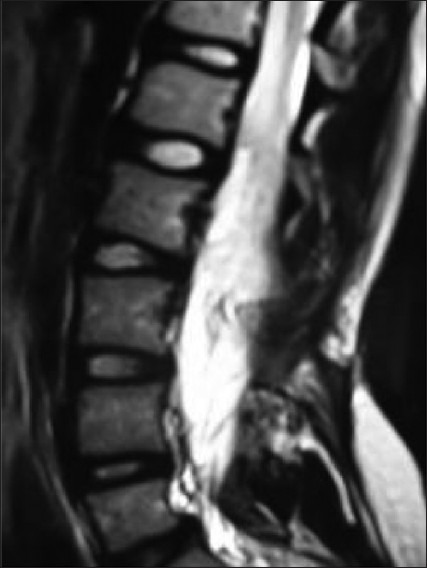
Retethering
Lipomyelomeningocele
Lumbosacral lipomyelomeningocele is a subcutaneous fibrofatty mass that traverses the lumbodorsal fascia, causing a spinal laminar defect, penetrating dura and tethering the spinal cord [Figure 13]. Lipomas form one of the prominent causes of spinal cord tethering, almost all of them with low-lying conus. The term lipomyelomeningocele is used despite the overlying skin being normal. They arise from the disorder of embryogenesis, usually consist of single cell type but rather demonstrate fibrous tissue, muscle cells, neural tissue and a variety of other cell types that arise from all embryonic layers. Incidence is approximately 1 in 4000 births in USA, with a slightly female preponderance. True incidence in terminal lipomas is not known. MR studies represent 13- 26% of lesions accounting for tethering.[41,42] Symptoms and signs are mainly related to the traction on the lower spinal cord, leading to motor deficits, sensory disturbance, spasticity, urodynamic, urorectal dysfunctions and skeletal deformities.[43] Pathogenesis and natural history of these complex anomalies are not clearly understood. But it is certain that almost all deteriorate in their neurological state over course of time. Proper treatment at the appropriate time prevents their neurological deterioration. But surgical treatment is complex as it poses several challenges and risk of neurological deterioration. Various factors that can influence the outcomes are as follows:
Figure 13.
Lipoma
Anatomical features like size of the lipoma, location (midline or paramedian), wide bony defect, defective muscles and fascia, poor cleavage at neurolipomatus junction.
Physiological factors like degree of traction, ability of cord to withstand the effects of traction.
Pathological factors like vascularity of fibrolipomatous structures and associated anomalies.[44–46]
Lumbosacral lipomyelomeningoceles have been classified by Chapman as dorsal, transitional and terminal types, and by Aris into five types as dorsal, caudal, combined, filar and lipomeningomyelocele.[47,48]
Dorsal variety lipomas enter into the segment of spinal cord dorsally through fibrofatty pedicle, below which normal cord and dura exists. Transitional variety is essentially a dorsal type which covers the entire conus and extends up to the filum. Distally, no normal cord exists, whereas in terminal variety the normal looking conus ends into lipoma through a dural defect and all the sacral roots are cranial to the lipoma.
Diagnosis in 90% of children is made by the presence of lipomatous mass or a cutaneous marker. Associated hydrocephalus or cranial anomalies are extremely low though other spinal anomalies can be associated. MRI (craniospinal screening) is the choice of investigation for a comprehensive assessment [Figures 14–16]. The surgical treatment is aimed at complete untethering and prevention of retethering of the cord. Based on the progressive irreversible worsening, prophylactic surgery has been recommended by many authors.[49] There is no controversy regarding lipomas of filum terminale. But a report from Paris group showing 18.7% delayed deterioration on 10-year follow-up of asymptomatic conus lipomas and a report from Osaka group stating that 88% of their children operated prophylactically deteriorated with time had created further debate on this issue.[41,49–53]
Figure 14.
Terminal lipoma
Figure 16.
Lipomyelomeningocele
Figure 15.
Lipomyelomeningoceles
Surgical technique
Broad principles include vertical skin incision, dissection of subcutaneous lipoma up to the fascia, excision of extraspinal component of lipoma, identification of upper and lower laminae and performing laminectomy, identification of normal dura above and below and separation of dural tube from the thoracolumbar fascia all around. These are followed by dural opening (circumferential) around the fibrofatty stalk, removal of intradural lipoma, gentle dissection of fibrofatty pedicle up to the neural structures over the dorsal surface of cord and removal of fibrous tissue up to the neuro fibrolipomatous junction, removal of other fibrous bands and finally detachment of filum.
All known precautions to prevent retethering, like pial closure to cover the raw dorsal surface of the cord and duraplasty to enlarge the subarachnoid spaces and to facilitate free CSF circulation around the repaired surface of the cord, are to be adopted. Replacement of free fat over the dura fills the dead space, protects the soft tissue, prevents CSF leak and prevents the skin edge necrosis. The spinal cord should be eventually made free from five structures, i.e. skin, subcutaneous tissue, thoracolumbar fascia, dura and filum terminale, to achieve complete untethering. Surgery for terminal lipomas is relatively easy. One needs to separate dura from the lipoma and disconnect the neural tissue at neurolipomatous junction which is often evident clearly.[18,53–59]
Myelocystocele is an occult form of spinal dysraphism with a localized, cystic dilatation of the central canal of the spinal cord herniated through a posterior spina bifida. Terminal myelocystocele truly is an anomaly of the caudal cell mass associated with anomalies of anorectal system, lower genitourinary system and vertebrae such as anal atrecia, cloacal extrophy, lordosis, scoliosis and variable degree of sacral agenesis. It constitutes 4-8% of occult dysraphism and rarely occurs in lower thoracic region. The lesion consists of skin-covered lumbosacral spina bifida, arachnoid lined meningocele directly continuous with subarachnoid space and a low-lying hydromyelic spinal cord that traverses the meningocele and forms a distal sac which does not communicate with the subarachnoid space. MR appearance is distinctive and characterized by trumpet-like flaring of the distal cord central canal into an ependyma-lined terminal cyst. Surgical correction can not only release untethering, but also prevent the complications.[2,60–62]
Dermal sinus
Congenital dermal sinuses are a unique form of occult dysraphism presenting with meningitis, tethering or neurological compression, with an incidence of 1 in 1500 births. The dermal sinus tracts are lined by squamous epithelium and may penetrate anywhere in the midline from the lumbosacral region to the occiput or nasion. Despite innocuous external look, the tract may extend over several spinal levels before entering the dura or getting attached to the filum of the spinal cord. Dermoid and epidermoid nodules are frequently associated with these tracts. The small sinus osteum is quite often overlooked unless discharge is noted by the parents. The neurological examination is nearly always intact. The MRI is diagnostic. Embryologically, the formation of the dermal sinuses may reflect incomplete dysjunction. Focal, incomplete separation of the cutaneous ectoderm from the neural ectoderm during the 4th week of fetal development retains adherence of these layers. Altered dysjunction can lead to dermal sinus termination from the subcutaneous tissues to the intramedullary location. The most common location is lumbosacral area. Thoracic and cervical dermal sinuses are present in 10%. There are usually in midline, providing a portal for infection leading to meiningitis or intraspinal abscesses. The most common organisms are Staphylococcus aureus and Escherichia coli followed by Proteus species and anaerobes. Multiple organisms may be cultured, and recurrent meningitis is one of the classical presentations. Neurological examination is normal except in situations where the dermoid or epidermoids lead to cord compression. The treatment includes antibiotics and complete surgical excision of the sinus, tract and the associated lesions. Incomplete removal is the usual cause for recurrences.[63–69]
Syndromes of the tethered cord
Tethered cord syndrome may occur with conus in normal position with or without associated neurocutanoeus markers. The affected children usually present with hyperreflexic neurogenic bladder which is earlier referred as nonneurogenic bladder. Urodynamic studies often reveal detrusor hyperreflexia. There is growing evidence that sectioning of filum improves the bladder function in 96% of patients.[70–72]
Tethered cord syndrome in adults
Though unusual, it is a well-established entity. The late onset of presentation may be related to the cumulative effects of repeated microtrauma. Distinct precipitating events proceeding to the symptoms like heavy weight lifting, trauma and lithotomy position have been described in 60% In many respects, it is similar to the pediatric population except that the incidence of the low back pain and leg pain is significantly high in adults. MRI is diagnostic [Figure 17]. Though indications for surgery are still controversial and not clear, majority improve with proper surgical sectioning of the filum.[36,73–76]
Figure 17.
Dermal sinus
Neurogenic bladder with conus at normal position
Children with this condition usually present to the urologists with urinary incontinence and/or the associated complications. Tethered cord syndrome has been postulated as a cause of neurogenic hyperreflexic bladder even in the presence of a conus medullaris at a normal position. These conditions were earlier referred by different names like nonneurogenic-neurogenic bladder. They usually present in children less than 10 years. They do not have associated regional, neurological or any vertebral associated anomalies. Urodynamics are highly suggestive. Release of filum terminale has shown significant improvement in the bladder control and also the urodynamic abnormalities. Selcuki et al., described 96% improvement in bladder function.[77–80]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Harwood-Nash DC, McHugh K. Diastematomyelia in 172 children: The impact of modern neuroradiology. Pediatr Neurosurg. 1991;16:247–51. doi: 10.1159/000120535. [DOI] [PubMed] [Google Scholar]
- 2.De Jong TP, Boemers TM, Schouten A, van Gool JD, de Maat-Bleeker F, Bruijnzeel-Koomen CA. Peroperative anaphylactic reactions due to latex allergy. Ned Tijdschr Geneeskd. 1993;137:1934–6. [PubMed] [Google Scholar]
- 3.Morrow JD, Kelsey K. Folic acid for prevention of neural tube defects: Pediatric anticipatory guidance. J Pediatr Health Care. 1998;12:55–9. doi: 10.1016/s0891-5245(98)90222-x. [DOI] [PubMed] [Google Scholar]
- 4.Pal-de Bruin KM, Buitendijk SE, Hirasing RA, den Ouden AL. Prevalence of neural tube defects in births before and after promotion of periconceptional folic acid supplementation. Ned Tijdschr Geneeskd. 2000;144:1732–6. [PubMed] [Google Scholar]
- 5.Steinbok P. Dysraphic lesions of the cervical spinal cord. Neurosurg Clin N Am. 1995;6:367–76. [PubMed] [Google Scholar]
- 6.Boyd PA, Wellesley DG, De Walle HE, Tenconi R, Garcia-Minaur S, Zandwijken GR, et al. Evaluation of the prenatal diagnosis of neural tube defects by fetal ultrasonographic examination in different centers across Europe. J Med Screen. 2000;7:169–74. doi: 10.1136/jms.7.4.169. [DOI] [PubMed] [Google Scholar]
- 7.Candenas M, Villa R, Fernandez Collar R, Moina MJ, Pintado S, Garcia Saez F, et al. Maternal serum alpha-fetoprotein screening for neural tube defects. Report of a program with more than 30,000 screened pregnancies. Acta Obstet Gynecol Scand. 1995;74:266–9. doi: 10.3109/00016349509024447. [DOI] [PubMed] [Google Scholar]
- 8.Anderson NG, Jordan S, MacFaelane MR, Lovell-Smith M. Diastematomyelia: Diagnosis by prenatal sonography. AJR Am J Roentgenol. 1994;163:911–4. doi: 10.2214/ajr.163.4.8092034. [DOI] [PubMed] [Google Scholar]
- 9.Chan A, Robertson EF, Haan EA, Ranieri E, Keane RJ. The sensitivity of ultrasound and serum alpha-fetoprotein in population-based antenatal screening for neural tue defects. South Australia 1986-1991. Br J Obstet Gynaecol. 1995;102:370–6. doi: 10.1111/j.1471-0528.1995.tb11287.x. [DOI] [PubMed] [Google Scholar]
- 10.Chitty LS. Ultrasound screening for fetal abnormalities. Prenat Diagn. 1995;15:1241–57. doi: 10.1002/pd.1970151306. [DOI] [PubMed] [Google Scholar]
- 11.Malinger G, Lerman-Sagie T, Watemberg N, Rotmensch S, Lev D, Glezerman M. A normal second-trimester ultrasound does not exclude intracranial structural pathology. Ultrasound Obstet Gynecol. 2002;20:51–4. doi: 10.1046/j.1469-0705.2002.00743.x. [DOI] [PubMed] [Google Scholar]
- 12.Pierre-Kahn A, Hanlo P, Sonigo P, Parisot D, McConnell RS. The contribution of prenatal diagnosis to the understanding of malformative intracranial cysts: State of the art. Childs Nerv Syst. 2000;16:619–26. doi: 10.1007/s003810000316. [DOI] [PubMed] [Google Scholar]
- 13.Vintzileos AM, Ananth CV, Fisher AJ, Smulian JC, Day-Salvatore D, Beazoglou T, et al. Cost-benefit analysis of targeted ultrasonography for prenatal detection of spina bifida in patients with an elevated concentration of second-trimester maternal serum alpha-fetoprotein. Am J Obstet Gynecol. 1999;180:1227–33. doi: 10.1016/s0002-9378(99)70621-6. [DOI] [PubMed] [Google Scholar]
- 14.Brunberg JA, Latchaw RE, Kanal E, Burk DL, Jr, Albright L. Magnetic resonance imaging of spinal dysraphism. Radiol Clin North Am. 1988;26:181–205. [PubMed] [Google Scholar]
- 15.Gupta RK, Sharma A, Jena A, Tyagi G, Prakash B, Khushu S. Magnetic resonance evaluation of spinal dysraphism in children. Childs Nerv Syst. 1990;6:161–5. doi: 10.1007/BF00308494. [DOI] [PubMed] [Google Scholar]
- 16.Charney EB, Weller SC, Sutton LN, Bruce DA, Schut LB. Management of the newborn with myelomeningocele: Time for a decision-making process. Pediatrics. 1985;75:58–64. [PubMed] [Google Scholar]
- 17.Johnson MP, Gerdes M, Rintoul N, Pasquariello P, Melchionni J, Sutton LN, et al. Maternal-fetal surgery for myelomeningocele: Neurodevelopmental outcomes at 2 years of age. Am J Obstet Gynecol. 2006;194:1145–50. doi: 10.1016/j.ajog.2006.01.072. discussion 1150-2. [DOI] [PubMed] [Google Scholar]
- 18.Pang D. 1st China ISPN Course on Pediatric Neurosurgery. Hong Kong: 2005. Aug, Total and near-total resection of spinal cord lipoma. [Google Scholar]
- 19.Reigel DH. In Modern Technique in Surgery. Mount Kisco, NY: Futura; 1979. Kyphectomy and Myelomeningocele repair. [Google Scholar]
- 20.Venkataramana NK, Anantheshwar YN. Tissue expansion technique for closure of myelomeningocele. J Pediatr Neurosci. 2009;4:25–9. doi: 10.4103/1817-1745.49104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrahamsson K, Arnell Vu-Minh M, Jodal U, Lindehall B, Sillen U, Sixt R. Evaluation of renal function in children with myelodysplasia. Br J Urol. 2004;93(Suppl 2):26–7. [Google Scholar]
- 22.Barry BP, Hall N, Cornford E, Rose DH. Improved ultrasound detection of renal scarring in children following urinary tract infection. Clin Radiol. 1998;53:747–51. doi: 10.1016/s0009-9260(98)80317-6. [DOI] [PubMed] [Google Scholar]
- 23.Bauer SB, Colodny AH, Retik AB. The management of vesicoureteral reflux in children with myelodysplasia. J Urol. 1982;128:102–5. doi: 10.1016/s0022-5347(17)52774-3. [DOI] [PubMed] [Google Scholar]
- 24.Capitanucci ML, Iacobelli BD, Silveri M, Mosiello G, De Gennaro M. Long-term urological follow-up of occult spinal dysraphism in children. Eur J Pediatr Surg. 1996;6(Suppl 1):25–6. doi: 10.1055/s-2008-1071033. [DOI] [PubMed] [Google Scholar]
- 25.Gordon I. Vesico-ureteric reflux, urinary-tract infection, and renal damage in children. Lancet. 1995;346:489–90. doi: 10.1016/s0140-6736(95)91328-9. [DOI] [PubMed] [Google Scholar]
- 26.Greig JD, Young DG, Azmy AF. Follow-up of spina bifida children with and without upper renal tract changes at birth. Eur J Pediatr Surg. 1991;1:5–9. doi: 10.1055/s-2008-1042449. [DOI] [PubMed] [Google Scholar]
- 27.McLone DG. Technique for closure of myelomeningocele. Childs Brain. 1980;6:65–73. doi: 10.1159/000119887. [DOI] [PubMed] [Google Scholar]
- 28.Muller T, Arbeiter K, Aufricht C. Renal function in myelomeningocele: Risk factors, chronic renal failure, renal replacement therapy and transplantation. Curr Opin Urol. 2002;12:479–84. doi: 10.1097/00042307-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Boop FA, Russell A, Chadduck WN. Diagnosis and management of the tethered cord syndrome. J Ark Med Soc. 1992;89:328–31. [PubMed] [Google Scholar]
- 30.Jones PH, Love JG. Tight filum terminale. Arch Surg. 1956;73:556–66. doi: 10.1001/archsurg.1956.01280040010002. [DOI] [PubMed] [Google Scholar]
- 31.Yamada S, lacono R, Yamada B. Pathophysiology of the tethered spinal cord. In: Yamada S, editor. Tethered cord syndrome. Park ridge, IL: American Association of Neurological Surgeons; 1996. pp. 29–45. [Google Scholar]
- 32.Warder DE, Oakes WJ. Tethered cord syndrome- The low-lying and normally positioned conus. Neurosurgery. 1994;34:597–600. doi: 10.1227/00006123-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Warder DE, Oakes WJ. Tethered cord syndrome and the conus in a normal position. Neurosurgery. 1993;33:374–8. doi: 10.1227/00006123-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Chakrabortty S, Oi S, Yoshida Y, Yamada H, Yamaguchi M, Tamaki N, et al. Myelomeningocele and thick filum terminale with tethered cord appearing as a human tail. Case report. J Neurosurg. 1993;78:966–9. doi: 10.3171/jns.1993.78.6.0966. [DOI] [PubMed] [Google Scholar]
- 35.Pang D, Dias MS, Ahab-Barmada M. Split cord malformation. I. Aunified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 1992;31:451–81. doi: 10.1227/00006123-199209000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Pang D, Wilberger JE., Jr Tethered cord syndrome in adults. J Neurosurg. 1982;57:32–47. doi: 10.3171/jns.1982.57.1.0032. [DOI] [PubMed] [Google Scholar]
- 37.Pang D. Tethered cord syndrome. Neurosurgery: States of the Arts Review. 1986;1:45–79. [Google Scholar]
- 38.Schneider S, Rosenthal A, Greenberg B. A prelimnary report on the use of laser-Doppler flowmetry during tethered spinal cord release. Neurosurgery. 1993;32:214–7. doi: 10.1227/00006123-199302000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Breig A. Overstretching of and circumscribed pathological tension in the spinal cord: A basic cause of symptoms in cord disorders. J Biomech. 1970;33:7–9. doi: 10.1016/0021-9290(70)90046-1. [DOI] [PubMed] [Google Scholar]
- 40.Kang J, Kim King D. Effects of tethering on regional spinal cord blood flow and sensory evoked potentials in growing cats. Childs Nerv Syst. 1987;3:35–9. doi: 10.1007/BF00707191. [DOI] [PubMed] [Google Scholar]
- 41.Aoki N. Rapid growth of intraspinal lipoma demonstrated by magnetic resonance imaging. Surg Neurol. 1990;34:107–10. doi: 10.1016/0090-3019(90)90105-x. [DOI] [PubMed] [Google Scholar]
- 42.Bruce DA, Schut L. Spinal lipomas in infancy and childhood. Childs Brain. 1979;5:192–203. doi: 10.1159/000119818. [DOI] [PubMed] [Google Scholar]
- 43.Foster LS, Kogan BA, Cogen PH, Edwards MS. Bladder function in patients with lipomyelomeningocele. J Urol. 1990;143:984–6. doi: 10.1016/s0022-5347(17)40159-5. [DOI] [PubMed] [Google Scholar]
- 44.Lellouch-Tubiana A, Zerah M, Catala M, Brousse N, Kahn AP. Congenital intraspinal lipomas: Histological analysis of 234 cases and review of the literature. Pediatr Dev Pathol. 1999;2:346–52. doi: 10.1007/s100249900133. [DOI] [PubMed] [Google Scholar]
- 45.Lhowe D, Ehrlich MG, Chapman PH, Zaleske DJ. Congenital intraspinal lipomas: Clinical presentation and response to treatment. J Pediatr Orthop. 1987;7:531–7. [PubMed] [Google Scholar]
- 46.Venkataramana . In text book of operative Neurosurgery. Vol. 2. New Delhi: BI Publications Pvt. Ltd; 2005. Surgery of lumbar lipomeningocele; pp. 1119–23. [Google Scholar]
- 47.Chapman PH. Congenital intraspinal lipomas: Anatomic considerations and surgical treatment. Childs Brain. 1982;9:37–47. [PubMed] [Google Scholar]
- 48.Xenos C, Sgouros S, Walsh R, Hockley A. Spinal lipomas in children. Pediatr Neurosurg. 2000;32:295–307. doi: 10.1159/000028958. [DOI] [PubMed] [Google Scholar]
- 49.Cochrane DD, Finley C, Kestle J, Steinbok P. The patterns of late deterioration in patients with transitional lipomyelomeningocele. Eur J Pediatr Surg. 2000;10(Suppl 1):13–7. doi: 10.1055/s-2008-1072406. [DOI] [PubMed] [Google Scholar]
- 50.Koyanagi I, Iwasaki Y, Hida K, Abe H, Isu T, Akino M. Surgical treatment supposed natural history of the tethered cord with occult spinal dysraphism. Childs Nerv Syst. 1997;13:268–74. doi: 10.1007/s003810050081. [DOI] [PubMed] [Google Scholar]
- 51.Mclone D, Mutluer S, Naidich TP, Alvard EC. Concepts in pediatric neurosurgery. Basel: Krager; 1983. Lipomeningoceles of the conus medullaris; pp. 170–7. [Google Scholar]
- 52.Pierre-Kahn A, Zerah M, Renier D, Cinalli G, Sainte-Rose C, Lellouch-Tubiana A, et al. Congenital lumbosacral lipomas. Childs Nerv Syst. 1997;13:298–334. doi: 10.1007/s003810050090. discussion 335. [DOI] [PubMed] [Google Scholar]
- 53.Van Calenbergh F, Vanvolsem S, Verpoorten C, Lagae L, Casaer P, Plets C. Results after surgery for lumbosacral lipoma: The significance of early and late worsening. Childs Nerv Syst. 1999;15:439–42. doi: 10.1007/s003810050433. discussion 443. [DOI] [PubMed] [Google Scholar]
- 54.La Marca F, Grant JA, Tomita T, McLone DG. Spinal lipomas in children: Outcome of 270 procedures. Pediatr Neurosurg. 1997;26:8–16. doi: 10.1159/000121155. [DOI] [PubMed] [Google Scholar]
- 55.Oakes W. Management of spinal cord lipomas and lipomeningoceles. In: Wilkins RH, Rengachary SS, editors. Neurosurgery Update II. Vol. 3. New York: McGraw-Hill; 1991. pp. 3497–504. [Google Scholar]
- 56.AQ17Schimidek, Sweet Operative Neurosurgical Techniques. R. Shane tubs and w.Jerry doaker. (5th Edition) 2009;2:2201–2214. [Google Scholar]
- 57.Surgery of the developing nervous system. 4th ed. W. B. Saunders Company; 2001. Text Book of Pediatric Neurosurgery. [Google Scholar]
- 58.Tse YH, Fong D. Treatment and Outcome Review of Spinal Dysraphism. 11th Annual Scientific Meeting of the Hong Kong Neurosurgical Society. 2004 [Google Scholar]
- 59.Venkataramana NK. Tethered cord Syndrome: Progress in clinical Neurosciences. Neurol Soc India. 2003;18:56–70. [Google Scholar]
- 60.Choi S, McComb JG. Long-term outcome of terminal myelocystocele patients. Pediatr Neurosurg. 2000;32:86–91. doi: 10.1159/000028905. [DOI] [PubMed] [Google Scholar]
- 61.Rickwood AM, Thomas DG. The upper renal tracts in adolescents and young adults with myelomeningocele. Z Kinderchir. 1984;39(Suppl 2):104–6. doi: 10.1055/s-2008-1044296. [DOI] [PubMed] [Google Scholar]
- 62.Mclone D, Naidich T. Terminal myelocystocele. Neurosurgery. 1985;16:35–41. [PubMed] [Google Scholar]
- 63.Peacock WJ, Murovic JA. Magnetic resonance imaging in myelocystoceles. Report of two cases. J Neurosurg. 1989;70:804–7. doi: 10.3171/jns.1989.70.5.0804. [DOI] [PubMed] [Google Scholar]
- 64.Cheek WR, Laurent JP. Dermal sinus tracts. In: Marlin AE, editor. Concepts in Pediatric Neurosurgery. Basel: S. Krager; 1985. pp. 63–75. [Google Scholar]
- 65.Gindi SE, Fairburn B. Intramedullary spinal abscess as a complication of a congenital dermal sinus. J Neurosurg. 1969;30:494–7. doi: 10.3171/jns.1969.30.4.0494. [DOI] [PubMed] [Google Scholar]
- 66.Lemire RJ, Loeser JD, Leech RW, et al. Normal and Abnormal development of the Human Nervous System. New York: Harper and Row; 1975. [Google Scholar]
- 67.Muraszko K, Youkilis A. Intramedullary spinal tumors of disordered embryogenesis. J Neurooncol. 2000;47:271–81. doi: 10.1023/a:1006474611665. [DOI] [PubMed] [Google Scholar]
- 68.Kanev PM, Park TS. Dermoids and dermal sinus tracts of the spine. Neurosurg Clin N Am. 1995;6:359–66. [PubMed] [Google Scholar]
- 69.Powell KR, Cherry JD, Hougen TJ, Blinderman EE, Dunn MC. A prospective search for congenital dermal abnormalities of the craniospinal axis. J Pediatr. 1975;87:744–50. doi: 10.1016/s0022-3476(75)80298-8. [DOI] [PubMed] [Google Scholar]
- 70.Venger B, Laurent JL, Cheek WR, et al. Congenital thoracic dermal sinus tracts. In: Marlin AE, editor. Concepts in Pediatric Neurosurgery. Basel: S. Krager; 1989. pp. 161–72. [Google Scholar]
- 71.Kondo A, Kato K, Kanai S, Sakakibara T. Bladder dysfunction secondary to tethered cord syndrome in adults: Is it curable? J Urol. 1986;135:313–6. doi: 10.1016/s0022-5347(17)45622-9. [DOI] [PubMed] [Google Scholar]
- 72.Selçuki M, Unlü A, Uğur HC, Soygür T, Arikan N, Selçuki D. Patients with urinary incontinence often benefit from surgical detethering of tight fulum terminale. Childs Nerv Syst. 2000;16:150–5. doi: 10.1007/s003810050482. [DOI] [PubMed] [Google Scholar]
- 73.Kondo A, Kobayashi M, Otani T, Takita T, Mitsuya H. Children with unstable bladder: Clinical and urodynamic observation. J Urol. 1983;129:88–91. doi: 10.1016/s0022-5347(17)51932-1. [DOI] [PubMed] [Google Scholar]
- 74.Caruso R, Cervoni L, Fiorenza F, Vitale AM, Salvati M. Occult dysraphism in adulthood. A series of 24 cases. J Neurosurg Sci. 1996;40:221–5. [PubMed] [Google Scholar]
- 75.Gupta SK, Khosla VK, Sharma BS, Mathuriya SN, Pathak A, Tewari MK. Tethered cord syndrome in adults. Surg Neurol. 1999;52:362–70. doi: 10.1016/s0090-3019(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 76.Kim SK, Chung YS, Wang KC, Cho BK, Choi KS, Han DH. Diastematomyelia. Clinical manifestation and treatment outcome. J Korean Med Sci. 1994;9:135–44. doi: 10.3346/jkms.1994.9.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLone DG. The adult with a tethered cord. Clin Neurosurg. 1996;43:203–9. [PubMed] [Google Scholar]
- 78.Khoury AE, Hendrick EB, McLorie GA, Kulkarni A, Churchill BM. Occult spinal dysraphism: Clinical and urodynamic outcome after division of the filum terminale. J Urol. 1990;144:426–8. doi: 10.1016/s0022-5347(17)39481-8. discussion 428-9, 443-4. [DOI] [PubMed] [Google Scholar]
- 79.Kondo A. Cystourethrograms characteristic of bladder instability in children. Urology. 1990;35:242–6. doi: 10.1016/0090-4295(90)80041-k. [DOI] [PubMed] [Google Scholar]
- 80.Selcuki M, Coskun K. Management of tight filum terminale syndrome with special emphasis on normal level conus medullaris (NLCM) Surg Neurol. 1998;50:318–22. [PubMed] [Google Scholar]


