Abstract
Aim:
Medulloblastoma is one of the most common posterior fossa tumors in childhood. The treatment-related side effects as well as predictive outcome still remain as a major challenge. The improved understanding of the disease and advances in molecular biology is changing the treatment paradigms from Chang's staging system to molecular risk stratification. However, surgery still remains as an important mainstay of therapy and is formidable. The role of radical surgery has always been a crucial factor in the outcome of these patients, the best survival being reported in patients who had total excision of the tumor and with no metastasis.
Patient and Methods:
An analysis of 365 patients (age<18 years) of medulloblastoma who underwent treatment at the Seth G.S. Medical College and King Edward VII Memorial hospital (KEM), Mumbai over a 25- year period (1985-2000 and 2001-2010) is presented. The clinical profile, radiological features, pathology and surgical nuances are discussed.
Results:
The most common age group affected was between 3 and 12 years. 75.3% presented with headaches, vomiting and 63.2% with papilledema. Sitting position was used in majority of cases. A total of 8 patients underwent shunting; all of them were in the postoperative period (5.19%). 92.2% (142 cases) had classical medulloblastoma, 5.1% (8 cases) had desmoplastic variant, 1.9% (3 cases) had anaplastic changes and 0.6% (1 case) had glial differentiation. The 5-year and 10-year progression free survival rate was 73 and 41% for average risk disease while for high risk disease rate it was 34%. The mortality rate was 2%. The quality of life was enhanced in patients who survived 5-10 years after treatment.
Conclusion:
Surgery for medulloblastoma is formidable. The option of sitting position for medulloblastoma surgery is still viable. A vigilant neuroanesthesiologist and a safe surgery are necessary to achieve a good postoperative result. Radiological characteristics are helpful adjuncts for determining effective surgical strategy. Permanent CSF drainage can be avoided in majority of patients and can be definitively considered in progressive symptomatic hydrocephalus. A safe maximal resection and a good Karnofsky score are paramount to ensure compliance with adjuvant therapy and contribute to an overall survival advantage.
Keywords: Medulloblastoma, posterior fossa, sitting position, surgery, tumor
Introduction
Amongst the posterior fossa tumors in children, medulloblastoma has challenged the interest of clinicians to a large extent. The overwhelming response to radiation treatment resulting in a distinct survival advantage was the key factor. A multidisciplinary approach involving neurosurgeons, radiologists, neuropathologists, radiation and medical oncologists resulted in rapid advances in the understanding of the disease as well as effective treatment protocols. In addition, the encouraging response of chemo radiation manifesting as survival benefit is an added incentive. The current area of advanced research in medulloblastoma is in the molecular biology which aims at better understanding of the growth control mechanisms involved in the development and progression of medulloblastoma.[1–3] It will allow a better classification, leading to the improvement of the existing therapies, as well as to the development of new therapeutic approaches. There are various reports in literature discussing the molecular genetics of medulloblastoma.[1–6] However, there are only few reports discussing surgical challenges in medulloblastoma.[7–15] Surgery for medulloblastoma is formidable. The massive tumor size, advanced clinical presentation and potential risk of enhanced morbidity and mortality are peculiar factors influencing immediate surgical outcome. Moreover, in most centers posterior fossa surgery is performed in prone position or supine position with head turned to opposite side. We describe our experience of 365 patients (age<18 years) of medulloblastoma who had undergone surgery in sitting position. The surgical technique, nuances, results and outcome are discussed and the relevant literature is reviewed in that context.
Materials and Methods
Analysis of our series of 365 cases (age<18 years) of surgically treated medulloblastoma (1985-2010)
A retrospective comparative analysis of 365 cases (age<18 years) of medulloblastoma treated at the Seth G.S. Medical College and King Edward VII Memorial hospital (KEM) over the past 25 years (1985-2010) is presented. The clinical and radiological records were searched and information was retrieved with respect to age, sex, duration of symptoms, clinical profile, radiological imaging, surgical therapy, cerebrospinal fluid (CSF) diversion procedure, adjuvant therapy and quality of life. The risk stratification was measured into average and poor risk using the Chang's staging system.[16] CT scan was performed as a sole investigation prior to availability of MR imaging. In the later part of the series beyond the year 2000, a complete MR imaging study was performed in all patients. The size of the tumor, signal intensity characteristics, presence of calcification, extension into neighboring critical neural and vascular structures were assessed on MR imaging. Steroids (dexamethasone intravenously in three divided doses along with H2 receptor blocking drugs) were instituted in patients with preserved consciousness but incipient tentorial herniation on imaging as well as large-sized tumors with moderate to severe hydrocephalus. Cerebral decongestants were administered preoperatively if patients had deteriorating level of consciousness or in a comatose state and patients in critical neurological condition awaiting surgery. All patients underwent surgery within 24 to 48 h following admission. Surgery was performed in sitting position in all patients. Perioperative shunt surgery was considered in extremely sick patients with raised intracranial pressure, deteriorating level of consciousness with spontaneous decerebration, postoperative persistent or progressive symptomatic hydrocephalus or there is significant residual tumor associated with symptomatic hydrocephalus. Intraoperative parietal burr hole drainage of CSF was performed as a temporary measure to aid extirpation of tumor. A safe maximal resection of the tumor without endangering critical neurovascular structures was the goal. Postoperative CT or MR imaging was performed depending upon the logistics. The patients were stratified into average and high risk according to Chang's classification. The quality of life issues were assessed using Bloom's criteria.[17] Statistical analysis was performed using the SPSS 13 program for Windows and the statistical significance was established at a probability value 0.05.
Results
Analysis of our series of 211 patients (age<18 years) of surgically treated medulloblastoma (1985-2000)
The most common age group affected was between 3 and 12 years amounting for 49.6%. There were 15 patients below the age of 1 year. In our series 147 (69.7%) were males and 64 (30.3%) females, with a male to female ratio of 2.3: 1. It was also observed that males were affected more than females in all age groups. 77.7% presented with headaches, vomiting and 69.7% with papilledema. 20 patients presented solely with vomiting. Nuchal pain as one of the presenting features was seen in 8% of patients. Diminution of vision and diplopia was noted in 17.3 and 15.9% respectively. 9 patients were blind amounting for 4.1%. Cerebellar symptoms were seen in 51% cases. 14.8% patients presented with lower cranial nerve symptoms. 93 (44.3%) patients were having symptoms of less than one-month duration. There was no correlation between the duration of symptoms (more or less than 6 weeks) and survival. Truncal ataxia in association with nystagmus was most prevalent in 40.8%, followed by appendicular ataxia with nystagmus in 17.9%. 207 patients underwent preoperative CT imaging. Out of this, 48% cases had the typical picture of hyperdense lesion on plain imaging and an intense enhancement on contrast administration. Atypical findings included cystic changes in 26%, isodense attenuation in 9% and absent contrast enhancement in 4% of cases. Amongst the 207 patients, 183 patients had a midline vermian lesion, which accounted for 88.4%. The other sites in our series were cerebellar hemisphere 10.8%, cerebellopontine angle 0.4%, and petrous region 0.4%. Involvement of brainstem and leptomeningeal spread was seen in 20.4 and 8.3% respectively. Metastasis was seen in 3.7% of patients. The different sites of metastasis were suprasellar, temporal, parietal, frontal and paramedian. Hydrocephalus was noted in 92.5% patients, out of which 88% were of moderate to severe type. The total number of patients who underwent MR imaging preoperatively were 41. The typical finding was low signal intensity on T1-weighted images and variable on T2-weighted images. A total of 112 patients underwent shunting. Out of which, 61% were preoperative and 11 postoperative. In 197 (93.4%), the approach was midline suboccipital craniectomy, paramedian in 11 (5.6%) and retrosigmoid in 3 (1.3%) cases. 209 patients underwent surgery (99.2%) out of which 111 (53%) underwent total resection, 61 (28.6%) near total, and 37 (17.6%) partial. 90 (42.42%) patients had brainstem involvement. 26 (12.12%) cases had cerebellar peduncle involvement, 26 (12.4%) cases had subarachnoid spread, 40 (16.5%) had PICA attachment, 8 (3.2%) had cervical canal extension. In 3 patients (1.2%), the tumor had encased the 7th/8th complex and plugged the internal auditory meatus. 167 patients underwent post CT imaging. Out of this 21 were immediate post operative and 146 were on various stages of follow up. Postoperative MR imaging was performed in 20 patients and out of whom 5 were positive for spinal metastasis. 9 patients developed air embolism during surgery which was managed efficiently by our anesthesiologist. 28 had pneumocephalus and out of which 5 patients required tapping. 15 patients developed hematoma, 1 of which was local, and 1 was subdural. All local hematomas were re-explored, and subdural hematoma required tapping. Cranial nerve injuries were noted in 56 patients, out of which 6th and 7th nerve injuries were the commonest. Brainstem injury was noted in 6 patients. CSF leak and meningitis were noted in 22 and 18 patients respectively and all of which were managed conservatively. 3 patients developed pseudomeningocele and out of which 2 required theco-peritoneal shunt. 2 patients became blind in the immediate postoperative period, and 5 patients developed persistent cerebellar mutism. The surgical mortality was 8% in this series. 2 patients died following shunt surgery without the tumor being removed. In our series, 90.5% (190) cases had classical medulloblastoma, 8.6% (18 cases) had desmoplastic variant, and 0.8% (2) cases were melanotic type.
The radiation therapy delivered is as follows: 3500 cGy to craniospinal axis and 2000 cGy to posterior fossa over a period of four to six weeks. 117 (67.3%) patients out of 174 received postoperative radiotherapy. A total of 57 (32.7%) patients received chemotherapy. Out of these, 11 (6.6%) patients received exclusive chemotherapy because they were below 3 years of age.
One-year survival rate in our series was 40.6% (85 patients). Out of the 85 patients, 52 were asymptomatic at 1-year follow up and 33 presented with some form of symptoms. 11 of the 33 underwent repeat surgery, 7 for local recurrence and 4 for residual tumor. 3 cases were operated for spinal metastasis. The 5-year survival rate in our series was found to be 33.2% (44 patients). 11.9% (29 patients) were recurrence-free at 5 years. The remaining 21.2% (16 patients) had some form of recurrence or metastasis, supratentorial/spinal/systemic. Local recurrence was seen in 13.9% patients and all of which were re-explored. 5 were operated for spinal metastasis. The 10-year survival rate in our series was 14.9%. In our series, supratentorial metastasis was seen in 4.9% (10 cases) Spinal metastasis was seen in 6.4% (13 patients) and of which 5 were operated in the first year and 8 after 5 year follow up. The rate of detection of spinal metastasis in our series was less as compared to others because patients were not investigated thorough. 8.2% (17 patients) presented with systemic metastasis in our series.
In patients following for long term, 6 had a low intellectual score, 4 had endocrinological abnormalities in form of hypopituitarism, and 3 had short stature. All the above sequelae were related to radiotherapy. In our series, the quality of life in 97 children who survived 5-10 years after treatment were grouped as follows: group 1-36 cases, group 2-50 cases, group 3-6 cases and group 4-5 cases.
Analysis of our series of 154 cases of surgically treated medulloblastoma (2001-2010)
The most common age group affected was between 3 and 12 years amounting for 84.4% [Figure 1]. There were 12 patients below the age of 1 year. In our series 103 (67%) were males and 51 (33%) females, with a male to female ratio of 2:1. 75.3% presented with headaches, vomiting and 63.2% with papilledema. Nuchal pain as one of the presenting features was seen in 10% of patients. Diminution of vision and diplopia was noted in 25.6 and 18.3% respectively. 3 patients were blind. Cerebellar symptoms were seen in 56% cases. 4.2% patients presented with lower cranial nerve symptoms. 62 (40.6%) patients were having symptoms of less than one-month duration. There was no correlation between the duration of symptoms (more or less than 6 weeks) and survival. Truncal ataxia in association with nystagmus was most prevalent (45.2%), followed by appendicular ataxia with nystagmus in 21.3%. 64 patients underwent preoperative CT imaging and 151 patients had MR imaging. 140 patients had a midline vermian lesion, which accounted for 91%. The other sites in our series were cerebellar hemisphere 8%, cerebellopontine angle and petrous region 0.5%, superior vermian/pineal region 0.5%. Involvement of brainstem and leptomeningeal spread was seen in 31.4 and 5.2% respectively. Metastasis was seen in 2.7% of patients. Hydrocephalus was noted in 96.5% patients, out of which 92% were of moderate to severe type. The total number of patients who underwent MR imaging preoperatively were 151. A total of 8 patients underwent shunting; all of them were in the postoperative period (5.19%). In 140 (91%) the approach was midline suboccipital craniectomy, paramedian in 12 (8%) and retrosigmoid in remaining 2 cases. 154 patients underwent surgery (100%) and out of which 110 (72%) underwent total resection and 44 patients (28%) had near total resection. In patients who underwent near total resection, 17 (38%) patients had brainstem involvement. 12 (28.2%) cases had cerebellar peduncle involvement, 5 (11.8%) cases had subarachnoid spread, 6 (13.6%) had PICA attachment, 5 (11.3%) had cervical canal extension. 84 patients underwent MR imaging in the immediate postoperative period while 101 patients underwent CT imaging. In our series, 92.2% (142 cases) had classical medulloblastoma, 5.1% (8 cases) had desmoplastic variant, 1.9% (3 cases) had anaplastic changes and 0.6% (1 case) had glial differentiation [Figure 2]. 151 patients underwent MR imaging in various stages of follow up. Postoperative MR imaging was performed in all patients and out of whom 8 were positive for spinal metastasis. 4 patients developed air embolism during surgery. 13 had pneumocephalus and out of which 4 patients required tapping. There was no postoperative hematoma in any patient. Cranial nerve injuries were noted in 14 patients. Brainstem injury was noted in 3 patients. CSF leak and meningitis were noted in 5 and 11 patients respectively and all of which were managed conservatively. 5 patients developed pseudomeningocele and of whom 3 required theco-peritoneal shunt. Two patients had persistent cerebellar mutism. The mortality rate was 2%.
Figure 1.
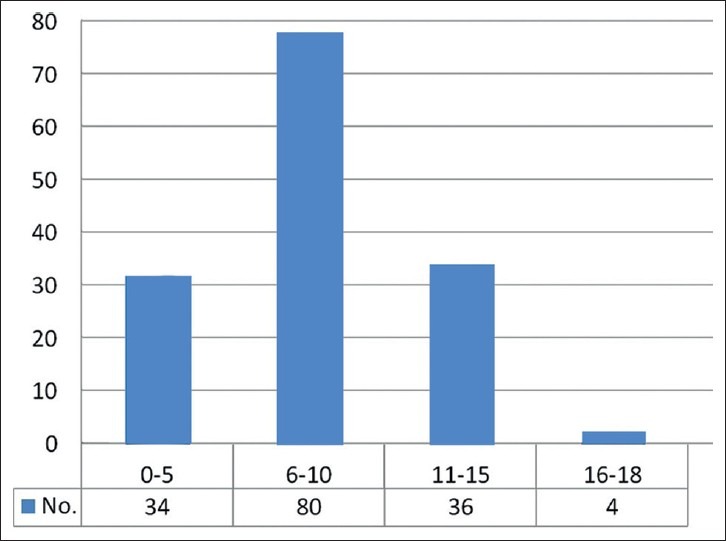
Age characteristics of medulloblastoma patients (n = 154) [2001-2010]
Figure 2.
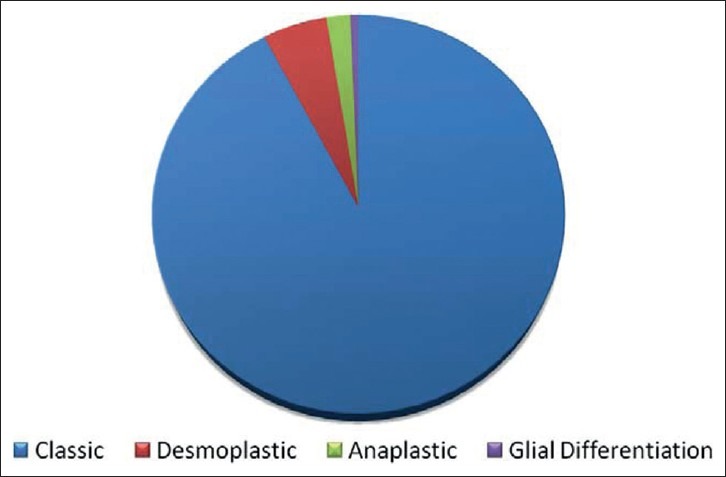
Histological subtype (n = 154)-(2001-2010)
The radiation therapy dose for average risk medulloblastoma was 3500 cGy to craniospinal axis in 21 fractions and 19.8 Gy posterior fossa boost with a total dose of 54 Gy to the posterior fossa over a period of 4-6 weeks. Similarly for the high risk medulloblastoma, in addition to craniospinal irradiation, a dose intensive chemotherapy including cyclophosphamide, vincristine and cisplatinum is administered. 108 (70%) patients out of 154 received postoperative radiotherapy. A total of 52 (34%) patients received chemotherapy. 14 patients (9%) patients received exclusively chemotherapy, because they were below 3 years of age. CSF sampling 2 weeks following surgical procedure has yielded positive results in less than 50% of the patients.
The 5-year and 10-year progression free survival rate was 73 and 41% for average risk disease while for high risk disease rate it was 34%. The follow up in our series was 60% (92 patients) at the end of 2 years. Patients under 3 years old at the time of diagnosis (24 cases) who had undergone surgery had a 5-year survival rate of 32.6%. The difference in survival between the two age groups was statistically significant (P<0.05). 18 patients amongst 92 had disease relapse at the end of 2 years. 6 amongst the 18 underwent repeat surgery, 2 for local recurrence and 4 for residual tumor. One patient, a 10-year-old male child who presented with raised intracranial pressure, showed additional two separate isodense well-enhancing tumors, one in the posterior third ventricle in the region of the suprapineal recess and other in the floor of the third ventricle which obliterated all the suprasellar cisternal spaces in addition to the vermian mass.[18] Another long-term survivor of medulloblastoma, a 13-year-old female child, presented with a left Broca's area tumor 8 years later which was diagnosed as gliosarcoma.[19]
Quality of life
Quality of life was divided into following groups[17]
-No disability, active life.
-Mild disability, active life. (there may be ocular paresis, limited intention tremor and mild ataxia)
-Partial disability (severely ataxic or have seriously reduced vision, but all are capable of self-care).
-Total disability (incapable of self-care).
It was enhanced in patients who survived 5-10 years after treatment. They were grouped as follows: Group I- 42 cases, group II- 43 cases, group III- 4 cases and group IV- 3 cases.
Surgical technique
The surgery is performed in a sitting position. In children between 1 and 3 years of age, a special indigenously designed chair is used for sitting position. Pediatric size pins are used for fixation if the skull is adequately thick or plaster of paris mould technique is used as described by us earlier.[20,21] An esophageal stethoscope and end tidal CO2 monitoring is done to detect air embolism. Steroids and broad-spectrum antibiotics were administered. An intraoperative controlled drainage of CSF through a parietal burr hole is carried out depending upon surgeon's preference. It maintains brain relaxation and eases cerebellar retraction. The skin incision is taken from the inion till C2. The subocciptial muscles are retracted and a midline suboccipital craniectomy is performed. C1 arch was not excised in any case. The wound is continuously irrigated with saline to prevent any episode of air embolism. The dura mater is reflected superiorly and the cisterna magna is opened to drain cerebrospinal fluid and slacken the brain. The surgery is performed under high magnification microscope using microsurgical techniques. The shortest and most linear approach is taken. Usually, the inferior vermian is spilt and internal debulking of the tumor is performed. The tumor is soft, suckable and moderately vascular. After adequate debulking is performed, the superior pole of the tumor is held with forceps and the tumor capsule is separated from the region around the aqueduct. As soon as the aqueduct is seen following the decompression of the superior pole of the tumor, a gelfoam or a small cottonoid should be kept to plug the aqueduct. This will prevent large amount of mixture of air and nitrous oxide anesthetic gas from entering the ventricles and the subarachnoid spaces causing expansion enough to cause raised intracranial pressure in the postoperative period. Tumor decompression slackens both the cerebellar hemispheres, consequently reducing the need for prolonged cerebellar retraction. In some situations, the placement of malleable retractors can be completely avoided. The dissection is then carried inferiorly and laterally in the region of the middle cerebellar peduncles and finally around the lower pons and the medullary region. A safe maximal excision of the tumor is performed. It is prudent to leave behind a small part of the tumor which is densely adherent to the brainstem or engulfing the posteroinferior cerebellar arteries than to risk injury to these vital structures. The entire dissection is performed by restricting the use of suction force near the brainstem and critical neurovascular structures. Following excision of the tumor, the cavity is irrigated with lukewarm saline cause egress of nitrous oxide anesthetic gas. A telovelar approach is performed for tumors presenting in the cerebellomedullary fissure. A Stein's approach is undertaken if the tumor extends in the superior vermian region and aqueduct of sylvius into the third ventricle.
Discussion
Medulloblastoma is one of the common posterior fossa tumors, predominantly seen in childhood. It has been classified as Primitive neuroectodermal tumors (PNET) since there is no identifiable precursor cell described as a ‘medulloblast’. However, the term medulloblastoma is classified as a separate entity due to its distinctive and readily recognizable histopathological features and a better prognosis compared with the supratentorial PNET's. Since its description by Cushing[22] in 1925, survival in medulloblastoma has improved tremendously due to advances in radiological diagnosis, microsurgical techniques and chemoradiation therapy. Amongst all treatment modalities, radiation therapy has been the most significant advance impacting overall survival.[23] However, the treatment-related long-term side effects including cognitive deficits, impaired psychosocial interaction and poor scholastic skills and endocrinological deficiences have led to the introduction of attenuated radiotherapy dosage and induction of chemotherapy.[23] The Chang's classification for tumor staging has recently been shown not to have an impact on survival. The advances in molecular genetics and better understanding of the biology of the disease have led to identification of molecular markers which are being currently investigated for a better risk stratification and development of new molecular targeted therapies.
A multi-institutional data on central nervous system tumors in the pediatric age group (<18 years of age) collected from the neuropathology records of seven tertiary hospitals in India report medulloblastoma as the most common brain tumors in the pediatric age group only preceded by astrocytoma.[24] The incidence of pediatric posterior fossa medulloblastoma is higher in the first decade of life, and there is a well-known male predominance.[25,26] Our series also demonstrated that the highest incidence was in the age group of 3-12 years and there was a male predominance, almost twice more common than in females.
The nosology of the tumor was established in 1925 by Cushing and Bailey.[27] Cushing[27] operated on 61 medulloblastomas by 1930 and it was clear that surgery alone did not impact survival. He was only cautiously optimistic about a posterior fossa medulloblastoma at the end of his career. Improved patient survival was only possible after the advent of craniospinal irradiation, described by Patterson and Farr in 1953.[28] Since 1960, there have been many case series which have reported an average 1 year survival rate of 63-71.4% and 5 year survival rate of 19.5-34.5%.[7–15] The recent advances in medulloblastoma stress upon the attenuation of radiotherapy dosage and dose intensive chemotherapy and the identification of molecular markers, and surgery still plays an important role in the understanding and management of the disease.
Surgery for medulloblastoma is formidable and has an inherent risk of enhanced morbidity and mortality if performed incorrectly. The surgical approach, handling of the tissues and the maneuver in which tumor is removed has impact on the ultimate outcome. Majority of the surgical series reported in literature agree upon safe maximal excision of the tumor, thus rendering survival advantage. In our series of 365 cases of medulloblastoma, there have been distinct differences in the surgical approaches and techniques from the earlier reported case series.
In major neurosurgical centers all over the world, prone or oblique position for surgery is most favored.[1,29–33] Sitting position in neurosurgery has always been a concern for the neurosurgeons and anesthesiologists since its inception. The potential for serious complications after venous air embolism and increasing litigious social environment are the main reasons for the dramatic decline in the use of the sitting position in neurosurgical practice.[33,34] There are few premier neurosurgical centers in Europe and Asia which have persisted with the practice to use sitting position for posterior fossa tumor surgery. They impress upon that posterior fossa tumor surgery in the sitting position in children is not associated with an increased number or severity of perioperative complications and the postoperative course appears better in this position.[30] In our series of 365 patients, all patients have been operated in sitting position and there has been no major compulsion to change our practice. The incidence of air embolism was 3.5%. The early detection and prompt treatment of air embolism in sitting position is possible largely due to useful adjuncts like precordial doppler, capnography, pulmonary artery catheter, and transesophageal echocardiography.[34,35] Brain stem handling requiring prolonged mechanical ventilation following surgery and seizures has been reported as a causation of sitting position during surgery.[32]
There are certain pecularities in our case series which have been pertinent to the pediatric population in the Indian subcontinent. Since ours is a tertiary care center in western India with free access to economically deprived section of the society, the patients and tumor characteristics are a unique challenge to the neurosurgeon. In our series, the size of the tumors has been massive ranging from 4 cm to 6 cm in size. The extent of hydrocephalus was moderate to severe and the patients often present with severely compromised vision and optic atrophy The patients are usually sick and there is always a greater risk of impending herniation in these patients. Limited resources allow a restricted time frame for providing effective treatment. Hence patients undergo definitive surgery for the tumor within 24 to 48 h after admission. External ventricular drainage is not a routine practice. In sitting position, following posterior fossa bone removal, the ventriculomegaly provides gravity assisted centrifugal force allowing extrusion of tumor and aid its removal.[35] An intraoperative parietal burr hole drainage of CSF is an effective option in severe hydrocephalus. During surgery, the floor of the fourth ventricle is found early in the procedure and cottonoids are used to protect the floor of the fourth ventricle at all times.[36] In our experience, due to the massive size of the tumor as seen in most of our patients as well as due to the increased pressure in the posterior fossa, it is almost impossible and presumably hazardous to place cottonoids in the floor of the fourth ventricle. Hence rapid internal debulking of the tumor is performed, the superior pole of the tumor is reached initially, tumor excised and the aqueduct is decompressed. Thus there is rapid egress of CSF and the brain is slackened. Cessation of nitrous oxide whilst in proximity to removal of tumor blocking the aqueduct, plugging of the aqueduct with an adequate sized cottonoid or temporary placement of gelfoam across the aqueduct are some of the maneuvers which can prevent or significantly reduce the massive influx of air into the intracranial cavity.[35,37] The gelfoam swells and blocks the opening of the aqueduct, thus preventing air from entering into the intracranial space.
The radiological characteristics of the tumor need to be analyzed. Most of the medulloblastomas are hyperdense on plain CT imaging suggesting increased cellularity. The signal characteristics on T2-weighted imaging are helpful in devising the surgical strategy [Figure 3]. A hyperintense tumor on T2-weighted images can be rapidly internally debulked, thus allowing the tumor capsule to involute in the operative field and creates a corridor between the cerebellum and surrounding critical neural structures, thus facilitating microneurosurgical dissection. In such cases, the need for cerebellar retraction also decreases to a significant extent which has an important function of preventing cerebellar mutism in the postoperative period. In our series, about 90% of the tumors were iso-hyperintense on T2-weighted images. Hypointensity on T2-weighted images suggests a hard consistency or calcific tumor which requires sequential piecemeal excision.
Figure 3.
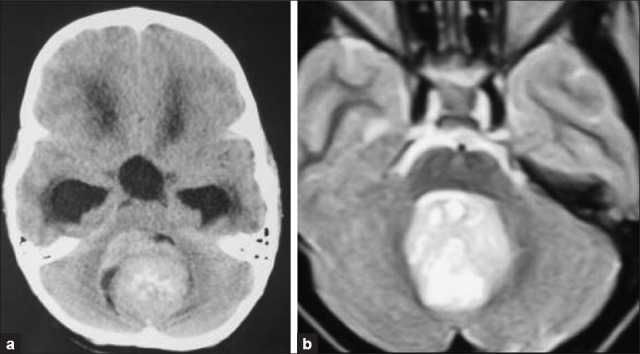
Radiological features (a) Hyperdense on plain CT imaging; (b) Hyperintense signal intensity tumor on T2-weighted MRI -soft consistency
Management of hydrocephalus in medulloblastoma
Supratentorial hydrocephalus is sequelae of posterior fossa medulloblastoma. It occurs due to aqueductal blockade. It is usually perceived that one third of patients will require CSF diversion procedure preoperatively and one third postoperatively. The remaining one third would not require any form of CSF diversion. In the earlier years, preoperative placement of a ventriculoperitoneal shunt was widely practiced.[1,7–15,36] Pre- resection endoscopic third ventriculostomy is performed in few centers.[1] However, the tangible risk of upward herniation ‘reverse coning’, hemorrhage in tumor, seedling or metastasis has lowered the incidence of preoperative CSF diversion.[38–41] A critical equilibrium is maintained between the CSF pathways and the tumor to preserve the diencephalic and brainstem function.[38,39] Supratentorial ventriculomegaly with increased CSF pressure allows the tumor to confine within a specified space. CSF diversion procedure can be suboptimal and sometimes upset this critical equilibrium, thus increasing morbidity and ensuing potential mortality. The outcome following CSF diversion procedure remains uncertain since there are no defined preoperative radiological imaging criteria to assess tumor biology. Preoperative ventricular volume has been suggested to be of predictive value for the later need for shunt placement. It is believed that following tumor removal, obstructive hydrocephalus is converted into communicating hydrocephalus, which may require surgical treatment if it exceeds a certain level of CSF volume.[42] In our early experience (1985-2000), the incidence of preoperative and postoperative CSF diversion procedure was 53.8 and 21.6% respectively. Since the last decade (2001-2010), no preoperative CSF diversion procedure was performed while the incidence of postoperative CSF diversion procedure was only 5.1%. CSF diversion procedure was indicated only in postoperative symptomatic persistent or progressive hydrocephalus. The proportion of patients requiring permanent CSF drainage for symptomatic hydrocephalus in cases with total extirpation of medulloblastoma is low. Hydrocephalus at presentation was not a statistically significant factor affecting the 5-years survival (P = 0.513).
Cushing[27] in 1930 has an operative mortality of 32% in his experience with 61 cases of medulloblastoma. On comparative analysis of our case series between 1985 and 2000 and 2000-2010, the surgical mortality has significantly reduced from 8% to 2%. The rapid advances in neuroanesthesia monitoring, refinement in microsurgical techniques and the postoperative care has been instrumental in reducing the mortality. The surgical morbidity has also significantly reduced over the last decade. Safe excision of the tumor to prevent inadvertent injury to critical neurovascular structures and judicious use of cerebellar retraction has also reduced the incidence of postoperative cerebellar mutism from 2.3% to 1.2%. The incidence of total resection has also improved from 53% to 72% which is similar to those reported in most case series.[1,7–15,43] The need to achieve safe maximal resection of the tumor was crucial since it added to a survival advantage. Consequently, the 5 and 10 year survival for average risk medulloblastoma increased from 33.2% and 14.9% to 73% and 41% respectively. This has been largely due to the advent of craniospinal radiation therapy along with a posterior fossa boost. The survival rate for high risk disease with children under the age of 3 years has been 34% due to dose intensive chemotherapy. The long-term survival for recurrent and metastatic medulloblastoma could not be commented in our series due to limited duration of follow up of these patients. The aggressive clinical behavior in patients with medulloblastomas is related to severe anaplasia and hence pathologic grading of medulloblastomas with respect to anaplasia may be useful.[44] The majority of histopathological type in our series was reported to be classical medulloblastoma followed by desmoplastic variant. It was surprising that medulloblastoma with extensive nodularity was not noted in our series in children under the age of 18 years and anaplastic medulloblastoma was not common. Desmoplastic variety had better survival rate than the classical one in our series. Hoffman[45] reported that desmoplastic subtype had poor prognosis. There was no significant difference in the total survival rate of classical to desmoplastic variety.[46]
Survivors of childhood medulloblastoma have long-term deficits in intelligence, memory, language, attention, academic skills, psychosocial function, and a compromised quality of life.[47,48] The quality of life issue was inversely proportional to the survival advantage gained by the improvements in craniospinal radiotherapy and dose intensive chemotherapy. Majority of the patients led an independent life but were marred by issue relating to endocrinological dysfunction, decline in scholastic and academic performances and cognitive function. However, due to intensive rehabilitation measures and help from the Brain tumor foundation, these children are leading an independent life with enhanced interactive abilities. The current change in treatment protocols viz. attenuation of radiation dosage for craniospinal irradiation, autologous stem cell rescue and induction of dose intensive chemotherapy will determine long-term results.[49]
Conclusion
Surgery for medulloblastoma is formidable. The option of sitting position for medulloblastoma surgery is still viable. A vigilant neuroanesthesiologist and a safe surgery are necessary to achieve a good postoperative result. Radiological characteristics are helpful adjuncts for determining effective surgical strategy. Permanent CSF drainage can be avoided in majority of patients and can be definitively considered in progressive symptomatic hydrocephalus. A safe maximal resection and a good Karnofsky score are paramount to ensure compliance with adjuvant therapy and contribute to an overall survival advantage.
Acknowledgments
The authors are grateful to Dr. Rakesh Jalali and Dr. Tejpal Gupta from Tata Memorial hospital for sharing valuable information regarding adjuvant therapy of medulloblastoma.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Muzumdar D, Ventureyra EC. Treatment of posterior fossa tumors in children. Expert Rev Neurother. 2010;10:525–46. doi: 10.1586/ern.10.28. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar C, Deb P, Sharma MC. Medulloblastomas: New directions in risk stratification. Neurol India. 2006;54:16–23. doi: 10.4103/0028-3886.24696. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Rood BR, MacDonald TJ. Medulloblastoma: Present concepts of stratification into risk groups. Pediatr Neurosurg. 2003;39:60–7. doi: 10.1159/000071316. [DOI] [PubMed] [Google Scholar]
- 4.Ellison DW, Clifford SC, Gajjar A, Gilbertson RJ. What's new in neuro-oncology. Recent advances in medulloblastoma? Eur J Paediatr Neurol. 2003;7:53–66. doi: 10.1016/s1090-3798(03)00014-x. [DOI] [PubMed] [Google Scholar]
- 5.Gilbertson RJ. Medulloblastoma: Signalling a change in treatment. Lancet Oncol. 2004;5:209–18. doi: 10.1016/S1470-2045(04)01424-X. [DOI] [PubMed] [Google Scholar]
- 6.Kombogiorgas D, Sgouros S, Walsh AR, Hockley AD, Stevens M, Grundy R, et al. Outcome of children with posterior fossa medulloblastoma: A single institution experience over the decade 1994-2003. Childs Nerv Syst. 2007;23:399–405. doi: 10.1007/s00381-006-0258-5. [DOI] [PubMed] [Google Scholar]
- 7.Modha A, Vassilyadi M, George A, Kuehn S, Hsu E, Ventureyra E. Medulloblastoma in children-the Ottawa experience. Childs Nerv Syst. 2000;16:341–50. doi: 10.1007/s003810050529. [DOI] [PubMed] [Google Scholar]
- 8.Kuhl J. Modern treatment strategies in medulloblastomas. Childs Nerv Syst. 1998;14:2–5. doi: 10.1007/s003810050164. [DOI] [PubMed] [Google Scholar]
- 9.David KM, Casey AT, Hayward RD, Harkness WF, Phipps K, Wade AM. Medulloblastoma: Is the 5-year survival rate improving. A review of 80 cases from a single institution? J Neurosurg. 1997;86:13–21. doi: 10.3171/jns.1997.86.1.0013. [DOI] [PubMed] [Google Scholar]
- 10.Di Rocco C, Ianelli A, Pappaci F, Tamburrini G. Prognosis of medulloblastoma in children. Child Nerv Syst. 1997;13:388–96. doi: 10.1007/s003810050106. [DOI] [PubMed] [Google Scholar]
- 11.Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P. Effects of medulloblastoma resections on outcome in children: A report from the Children's Cancer Group. Neurosurgery. 1996;38:265–71. doi: 10.1097/00006123-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Sutton LN, Phillips PC, Molloy PT. Surgical management of medulloblastoma. J Neurooncol. 1996;29:9–21. doi: 10.1007/BF00165514. [DOI] [PubMed] [Google Scholar]
- 13.Gajjar A, Sanford RA, Bhargava R, Heideman R, Walter A, Li Y, et al. Medulloblastoma with brain stem involvement: The impact of gross total resection on outcome. Pediatr Neurosurg. 1996;25:182–7. doi: 10.1159/000121121. [DOI] [PubMed] [Google Scholar]
- 14.Vassilyadi M, Farmer JP, Montes JL, Choi S, Blundell JE, Meagher-Villemure K. Medulloblastoma in children. Can J Neurol Sci. 1995;22:S34. [Google Scholar]
- 15.Rutka HT, Hoffman HJ. A critical review of medulloblastoma: From a difficult past to a promising future. Neurosurg Q. 1991;1:54–78. [Google Scholar]
- 16.Chang CH, Housepian EM, Herbert C., Jr An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–9. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 17.Bloom HJ, Wallace EN, Henk JM. The treatment and prognosis of medulloblastoma in children. A study of 82 verified cases. Am J Roentgenol Radium Ther Nucl Med. 1969;105:43–62. doi: 10.2214/ajr.105.1.43. [DOI] [PubMed] [Google Scholar]
- 18.Chagla A, Muzumdar DP. Retrograde cerebrospinal fluid metastasis in a vermian medulloblastoma. Neurol India. 2001;49:415. [PubMed] [Google Scholar]
- 19.Malde R, Jalali R, Muzumdar D, Shet T, Kurkure P. Gliosarcoma occurring 8 years after treatment for a medulloblastoma. Childs Nerv Syst. 2004;20:243–6. doi: 10.1007/s00381-003-0850-x. [DOI] [PubMed] [Google Scholar]
- 20.Muzumdar DP. Simple technique of head fixation for image-guided neurosurgery in infants. Childs Nerv Syst. 2007;23:611. doi: 10.1007/s00381-007-0318-5. [DOI] [PubMed] [Google Scholar]
- 21.Muzumdar DP, Bhatjiwale MG, Goel A. Plaster of Paris mould for stereotactic frame fixation. Pediatr Neurosurg. 2005;41:229–32. doi: 10.1159/000087478. [DOI] [PubMed] [Google Scholar]
- 22.Bailey P, Cushing H. Medulloblastoma cerebelli. A common type of midcerebellar glioma of childhood. Arch Neurol Neurosurg Psychiatry. 1925;14:192–224. [Google Scholar]
- 23.Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group Study. J Clin Oncol. 1999;17:2127–36. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 24.Jain A, Sharma MC, Suri V, Kale SS, Mahapatra AK, Tatke M, et al. Spectrum of pediatric brain tumors in India: A multi-institutional study. Neurol India. 2011;59:208–11. doi: 10.4103/0028-3886.79142. [DOI] [PubMed] [Google Scholar]
- 25.Kaderali Z, Lamberti-Pasculli M, Rutka JT. The changing epidemiology of paediatric brain tumours: A review from the Hospital for sick children. Childs Nerv Syst. 2009;25:787–93. doi: 10.1007/s00381-008-0771-9. [DOI] [PubMed] [Google Scholar]
- 26.Makino K, Nakamura H, Yano S, Kuratsu J Kumamoto Brain Tumor Group. Population-based epidemiological study of primary intracranial tumors in childhood. Childs Nerv Syst. 2010;26:1029–34. doi: 10.1007/s00381-010-1126-x. [DOI] [PubMed] [Google Scholar]
- 27.Kunschner LJ. Harvey Cushing and medulloblastoma. Arch Neurol. 2002;59:642–5. doi: 10.1001/archneur.59.4.642. [DOI] [PubMed] [Google Scholar]
- 28.Paterson E, Farr RF. Cerebellar medulloblastoma: Treatment by irradiation of the whole central nervous system. Acta Radiol. 1953;39:323–36. doi: 10.3109/00016925309136718. [DOI] [PubMed] [Google Scholar]
- 29.Porter JM, Pidgeon C, Cunningham AJ. The sitting position in neurosurgery: A critical appraisal. Br J Anaesth. 1999;82:117–28. doi: 10.1093/bja/82.1.117. [DOI] [PubMed] [Google Scholar]
- 30.Orliaguet GA, Hanafi M, Meyer PG, Blanot S, Jarreau MM, Bresson D, et al. Is the sitting or the prone position best for surgery for posterior fossa tumours in children? Paediatr Anaesth. 2001;11:541–7. doi: 10.1046/j.1460-9592.2001.00733.x. [DOI] [PubMed] [Google Scholar]
- 31.Kikuta KI, Miyamoto S, Kataoka H, Satow T, Yamada K, Hashimoto N. Use of the prone oblique position in surgery for posterior fossa lesions. Acta Neurochir (Wien) 2004;146:1119–24. doi: 10.1007/s00701-004-0337-x. [DOI] [PubMed] [Google Scholar]
- 32.Rath GP, Bithal PK, Chaturvedi A, Dash HH. Complications related to positioning in posterior fossa craniectomy. J Clin Neurosci. 2007;14:520–5. doi: 10.1016/j.jocn.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Engelhardt M, Folkers W, Brenke C, Scholz M, Harders A, Fidorra H, et al. Neurosurgical operations with the patient in sitting position: Analysis of risk factors using transcranial Doppler sonography. Br J Anaesth. 2006;96:467–72. doi: 10.1093/bja/ael015. [DOI] [PubMed] [Google Scholar]
- 34.Aleksic V, Radulovic D, Milakovic B, Nagulic M, Vucovic D, Antunovic V, et al. A retrospective analysis of anesthesiologic complications in pediatric neurosurgery. Paediatr Anaesth. 2009;19:879–86. doi: 10.1111/j.1460-9592.2009.03096.x. [DOI] [PubMed] [Google Scholar]
- 35.Muzumdar DP. Ventricular CSF drainage and medulloblastoma. Pediatr Neurosurg. 2007;43:74–5. doi: 10.1159/000097533. [DOI] [PubMed] [Google Scholar]
- 36.Raimondi AJ, Tomita T. Medulloblastoma in childhood: Comparative results of partial and total resection. Childs Brain. 1979;5:310–28. [PubMed] [Google Scholar]
- 37.Muzumdar D. Surgery in sitting position in patient with ventriculoperitoneal shunt in situ may be hazardous! Childs Nerv Syst. 2009;25:1531–2. doi: 10.1007/s00381-009-0992-6. [DOI] [PubMed] [Google Scholar]
- 38.Elgamal EA, Richards PG, Patel UJ. Fatal haemorrhage in medulloblastoma following ventricular drainage. Case report and review of the literature. Pediatr Neurosurg. 2006;42:45–8. doi: 10.1159/000089509. [DOI] [PubMed] [Google Scholar]
- 39.Muzumdar DP, Bhatjiwale MG, Goel A. Death following ventricular cerebrospinal fluid shunting in supratentorial malignant tumor associated with hydrocephalus. Neurol India. 2004;52:284–6. [PubMed] [Google Scholar]
- 40.Goel A. Preoperative shunts in thalamic tumours. Neurol India. 2000;48:347–50. [PubMed] [Google Scholar]
- 41.Goel A. Whither preoperative shunts for posterior fossa tumours? Br J Neurosurg. 1993;7:395–9. doi: 10.3109/02688699309103494. [DOI] [PubMed] [Google Scholar]
- 42.Kombogiorgas D, Natarajan K, Sgouros S. Predictive value of preoperative ventricular volume on the need for permanent hydrocephalus treatment immediately after resection of posterior fossa medulloblastomas in children. J Neurosurg Pediatr. 2008;1:451–5. doi: 10.3171/PED/2008/1/6/451. [DOI] [PubMed] [Google Scholar]
- 43.Rutka JT, Hoffman HJ. Medulloblastoma: A historical perspective and overview. J Neurooncol. 1996;29:1–7. doi: 10.1007/BF00165513. [DOI] [PubMed] [Google Scholar]
- 44.Eberhart CG, Kepner JL, Goldthwaite PT, Kun LE, Duffner PK, Friedman HS, et al. Histopathologic grading of medulloblastomas: A Pediatric Oncology Group study. Cancer. 2002;94:552–60. doi: 10.1002/cncr.10189. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman MD, Park T. Medulloblastoma: Clinical presentation and management. J Neurosurg. 1983;8:543–52. doi: 10.3171/jns.1983.58.4.0543. [DOI] [PubMed] [Google Scholar]
- 46.Anthony J, Caputy MD, David C. A review of the factors influencing the prognosis of medulloblastoma. J Neurosurg. 1987;66:80–7. doi: 10.3171/jns.1987.66.1.0080. [DOI] [PubMed] [Google Scholar]
- 47.Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: A longitudinal analysis. J Clin Oncol. 2001;19:2302–8. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 48.Dennis M, Spiegler BJ, Hetheringto CR, Greenberg M. Neuropsychological sequela of the treatment of children with medulloblastome. J Neurooncol. 1996;29:91–100. doi: 10.1007/BF00165522. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Martinez A, Lassaletta A, Gonzalez-Vicent M, Sevilla J, Diaz MA, Madero L. High-dose chemotherapy with autologous stem cell rescue for children with high risk and recurrent medulloblastoma and supratentorial primitive neuroectodermal tumors. J Neurooncol. 2005;71:33–8. doi: 10.1007/s11060-004-4527-4. [DOI] [PubMed] [Google Scholar]


