Abstract
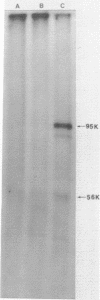
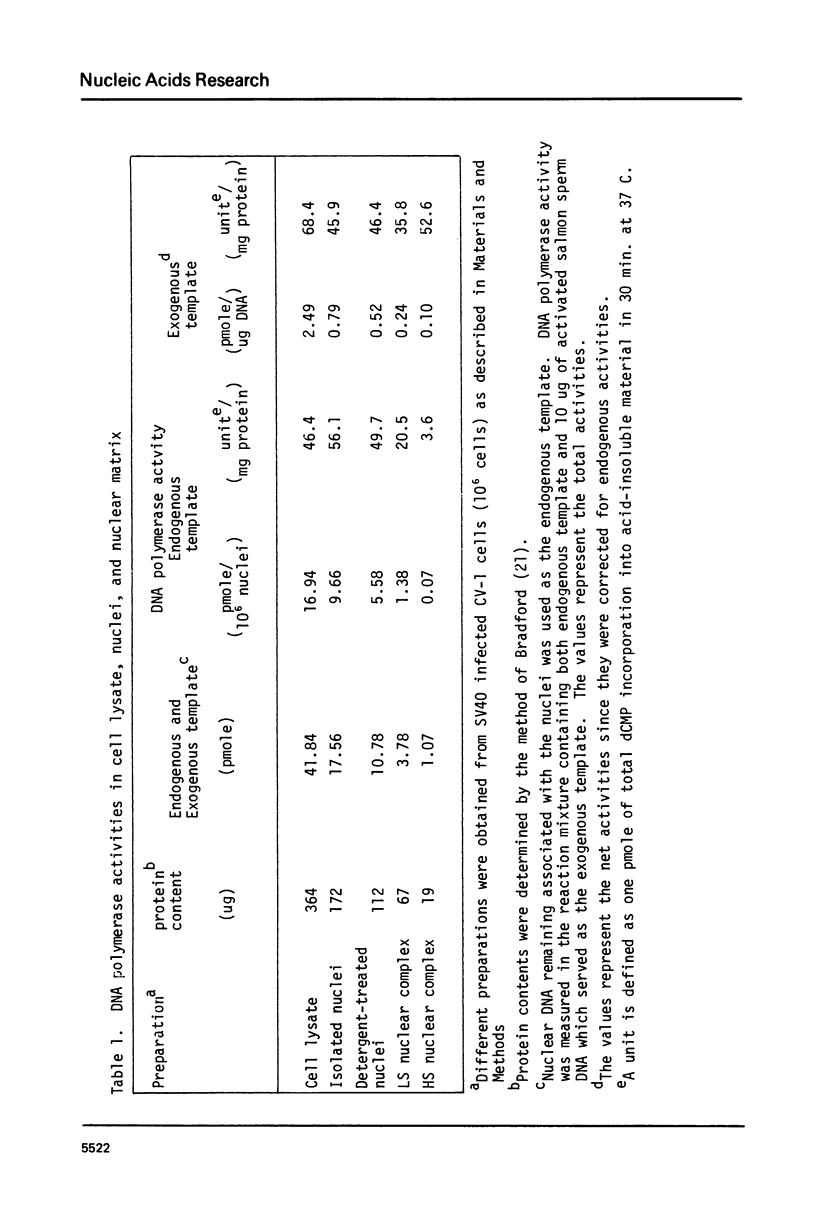
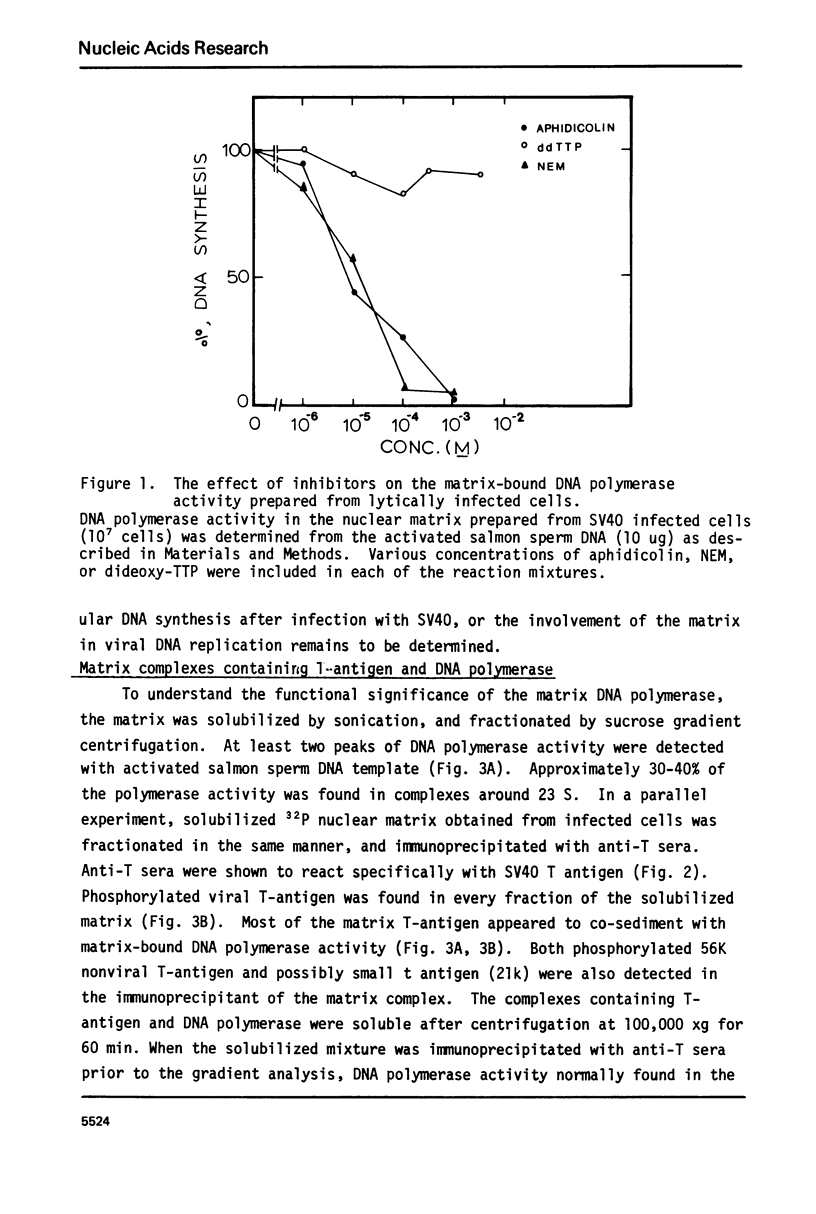
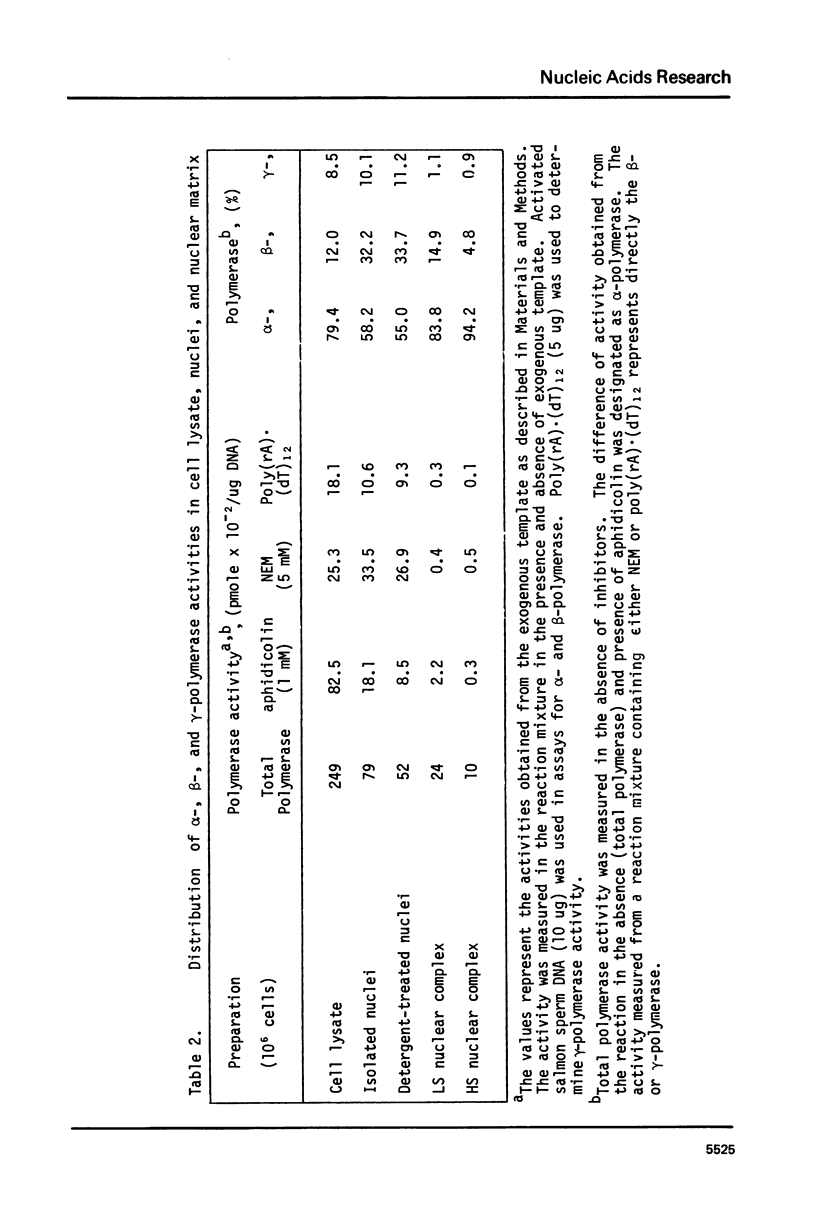
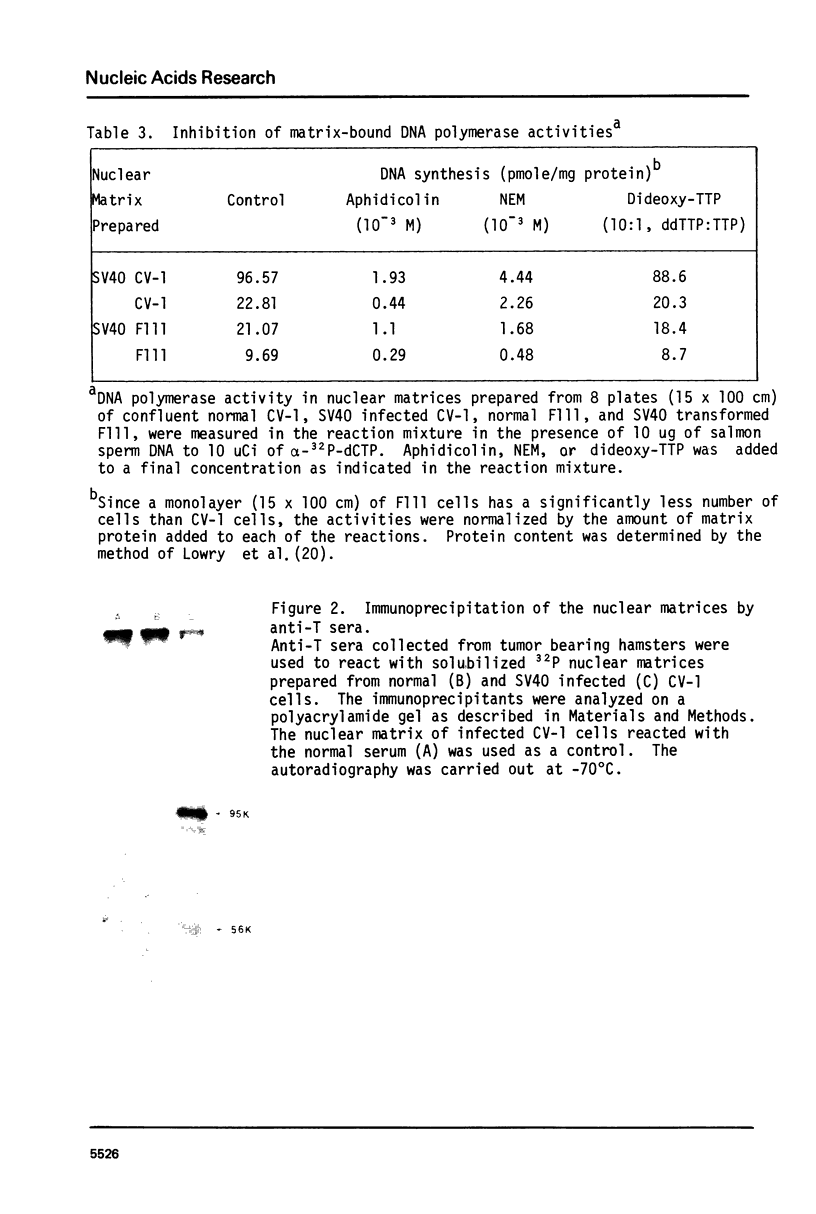
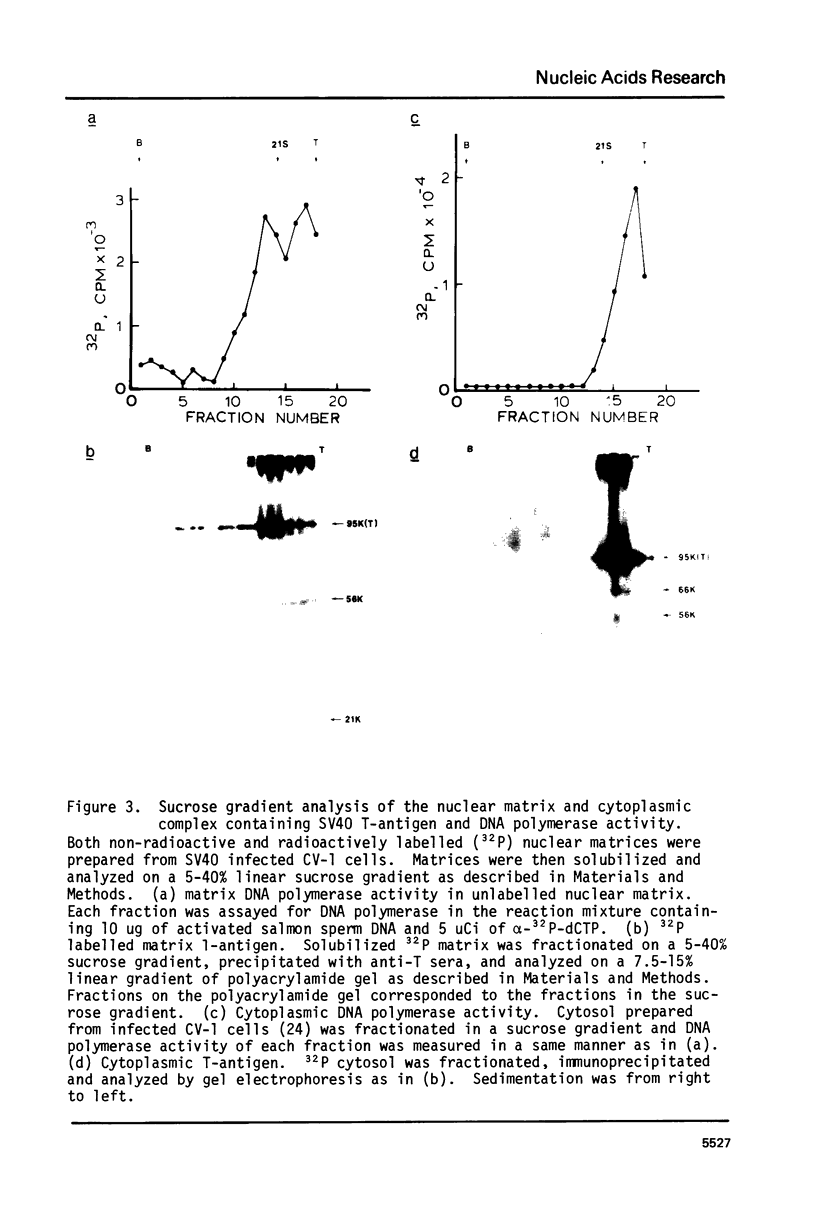
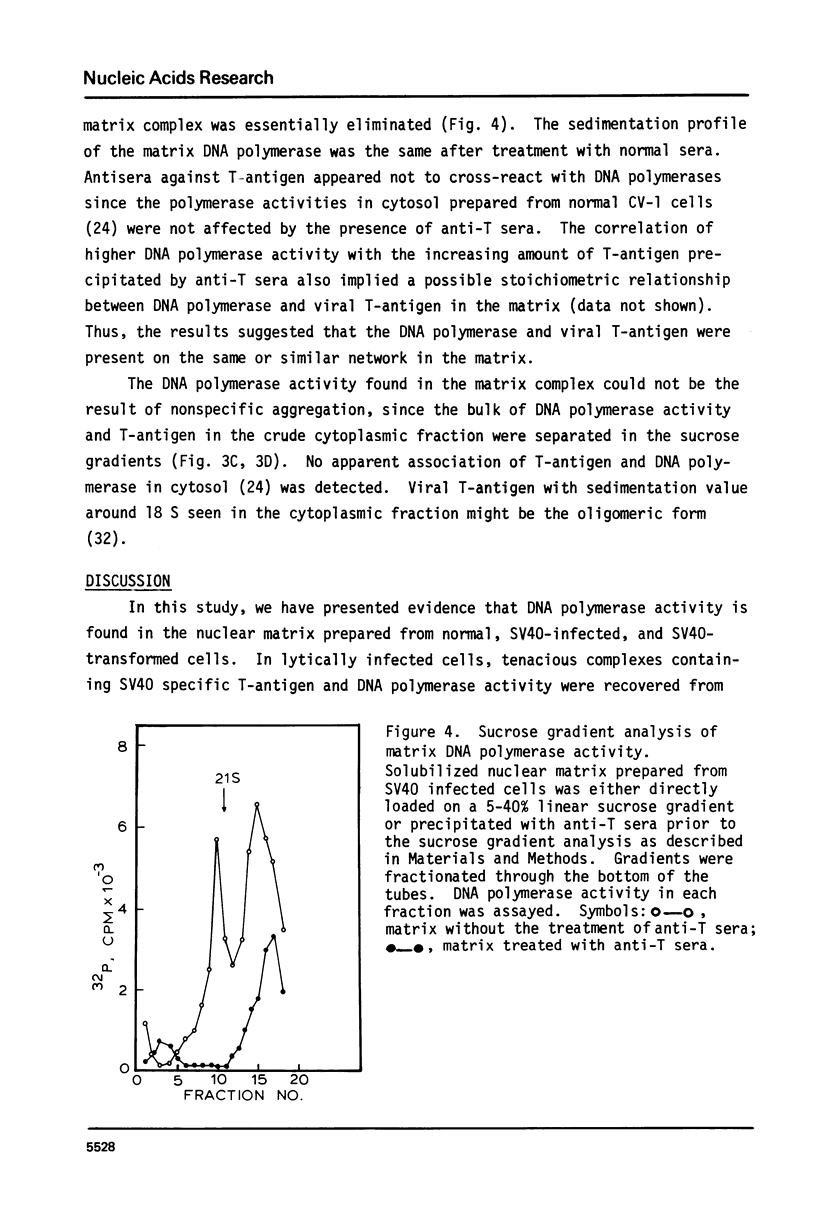
The nuclear matrix prepared from normal, simian virus 40 (SV40)-infected, and SV40-transformed cells contained DNA polymerase activities. Approximately 12% of the total DNA polymerase activities in isolated nuclei remained with the nuclear matrix. alpha-polymerase was the major matrix DNA polymerase activity as judged by sensitivity to various inhibitors: aphidicolin, dideoxy-TTP, and N-ethylmaleimide. Approximately 2-4 fold higher DNA polymerase activity was detected in matrices obtained from lytically infected and virus-transformed cells than that found in normal cells. In lytically infected cells, 30-50% of the matrix-bound DNA polymerase activity solubilized by sonication co-sedimented with majority of the matrix T-antigen, and was co-precipitated with anti-T sera. The results suggest that alpha-polymerase and viral T-antigen may form a functional complex in the matrix.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A., Horowitz M., Skolnik H., Abulafia R., Laub O., Aloni Y. The metabolism of SV40 RNA is associated with the cytoskeletal framework. Virology. 1981 Jun;111(2):475–487. doi: 10.1016/0042-6822(81)90350-0. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradley M. K., Griffin J. D., Livingston D. M. Relationship of oligomerization to enzymatic and DNA-binding properties of the SV40 large T antigen. Cell. 1982 Jan;28(1):125–134. doi: 10.1016/0092-8674(82)90382-8. [DOI] [PubMed] [Google Scholar]
- Brown M., Bollum F. J., Chang L. M. Intracellular localization of DNA polymerase alpha. Proc Natl Acad Sci U S A. 1981 May;78(5):3049–3052. doi: 10.1073/pnas.78.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler-White A. J., Humphrey G. W., Pigiet V. Association of polyoma T antigen and DNA with the nuclear matrix from lytically infected 3T6 cells. Cell. 1980 Nov;22(1 Pt 1):37–46. doi: 10.1016/0092-8674(80)90152-x. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crémisi C. Chromatin replication revealed by studies of animal cells and papovaviruses (simian virus 40 and polyoma virus). Microbiol Rev. 1979 Sep;43(3):297–319. doi: 10.1128/mr.43.3.297-319.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Berg P. Requirement of a Cytoplasmic Fraction for Synthesis of SV40 Deoxyribonucleic Acid in Isolated Nuclei*. J Biol Chem. 1975 Jun 10;250(11):4348–4354. [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Anderson S., DePamphilis M. L. Involvement of DNA polymerase alpha in simian virus 40 DNA replication. J Biol Chem. 1978 May 10;253(9):3273–3280. [PubMed] [Google Scholar]
- Filpula D., Fisher P. A., Korn D. DNA polymerase-alpha. Common polypeptide core structure of three enzyme forms from human KB cells. J Biol Chem. 1982 Feb 25;257(4):2029–2040. [PubMed] [Google Scholar]
- Frearson P. M., Crawford L. V. Polyoma virus basic proteins. J Gen Virol. 1972 Feb;14(2):141–155. doi: 10.1099/0022-1317-14-2-141. [DOI] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Spadari S. Variation of DNA polymerases-alpha, -beta. and -gamma during perinatal tissue growth and differentiation. Nucleic Acids Res. 1977 Aug;4(8):2917–2929. doi: 10.1093/nar/4.8.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Kaufmann S. H., Coffey D. S., Shaper J. H. Considerations in the isolation of rat liver nuclear matrix, nuclear envelope, and pore complex lamina. Exp Cell Res. 1981 Mar;132(1):105–123. doi: 10.1016/0014-4827(81)90088-4. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Knopf K. W., Yamada M., Weissbach A. HeLa cell DNA polymerase gamma: further purification and properties of the enzyme. Biochemistry. 1976 Oct 5;15(20):4540–4548. doi: 10.1021/bi00665a032. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Pardon J. F. Structure and function of chromatin. Annu Rev Genet. 1979;13:197–233. doi: 10.1146/annurev.ge.13.120179.001213. [DOI] [PubMed] [Google Scholar]
- Long B. H., Huang C. Y., Pogo A. O. Isolation and characterization of the nuclear matrix in Friend erythroleukemia cells: chromatin and hnRNA interactions with the nuclear matrix. Cell. 1979 Dec;18(4):1079–1090. doi: 10.1016/0092-8674(79)90221-6. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Nelkin B. D., Pardoll D. M., Vogelstein B. Localization of SV40 genes within supercoiled loop domains. Nucleic Acids Res. 1980 Dec 11;8(23):5623–5633. doi: 10.1093/nar/8.23.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B., Coffey D. S. A fixed site of DNA replication in eucaryotic cells. Cell. 1980 Feb;19(2):527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Parker R. J., Tobia A. M., Baum S. G., Schildkraut C. L. DNA replication in synchronized cultured mammalian cells. V. The temporal order of synthesis of component alpha DNA during monkey DNA synthesis induced by SV40 virus. Virology. 1975 Jul;66(1):82–93. doi: 10.1016/0042-6822(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Sheinin R., Humbert J. Some aspects of eukaryotic DNA replication. Annu Rev Biochem. 1978;47:277–316. doi: 10.1146/annurev.bi.47.070178.001425. [DOI] [PubMed] [Google Scholar]
- Smith B. J. Light satellite-band DNA in mouse cells infected with polyoma virus. J Mol Biol. 1970 Jan 14;47(1):101–106. doi: 10.1016/0022-2836(70)90405-5. [DOI] [PubMed] [Google Scholar]
- Smith H. C., Berezney R. DNA polymerase alpha is tightly bound to the nuclear matrix of actively replicating liver. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1541–1547. doi: 10.1016/s0006-291x(80)80041-6. [DOI] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. In vitro replication of simian virus 40 DNA in a nucleoprotein complex. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3466–3470. doi: 10.1073/pnas.73.10.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. T. Effect of 1,4-dihydroxy-5,8-bis (2-(2-hydroxyethylamino) ethyl amino)-9,10-anthracenedione (dihydroxyanthraquinone) on the replication of simian virus 40 chromosome. Biochem Biophys Res Commun. 1981 Nov 16;103(1):249–255. doi: 10.1016/0006-291x(81)91686-7. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Weissbach A. Eukaryotic DNA polymerases. Annu Rev Biochem. 1977;46:25–47. doi: 10.1146/annurev.bi.46.070177.000325. [DOI] [PubMed] [Google Scholar]