Abstract
Objective:
To examine vascular risk factors, as measured by the Framingham Stroke Risk Profile (FSRP), to predict incident cognitive impairment in a large, national sample of black and white adults age 45 years and older.
Methods:
Participants included subjects without stroke at baseline from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study with at least 2 cognitive function assessments during the follow-up (n = 23,752). Incident cognitive impairment was defined as decline from a baseline score of 5 or 6 (of possible 6 points) to the most recent follow-up score of 4 or less on the Six-item Screener (SIS). Subjects with suspected stroke during follow-up were censored.
Results:
During a mean follow-up of 4.1 years, 1,907 participants met criteria for incident cognitive impairment. Baseline FSRP score was associated with incident cognitive impairment. An adjusted model revealed that male sex (odds ratio [OR] = 1.59, 95% confidence interval [CI] 1.43–1.77), black race (OR = 2.09, 95% CI 1.88–2.35), less education (less than high school graduate vs college graduate, OR = 2.21, 95% CI 1.88–2.60), older age (10-year increments, OR = 2.11, per 10-year increase in age, 95% CI 2.05–2.18), and presence of left ventricular hypertrophy (LVH, OR = 1.29, 95% CI 1.06–1.58) were related to development of cognitive impairment. When LVH was excluded from the model, elevated systolic blood pressure was related to incident cognitive impairment.
Conclusions:
Total FSRP score, elevated blood pressure, and LVH predict development of clinically significant cognitive dysfunction. Prevention and treatment of high blood pressure may be effective in preserving cognitive health.
Vascular risk factors like hypertension and diabetes are common among older adults,1,2 affect brain structure,3 and have been associated with incident cognitive decline,4 incident cognitive impairment,5–7 and incident dementia.8–10 The Framingham Stroke Risk Profile (FSRP) provides an estimate of the 10-year risk for future stroke based on age and presence and severity of several cardiovascular risk factors.11,12 Among stroke-free individuals, high FSRP score is related to lower cognitive function.13,14
We examined the relation of the FSRP and its components in predicting incident cognitive impairment, using a brief and easily administered cognitive screening test, in a large, demographically and regionally diverse sample of older adults in the continental United States. The FSRP score and its components were selected by the Framingham group to be most predictive of stroke. While it is likely that the coefficients for cognitive impairment will differ somewhat, our interest is to determine if the formula will also capture cognitive impairment and if so which, if any, of the component scores selected to be predictive for stroke also perform well in the prediction of cognitive impairment. We hypothesized that FSRP total score and its components would be related to incident cognitive impairment.
METHODS
Design and sampling frame.
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study is a national, population-based, longitudinal cohort study designed to investigate the factors associated with the excess stroke mortality observed among African Americans and residents of the Southeastern stroke belt region (see15 for details). Participants were randomly selected from commercially available lists. Community-dwelling individuals aged 45 years or older, and either African American or white, were eligible for the study. Exclusion criteria included self-reported medical conditions (such as cancer) that would prevent long-term participation, or being on a waiting list for a nursing home. The sample size was calculated to provide sufficient incident stroke events to detect relatively small risk effects. The sample was recruited between January 2003 and December 2007 using mail and telephone contact (33% response rate, 49% cooperation rate16). Enrollment resulted in a cohort of 30,239 individuals with 56% residents in stroke belt states (NC, SC, GA, AL, MS, TN, AR, and LA), 45% men, and 42% African American.
Standard protocol approvals, registrations, and patient consents.
Study procedures were reviewed and approved by the institutional review boards at the collaborating institutions. All subjects provided informed consent to participate in the study.
Procedures.
Demographic data (age, education [years completed], race [African American or white], and sex), health history including use of antihypertensive medications, and depressive symptoms were gathered via telephone interview at baseline. An in-home examination was used to gather physical measures including blood pressure, blood and urine samples, EKG, and an inventory of current medications. Incident stroke was ascertained via telephone follow-up every 6 months using the Questionnaire for Verifying Stroke-free Status17 and verified by medical record review and adjudication by a panel of neurologist stroke experts.
Measures.
The Six-item Screener (SIS) is a global measure of cognitive status that assesses 3-item recall and orientation to year, month, and day of the week.18 Scores range from 0 to 6 with a score of 4 or fewer correct indicative of cognitive impairment. The SIS was first administered at baseline in REGARDS in December 2003 and then annually to all participants. Incident cognitive impairment was defined as decline from an initial score of 5 or better to the most recent follow-up score of 4 or less. The SIS has been validated against clinical diagnoses of dementia and mild cognitive impairment (74% sensitivity and 80% specificity for both groups combined vs cognitively normal elders).18 The SIS has been used to document cognitive impairment in older patients seen in emergency departments19 and older depressed patients in a large randomized controlled trial.20 SIS scores are related to self-reported stroke symptoms and health behaviors,21 cardiovascular risk factors,22,23 and kidney dysfunction.24 Self-reported depressive symptoms were measured with the Center for Epidemiologic Studies–Depression (CES-D) scale, 4-item version.25
The FSRP11,12 was calculated as an estimate of the 10-year risk of stroke. It incorporates age, measured systolic blood pressure (in mm Hg recoded into 10 groupings from 95 to 204 mm Hg), presence of diabetes mellitus, current cigarette smoking, history of heart disease, atrial fibrillation, LVH, and the use of antihypertensive medication. Diabetes was defined as fasting glucose greater than or equal to 126 mL/dL, nonfasting glucose greater than or equal to 200 mL/dL, or self-reported use of diabetes medications. Current cigarette smoking (at the baseline) and current use of antihypertension medications (at the baseline) were determined by interview. History of heart disease was determined by self-reported myocardial infarction (MI), coronary artery bypass graft, angioplasty or stenting, or evidence of MI from baseline ECG. Atrial fibrillation was defined as self-reported or via ECG evidence. LVH was defined as presence on ECG (12 lead or 7 lead).26 Given our dichotomous outcome, we did not log transform scores though some studies with a continuous outcome have used transformed scores.14
Statistical analyses.
Our aim was to relate vascular risk factors to incident cognitive impairment in an initially cognitively intact and stroke-free cohort. Of 30,239 REGARDS participants, we excluded 8 due to anomalous data, 1,931 due to self-reported stroke at baseline, 2,322 due to cognitive impairment at baseline (SIS score of 4 or fewer correct), 500 due to missing SIS measurements, 1,603 due to only 1 SIS assessment, and 113 due to incident stroke prior to first SIS assessment. Thus 23,752 participants remained for analysis. Of note, 196 participants in the remaining 23,752 subsequently had an adjudicated stroke during the follow-up. The SIS assessments for these participants were included until the time at which their stroke occurred, but were censored afterward. To examine the effect of a more stringent case definition on outcomes, we conducted a sensitivity analysis focusing on participants with a minimum of 3 SIS assessments, the last 2 with SIS <5 (n = 20,803); the pattern of results did not differ and are not presented below.
Analyses were conducted using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Descriptive statistics were computed for continuous categorical variables, and t tests or χ2 tests of association were used as appropriate to assess whether differences in baseline characteristics existed between those with and without incident cognitive impairment. Logistic regression models were used to examine whether the odds of cognitive impairment differed by demographic characteristics, FSRP total score, and by FSRP factors, in univariate models, in models adjusted only for demographic factors, and in a single multivariable model. We assessed interactions between the FSRP total score and each of race, region, and gender, in order to determine whether differences in the relationship between the FSRP total score and incident impairment differed as a function of each of these factors. In addition, we examined the interaction between SBP and LVH, and the interaction between SBP and antihypertensive medication use. A sensitivity analysis excluding those with LVH was conducted to determine the impact this had on results. Odds ratios and 95% confidence intervals were computed.
Baseline characteristics included age, sex, race, region, education (< high school, high school graduate, some college, college graduate or higher), alcohol use (National Institute on Alcohol Abuse and Alcoholism classification: none, moderate [0–7/week, women; 0–14/week, men], heavy [7+/week, women; 14+/week, men]), baseline SIS score, CESD-4, SBP, and FSRP indicators.
RESULTS
Table 1 presents the baseline characteristics overall and by final cognitive status. The average age was 64 (SD = 9.2) years and the average length of follow-up was 4.1 (SD = 1.4) years. Eleven percent of the participants completed 2 SIS assessments during the follow-up, 22% had 3 assessments, 24% had 4 assessments, 24% had 5, and 19% had 6 or more. The group of 1,907 participants with incident cognitive impairment was significantly older and more likely to be male, African American, resident in the stroke belt, and to have completed fewer years of education than the group without incident cognitive impairment. Just over 78% (1,497/1,907) of incident cognitive participants met criteria by a decline of 2 or more points in the SIS. Baseline SIS scores were only slightly lower in the incident cognitive impairment group (mean of 5.6 vs 5.8) compared to the no decline group. The incident cognitive impairment group also had a slightly higher depressive symptoms score, and less alcohol use than the group with no cognitive decline.
Table 1.
Baseline characteristics
Abbreviations: CES-D-4 = 4-item Center for Epidemiologic Studies–Depression scale; SIS = Six-item Screener.
Difference between incident cognitive impairment and no cognitive impairment.
Stroke belt = North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas.
Stroke buckle = coastal plains of North Carolina, South Carolina, and Georgia.
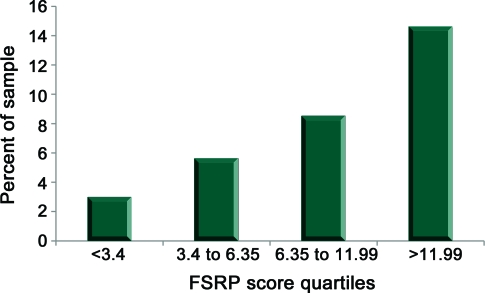
Table 2 shows the mean scores for the FSRP and systolic blood pressure and percent with component FSRP conditions for the whole sample and by incident cognitive impairment status. The total FSRP score and each of the FSRP factors, except current smoking, were related to incident cognitive decline. Specifically, the group with incident cognitive impairment had higher systolic blood pressure, more use of antihypertensive medications, and higher prevalence of diabetes, LVH, atrial fibrillation, and history of heart disease than the group that stayed cognitively intact. The figure depicts the frequency of incident cognitive impairment according to FSRP score quartile. A total of 21,936 participants had a FSRP score and 1,732 were cognitively impaired (1,816 participants did not have a total FSRP score due to missing one of the components). The rate of impairment increases in a nearly linear fashion across the FSRP quartiles to 14.5% in the highest quartile.
Table 2.
Framingham Stroke Risk Profile factors at baseline
Difference between incident cognitive impairment and no cognitive impairment.
Measured blood pressure in mm Hg recoded into 10 groupings from 95 to 204 mm Hg.
Figure. Percent incident cognitive impairment by Framingham Stroke Risk Profile (FSRP) score quartiles.
A total of 21,936 participants had a FSRP score and 1,732 were cognitively impaired (1,816 participants did not have a total FSRP score due to missing one of the components).
Table 3 presents the results from the fitted logistic regression models. In the univariate models, each of the demographic and FSRP factors, with the exception of current smoking, was related to incident cognitive impairment, and remained so after adjustment for demographic factors. After multivariable adjustment, the demographic factors (male sex, black race, stroke belt residence, and less education) and only the FSRP factors of older age and presence of LVH were significantly related to incident cognitive impairment. A separate multivariable analysis excluding subjects with LVH revealed that higher systolic blood pressure (odds ratio 1.04 for each 10-mm Hg increase, 95% confidence interval 1.02–1.06) and age (odds ratio 2.10 for each 10-year increase, 95% confidence interval 2.04–2.17) were related to incident cognitive impairment.
Table 3.
Odds of incident cognitive impairment as a function of demographics, region, and Framingham Stroke Risk Profile component scores (n = 23,752)
Abbreviations: CI = confidence interval; FSRP = Framingham Stroke Risk Profile; OR = odds ratio.
Includes sex, race, region, and education.
After adjusting for each of the other variables in the table.
95% CI that does not include 1.0.
The mean (SD) baseline FSRP score was 15.2 (13.3) in those who developed cognitive impairment and 9.1 (9.5) in those who did not. For each SD higher baseline FSRP score, the risk of incident cognitive impairment increased by 41% (95% confidence interval 37%–46%) after adjustment for demographic factors. None of the interactions assessed were statistically significant.
DISCUSSION
In a large, national sample that was stroke-free and cognitively normal at baseline, followed for an average of 4 years, and culled of participants who developed clinical stroke in the interval, FSRP score, which is composed of vascular risk factors, was linearly related to rate of incident cognitive impairment. In the highest FSRP quartile (scores >11.99), almost 3 in 20 participants developed incident cognitive impairment during the follow-up.
All the elements of the FSRP are significant predictors of cognitive impairment individually, and the more individual risk factors a person has, the greater the risk of cognitive impairment. Age and presence of LVH were the only FSRP component factors independently associated with future development of cognitive impairment. The association between LVH and cognitive impairment remained after controlling for age, sex, race, region of residence, and education. Consistent with the notion that LVH is a late developing marker of long-term exposure to high blood pressure,27 we also found that high systolic blood pressure was related to incident cognitive impairment in persons without LVH. This suggests that hypertension may be a very important risk factor to address in order to prevent cognitive impairment. Overall, it appears that the total FSRP score and its components, while initially derived to predict stroke, are also useful in the prediction of cognitive impairment.
Other studies have shown that increased stroke risk as measured by total FSRP score is related to lowered cognitive performance cross-sectionally13,14 and longitudinally.28 The longitudinal study28 consisted of 235 stroke- and dementia-free men at the baseline who were reassessed on a cognitive battery 3 years later. The FSRP was inversely related to verbal fluency but not word list learning, word list recall, pattern comparison, or digit span. Our study extends these findings by including a larger, more diverse population (23,752 participants, of whom 56% were female and 38% were African American), and longer follow-up (average of 4 and up to 6 years).
LVH is a pathologic reaction to cardiovascular disease including high blood pressure. Elevated blood pressure increases the load the heart contracts against and over time results in increased volume of heart muscle and functional degradation of the heart including heart failure. An earlier cross-sectional analysis of the Framingham Offspring Study cohort showed an inverse relation between left ventricular mass (as determined by heart wall thickness and chamber volume) and cognition.29 The relationship was attenuated when blood pressure was considered and eliminated when prevalent heart disease (coronary artery disease, claudication, and heart failure) and risk factors (diabetes, cholesterol, alcohol use, smoking, homocysteine, and depressed mood) were included in the modeling. Our study extends this finding by showing a longitudinal relationship between LVH and clinically significant incident cognitive impairment that is independent of other demographic and cardiovascular risk factors.
Our subgroup analysis suggested that elevations in systolic blood pressure were associated with incident cognitive impairment even in those without LVH. This is consistent with other studies of blood pressure and cognitive decline4,30,31 incident cognitive impairment,5–7 and incident dementia.9 Our data suggest an early role for elevated blood pressure in the relationship of LVH and longitudinal changes in cognition.
In contrast to other studies that reported a relationship of diabetes to cognitive decline,4,31–34 incident cognitive impairment,5,31–34 and incident dementia,8,10 diabetes was not independently associated with risk of incident cognitive impairment in this study (others have also failed to see an association35). This may be due to a limitation of the SIS in assessing cognitive impairment as a recent systematic review of prospective observational studies on diabetes and cognitive decline indicated that the broad measure MMSE (from which the SIS is derived) was less sensitive than a psychomotor speed-based cognitive test for diabetes-associated cognitive decline.36 It is also possible that diabetes as reflected in the FSRP (present vs absent), while sensitive to stroke risk, requires additional elaboration and specification in order to be a marker of cognitive decline. For example, it may be necessary to capture the duration of exposure to diabetes or the quality of treatment and control of diabetes.
Subclinical cerebrovascular disease including white matter abnormalities, silent cerebral infarction, and brain atrophy may underlie the association we saw between stroke risk factors and cognition. Other studies with neuroimaging verification in stroke-free participants with FSRP risk have found that FSRP scores are correlated with silent cerebral infarctions37 and changes in cerebral brain volume over time.38
This study has strengths including a very large, diverse sample that was free of clinical stroke at the baseline, censoring subjects at the time of incident stroke during the follow-up interval, longitudinal analysis with moderate length of follow-up interval, and use of a robust marker of clinically important cognitive dysfunction. Limitations include attrition over the follow-up interval. The attrition rate in REGARDS is about 3% per year which is not atypical for a large study with high proportion of older adults. To the extent that less cognitively able subjects were over-represented among the dropouts, a likely situation,39 our findings would underestimate the relation between cardiovascular risk factors and cognition. Our use of a global cognitive marker focused on memory means that we are unable to examine the effects of stroke risk factors on other cognitive domains sensitive to cardiovascular dysfunction including executive, psychomotor, and visuospatial function. Our definition of cognitive impairment is based on a screening test and not a clinical diagnosis of mild cognitive impairment or dementia. While screening tests such as the SIS do have reasonable correspondence to clinical diagnosis,18,40 there is some loss of precision, which would make it less likely that correlates of cognitive impairment could be detected. We found that current smoking (as coded in the FSRP) was not related to cognitive status. Since relatively few people are current smokers and former smokers are common, future research could examine smoking in a more differentiated way, for example, current smoker, former smoker, or never smoked, or smoking could be scaled in terms of pack-years. Finally, 25% of our participants received a 7-lead ECG which requires calculation of Cornell voltage using S-wave amplitude in the midsternal lead (SV) instead of SV3 in the formula to calculate LVH. While this approach to LVH has demographic and clinical associations that are similar to that calculated from a standard 12-lead ECG (using SV3),26 some loss of precision in that portion of the sample is possible, which would lead to underestimation of the relationships between LVH and cognition.
Our findings suggest that the vascular risk factors measured by the FSRP, elevated blood pressure and its long-term consequence, left ventricular hypertrophy, may provide a simple and efficient means of identifying adults who are at risk for future cognitive impairment and lends support to the notion that increased attention to prevention and treatment of high blood pressure may be effective in preserving cognitive health.
ACKNOWLEDGMENT
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
Footnotes
- CES-D
- Center for Epidemiologic Studies–Depression
- CI
- confidence interval
- FSRP
- Framingham Stroke Risk Profile
- LVH
- left ventricular hypertrophy
- MI
- myocardial infarction
- OR
- odds ratio
- REGARDS
- Reasons for Geographic and Racial Differences in Stroke
- SIS
- Six-item Screener
AUTHOR CONTRIBUTIONS
Dr. Unverzagt: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data. Dr. McClure: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, statistical analysis, obtaining funding. Dr. Wadley: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, obtaining funding. Dr. Jenny: drafting/revising the manuscript for content, including medical writing for content. Dr. Go: drafting/revising the manuscript for content, including medical writing for content. Dr. Cushman: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, obtaining funding. Dr. Kissela: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, obtaining funding. Dr. Kelley: drafting/revising the manuscript for content, including medical writing for content. Dr. Kennedy: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data. Dr. Moy: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, obtaining funding. Dr. V. Howard: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, acquisition of data, study supervision or coordination, obtaining funding. Dr. G. Howard: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision or coordination, obtaining funding.
DISCLOSURE
Dr. Unverzagt has served as a consultant to Eli Lilly and Company; serves on the editorial boards of the Journal of the International Neuropsychological Association and Neuropsychology; receives research support from the NIH and Posit Science Inc; and holds stock in Eli Lilly and Company. Dr. McClure serves on a Data Monitoring Committee for the NIH/NINDS and receives research support from Genzyme Corporation, the NIH (NINDS, NICHD, NHLBI), and NASA. Dr. Wadley has received funding for travel from Amgen; serves on the editorial board of Current Gerontology and Geriatrics Research; and receives research support from Genzyme Corporation, the NIH, and the Jefferson County Office of Senior Citizens Services. Dr. Jenny serves on the editorial board of Arteriosclerosis, Thrombosis and Vascular Biology; serves as a consultant for Tethys Bioscience, Inc.; receives research support from GlaxoSmithKline, the NIH, and the American Diabetic Association; and holds stock in Haematologic Technologies, Inc. Dr. Go receives research support from the NIH/NINDS. Dr. Cushman serves on the editorial boards of the Journal of Thrombosis and Haemostasis, Circulation, Archives of Internal Medicine, and the Journal of Thrombosis and Thrombolysis; and receives/has received research support from Amgen, GlaxoSmithKline, and the NIH. Dr. Kissela serves on scientific advisory boards for Northstar Neuroscience and Allergan, Inc.; has received funding for travel and speaker honoraria from Allergan, Inc.; has received research support from NexStim and the NIH; and provides medico-legal reviews. Dr. Kelley receives/has received research support from Novartis and the NIH. Dr. Kennedy receives research support from the NIH (NINDS, NIA, NIDDK). Dr. Moy reports no disclosures. Dr. V. Howard serves/has served on scientific advisory boards for Amgen, Boehringer-Ingelheim, Mitsubishi, PhotoThera, and MediciNova; her spouse serves on a scientific advisory boards for Bayer Schering Pharma; has received funding for travel from Amgen; serves as a consultant for NIH review committees; her spouse has provided legal consulting for Merck Serono; and receives research support from the NIH (NINDS, NIDDK, NIOSH). Dr. G. Howard serves/has served on scientific advisory boards for Bayer Schering Pharma, Abbott, Boehringer Ingelheim, BrainsGate, Cerevast Therapeutics, Inc., CoAxia, Inc., MediciNova, Inc., Mitsubishi Tanabe Pharma Corporation, and PhotoThera; serves as Stroke Section Editor for the Journal of The American Society of Hypertension; and receives research support from Amgen and the NIH (NINDS, NIAMS, NICHD, NHLBI).
REFERENCES
- 1. Ong KL, Cheung BMY, Man YB, Lau CP, Lam KSL. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 2007;49:69–75 [DOI] [PubMed] [Google Scholar]
- 2. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008 [Google Scholar]
- 3. Van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MMB. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan Study. Stroke 2008;39:2712–2719 [DOI] [PubMed] [Google Scholar]
- 4. Knopman DS, Mosley TH, Catellier DJ, Coker LH, Atherosclerosis Risk Communities Study Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement 2009;5:207–214 [DOI] [PubMed] [Google Scholar]
- 5. Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry 2006;63:273–280 [DOI] [PubMed] [Google Scholar]
- 6. Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology 2001;56:1683–1689 [DOI] [PubMed] [Google Scholar]
- 7. Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007;64:1734–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology 2003;22:13–22 [DOI] [PubMed] [Google Scholar]
- 9. Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000;21:49–55 [DOI] [PubMed] [Google Scholar]
- 10. Schrijvers EMC, Witteman JCM, Sijbrands EJG, Hofman A, Koudstaal PJ, Breteler MMB. Insulin metabolism and the risk of Alzheimer disease: The Rotterdam Study. Neurology 2010;75:1982–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication: The Framingham Study. Stroke 1994;25:40–43 [DOI] [PubMed] [Google Scholar]
- 12. Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22:312–318 [DOI] [PubMed] [Google Scholar]
- 13. Llewellyn DJ, Lang IA, Xie J, Huppert FA, Melzer D, Langa KM. Framingham stroke risk profile and poor cognitive function: a population-based study. BMC Neurol 2008;8:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elias MF, Sullivan LM, D'Agostino RB, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke 2004;35:404–409 [DOI] [PubMed] [Google Scholar]
- 15. Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–143 [DOI] [PubMed] [Google Scholar]
- 16. Howard VJ, Woolson RF, Egan BM, et al. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens 2010;4:32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meschia JF, Brott TG, Chukwudelunzu FE, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke 2000;31:1076–1080 [DOI] [PubMed] [Google Scholar]
- 18. Callahan CM, Unverzagt FW, Hui SL, Perkins A, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002;40:771–781 [DOI] [PubMed] [Google Scholar]
- 19. Wilber ST, Lofgren SD, Mager TG, Blanda M, Gerson LW. An evaluation of two screening tools for cognitive impairment in older emergency department patients. Acad Emerg Med 2005;12:612–616 [DOI] [PubMed] [Google Scholar]
- 20. Steffens DC, Snowden M, Fan MY, Hendrie H, Katon WJ, Unutzer J. Cognitive impairment and depression outcomes in the IMPACT study. Am J Geriatr Psychiatry 2006;14:401–409 [DOI] [PubMed] [Google Scholar]
- 21. Wadley VG, McClure LA, Howard VJ, et al. Cognitive status, stroke symptom reports, and modifiable risk factors among individuals with no diagnosis of stroke or transient ischemic attack in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke 2007;38:1143–1147 [DOI] [PubMed] [Google Scholar]
- 22. Tsivgoulis G, Alexandrov AV, Wadley VG, et al. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology 2009;73:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pullicino PM, Wadley VG, McClure LA, et al. Factors contributing to global cognitive impairment in heart failure: results from a population-based cohort. J Card Fail 2008;14:290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 2008;52:227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas 1993;53:1117–1125 [Google Scholar]
- 26. Soliman EZ, Howard G, Prineas RJ, McClure LA, Howard VJ. Calculating Cornell voltage from nonstandard chest electrode recording site in the Reasons for Geographic and Racial Differences in Stroke study. J Electrocardiol 2010;43:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vasan RS, Levy D. The role of hypertension in the pathogenesis of heart failure: a clinical mechanistic overview. Arch Intern Med 1996;156:1789–1796 [PubMed] [Google Scholar]
- 28. Brady CB, Spiro A, Glinchey-Berroth R, Milberg W, Gaziano JM. Stroke risk predicts verbal fluency decline in healthy older men: evidence from the Normative Aging Study. J Gerontol Ser B Psychol Sci Soc Sci 2001;56:340–346 [DOI] [PubMed] [Google Scholar]
- 29. Elias MF, Sullivan LM, Elias PK, et al. Left ventricular mass, blood pressure, and lowered cognitive performance in the Framingham Offspring. Hypertension 2007;49:439–445 [DOI] [PubMed] [Google Scholar]
- 30. Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: does age make a difference? Hypertension 2004;44:631–636 [DOI] [PubMed] [Google Scholar]
- 31. Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001;56:42–48 [DOI] [PubMed] [Google Scholar]
- 32. Crowe M, Sartori A, Clay OJ, et al. Diabetes and cognitive decline: Investigating the potential influence of factors related to health disparities. J Aging Health 2010;22:292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 2004;61:661–666 [DOI] [PubMed] [Google Scholar]
- 34. Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol 2007;64:570–575 [DOI] [PubMed] [Google Scholar]
- 35. Tervo S, Kivipelto M, Hanninen T, et al. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord 2004;17:196–203 [DOI] [PubMed] [Google Scholar]
- 36. Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes : systematic overview of prospective observational studies. Diabetologia 2005;48:2460–2469 [DOI] [PubMed] [Google Scholar]
- 37. Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham Offspring Study. Stroke 2008;39:2929–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: The Framingham Offspring Study. Neurology 2004;63:1591–1599 [DOI] [PubMed] [Google Scholar]
- 39. Euser SM, Schram MT, Hofman A, Westendorp RGJ, Breteler MMB. Measuring cognitive function with age: the influence of selection by health and survival. Epidemiology 2008;19:440–447 [DOI] [PubMed] [Google Scholar]
- 40. Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc 1992;40:922–935 [DOI] [PubMed] [Google Scholar]