Abstract
Objective:
To examine whether the association between clinical Alzheimer disease (AD) diagnosis and neuropathology and the precision by which neuropathology differentiates people with clinical AD from those with normal cognition varies by age.
Methods:
We conducted a cross-sectional analysis of 2,014 older adults (≥70 years at death) from the National Alzheimer's Coordinating Center database with clinical diagnosis of normal cognition (made ≤1 year before death, n = 419) or AD (at ≥65 years, n = 1,595) and a postmortem neuropathologic examination evaluating AD pathology (neurofibrillary tangles, neuritic plaques) and non-AD pathology (diffuse plaques, amyloid angiopathy, Lewy bodies, macrovascular disease, microvascular disease). We used adjusted logistic regression to analyze the relationship between clinical AD diagnosis and neuropathologic features, area under the receiver operating characteristic curve (c statistic) to evaluate how precisely neuropathology differentiates between cognitive diagnoses, and an interaction to identify effect modification by age group.
Results:
In a model controlling for coexisting neuropathologic features, the relationship between clinical AD diagnosis and neurofibrillary tangles was significantly weaker with increasing age (p < 0.001 for interaction). The aggregate of all neuropathologic features more strongly differentiated people with clinical AD from those without in younger age groups (70–74 years: c statistic, 95% confidence interval: 0.93, 0.89–0.96; 75–84 years: 0.95, 0.87–0.95; ≥85 years: 0.83, 0.80–0.87). Non-AD pathology significantly improved precision of differentiation across all age groups (p < 0.004).
Conclusion:
Clinical AD diagnosis was more weakly associated with neurofibrillary tangles among the oldest old compared to younger age groups, possibly due to less accurate clinical diagnosis, better neurocompensation, or unaccounted pathology among the oldest old.
Age is arguably the strongest risk factor for dementia.1 Those 85 years or older, referred to as the oldest old, accounted for less than 1% of the population in 1996 but comprised approximately 40% of dementia cases.2,3 Despite high prevalence of dementia among the oldest old, cognitive impairment in this age group is not well characterized by symptoms or neuropathology,4 with relatively few neuropathologic studies of Alzheimer disease (AD) in the oldest old.5–15
Preliminary studies that compared the strength of association between dementia diagnosis or cognitive performance and AD neuropathology (neuritic plaques and neurofibrillary tangles) between the young old and the oldest old suggest that the association is weaker among the oldest old6,13; however, these studies were limited by small to moderate size and considered neuropathologic features individually. It may be important to consider non-AD pathology such as Lewy bodies and cerebrovascular disease concurrently to AD pathology, particularly among the oldest old. A higher load of cerebrovascular pathology or other non-AD pathology among the oldest old may account for the reduced association between clinical diagnosis of AD and AD pathology with older age.
Here, we analyzed data from the National Alzheimer's Coordinating Center (NACC), which has a large sample of participants with clinical cognitive diagnoses. The objectives were twofold: 1) to examine whether the association between clinical AD diagnosis and neuropathologic features varied by age; and 2) to examine whether the precision by which neuropathologic features differentiated people with AD from people without clinical AD varied by age.
METHODS
NACC.
The National Institute of Aging (NIA) established the NACC in 1999 to facilitate collaborative research among the NIA-funded AD Centers (ADCs), as detailed elsewhere.16 The NACC maintains a standardized database that includes clinical and neuropathologic data for patients at each ADC. Although the criteria for diagnosis were not reported until 2005, clinical diagnoses of AD at each ADC were generally made using the most recent consensus guidelines.
Standard protocol approvals, registrations, and patient consents.
Patients or their proxies provided informed consent through an Institutional Review Board–approved protocol at each site.
Study sample.
For these analyses, we used data collected from 1984 to June 2009 in the minimum dataset (MDS), which contains standard information for all participants enrolled at the ADCs (n = 8,667). Inclusion criteria for our study were 1) clinical diagnosis of normal cognition within 1 year of death (n = 419) or clinical AD diagnosis at age 65 years or older (n = 1,821); 2) age at least 70 years at death; and 3) a postmortem neuropathologic examination that included Braak & Braak staging of neurofibrillary tangles, Consortium to Establish a Registry of Alzheimer's Disease (CERAD) rating of neuritic plaques, and an evaluation of diffuse plaques, Lewy bodies, amyloid angiopathy, macrovascular disease, and microvascular disease. To avoid confounding conditions, we eliminated people with neuropathologic reports of frontotemporal dementia (both tauopathies and TDP43-pathies). We also eliminated people with unclear neuropathologic diagnoses, such as unspecified Lewy body disease or nonspecific angiopathy. Data from 2,014 people were included in our analyses, of whom 27% (n = 552) were 70–74 years, 29% (n = 579) were 75–84 years, and 44% (n = 883) were 85 years or older at death.
Neuropathology.
The NACC requests specific information on neuropathologic findings from each ADC using a standard neuropathology data form. Neurofibrillary tangles were evaluated using Braak & Braak staging, which is based on spread of the neurofibrillary tangles in the brain.17 The CERAD rating was used to rate neuritic plaque as frequent, moderate, sparse, or no neuritic plaques.18 Diffuse plaques were rated on an identical 4-level scale. Presence of Lewy bodies and markers of Parkinson disease and dementia with Lewy bodies were indicated as brainstem predominant type, intermediate or transitional (limbic) type, diffuse (neocortical) type, indeterminate, absent, or not noted.19 The latter were excluded for the purpose of these analyses. Amyloid angiopathy was rated on a 4-level scale as severe, moderate, mild, or none. Other vascular pathology was reported only as present or absent. We defined macrovascular disease as the presence of large infarcts (>1 cm) and microvascular disease as the presence of microinfarcts, lacunes, or subcortical arteriosclerosis.
We dichotomized each neuropathologic variable for the purpose of this study. We compared those with advanced Braak & Braak stages of neurofibrillary tangles (stages IV–VI) to those with less severe stages (I–III). Neuritic and diffuse plaques were dichotomized as moderate/frequent vs sparse/no plaques. Lewy bodies were dichotomized as intermediate, transitional (limbic), and diffuse (neocortical) type vs brainstem predominant or none. Amyloid angiopathy was compared moderate/severe vs mild/none. Finally, macrovascular disease and microvascular disease were either present or absent.
Additional measures.
All patients in the MDS were described by age, sex, and education. The MDS also noted the presence or absence of depression at each visit. The Mini-Mental State Examination score, a test of global cognition, was administered at most visits.20 APOE genotype was assessed using standardized techniques.
Statistical analysis.
Patient characteristics at the last visit prior to death were analyzed by age group (70–74 years, 75–84 years, and ≥85 years) and clinical cognitive diagnosis using analysis of variance or χ2, as appropriate. The Cochrane-Armitage test for trend was used to evaluate the frequency of neuropathologic features across clinical cognitive diagnosis and age group.
We conducted multiple logistic regressions to evaluate the odds of a clinical AD diagnosis based on neuropathologic features both individually and in an aggregate model (all neuropathologic features in one model), adjusted for age, sex, education, and APOE ε4 allele. An interaction effect between each neuropathologic feature and age group was used to determine whether the odds of clinical AD diagnosis by level of the neuropathologic feature varied across age groups. A logistic regression determined the odds of clinical AD diagnosis based on levels of neuropathologic features in each age group.
The precision by which neuropathologic features differentiated those with from those without a clinical AD diagnosis was evaluated using the area under the receiver operator characteristic (ROC) curve c-statistic in each age group. The precision of differentiation of a model that included only AD pathology vs an aggregate model that considered all coexisting neuropathologic features was compared using Pearson χ2. The difference in precision between the 2 models was attributed to the inclusion of non-AD pathology in the model.
RESULTS
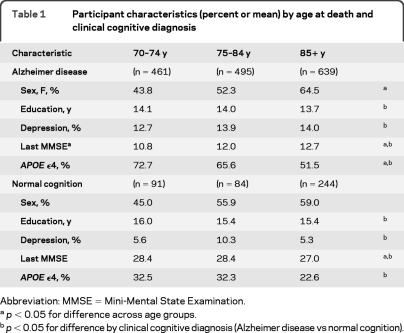
Those with clinical AD were less educated, had depression more often, had lower MMSE scores, and had an APOE ε4 allele more often than those with normal cognition prior to death. Among those with a clinical AD diagnosis, people who were older at death (≥85 years) were female more often, had higher MMSE scores, and had an APOE ε4 allele less often than those who were younger at death (table 1).
Table 1.
Participant characteristics (percent or mean) by age at death and clinical cognitive diagnosis
Abbreviation: MMSE = Mini-Mental State Examination.
p < 0.05 for difference across age groups.
p < 0.05 for difference by clinical cognitive diagnosis (Alzheimer disease vs normal cognition).
All neuropathologic features, except macrovascular disease (p = 0.48), were more frequent in those with a clinical diagnosis of AD compared to those with normal cognition (p < 0.007, figure 1). Among those with clinical AD, the frequency of Lewy bodies (p < 0.001) declined with increasing age whereas the frequency of macrovascular (p = 0.02) and microvascular disease (p = 0.02) increased with age. The frequency of amyloid angiopathy peaked in the middle age group. In people with AD, there was no significant trend across age groups in the frequency of neurofibrillary tangles (p = 0.36), neuritic plaques (p = 0.09), or diffuse plaques (p = 0.56). For people with normal cognition, the frequency of neurofibrillary tangles (p < 0.001) increased with age. The frequency of all other neuropathologic features was similar across age groups in people with normal cognition prior to death (p > 0.22).
Figure 1. Frequency of neuropathologic features by age group and Alzheimer disease (AD) diagnosis.
Participants with AD are represented by triangles and participants with normal cognition are represented by squares. The percent of participants with the moderate/severe levels (neurofibrillary tangles, neuritic plaques, diffuse plaques, amyloid angiopathy, Lewy bodies) or with pathology present (macrovascular disease, microvascular disease) is indicated.
When modeled in separate multivariate logistic regression models (adjusted for age, sex, education, and APOE ε4 status), those with a clinical diagnosis of AD were more likely to have all neuropathologic features (p < 0.02) except macrovascular disease (p = 0.97) compared to those with normal cognition. The association between the odds of clinical AD and neurofibrillary tangles and Lewy bodies was significantly attenuated with older age at death (table 2), though both neuropathologic features were associated with increased odds of a clinical AD diagnosis in all age groups. Age did not significantly modify the relationship between clinical diagnosis of AD and neuritic plaques, diffuse plaques, amyloid angiopathy, macrovascular disease, or microvascular disease.
Table 2.
Odds of dementia by severity or presence/absence of each neuropathologic feature by age groupa
Odds of Alzheimer disease were evaluated in either a separate model for each feature or an aggregate model that includes all neuropathologic features (aggregate model). The significance of the interaction of each neuropathologic feature with age group is noted. All models are adjusted for age, sex, education, and presence of APOE ϵ4 allele.
When controlling for all coexisting neuropathologic features in an aggregate logistic regression model, the odds of a clinical diagnosis of AD remained significantly associated with neurofibrillary tangles, neuritic plaque, Lewy bodies, and microvascular disease. However, in this aggregate model, only the relationship between clinical AD diagnosis and neurofibrillary tangles was significantly modified by age group (table 2). The relationship between the odds of clinical AD and neurofibrillary tangles was significantly attenuated with older age. The relationship between clinical AD diagnosis and Lewy bodies appeared to lessen with increasing age but this trend did not reach statistical significance in the aggregate model (p = 0.06). The relationship between clinical AD and other neuropathologic features was not significantly different across age groups when all neuropathologic features were included in a single model.
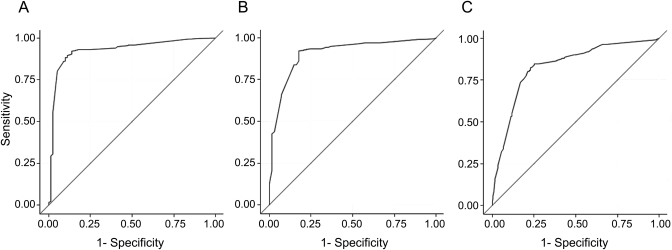
In ROC analyses, neuropathologic features differentiated people with from people without a clinical AD diagnosis more precisely among the younger age groups (70–74 years: c-statistic, 95% CI 0.93, 0.89–0.96; 75–84 years: 0.91, 0.87–0.95) than among the oldest old (≥85 years: 0.83, 0.80–0.87) (figure 2). Similarly, AD pathology (neurofibrillary tangles and neuritic plaques) also differentiated people with clinical AD from those with normal cognition more precisely among the younger age groups (70–74 years: c-statistic, 95% CI 0.89, 0.85–0.93; 75–84 years: 0.87, 0.82–0.92) than among the oldest old (0.81, 0.77–0.84). Inclusion of non-AD pathology improved the precision of differentiation in all age groups (70–74 years: Pearson χ2 8.46, p = 0.004; 75–84 years: Pearson χ2 7.52, p = 0.006; ≥85 years: Pearson χ2 8.68, p = 0.003).
Figure 2. Predictive value of neuropathologic features by age group: (A) youngest (70–74 years), (B) middle (75–84 years), and (C) oldest (≥85 years).
The aggregate model includes neurofibrillary tangles, neuritic plaques, diffuse plaques, Lewy bodies, amyloid angiopathy, macrovascular disease, and microvascular disease.
DISCUSSION
Of all the neuropathologic features that we evaluated, the odds of a clinical diagnosis of AD were most strongly associated with neurofibrillary tangles across all age groups; however, the strength of the association between clinical AD diagnosis and neurofibrillary tangles was significantly weaker among the oldest old, even when the presence of multiple pathologies was considered. Neuropathologic features, whether AD pathology only or the aggregate of all coexisting pathologic features, differentiated people with clinical AD from those with normal cognition more poorly with increasing age; however, consideration of non-AD pathology improved the precision of differentiation in all age groups.
It has long been understood that clinical cognitive diagnoses do not always correspond with neuropathologic findings postmortem.21 That is, some people with normal cognition at death have neuropathologic changes consistent with a pathologic diagnosis of AD or other dementia.21,22 Conversely, some people who are diagnosed with dementia show no obvious neuropathologic cause upon autopsy.23 However, this discord between clinical diagnosis of AD and neuropathologic features, and particularly AD pathology, seems to widen at higher ages.7
In line with most previous studies,6,7,13 the strength of the association between clinical diagnosis of AD and neurofibrillary tangles was weaker among the oldest old than the young old, even when the presence of coexisting neuropathologic features was taken into account. The reason why clinical AD diagnosis was more weakly associated with neurofibrillary tangles with greater age is unclear; however, there are several possible contributing factors. First, the level of cognitive impairment among those with AD was less severe among the oldest old than among the young old in our sample, as evidenced by higher MMSE scores prior to death (table 1). For that reason, the weaker relationship between neurofibrillary tangles and clinical cognitive diagnosis among the oldest old may be an artifact of disease severity, rather than age group. However, this is unlikely given that Haroutunian et al.6 found that the association between AD pathology and dementia severity was also diminished among the oldest old. Second, the frequency of neurofibrillary tangles among those with normal cognition was higher in the oldest old than in other age groups. This suggests that some of the oldest old who were classified with normal cognition may have subclinical AD. Third, in secondary analyses, the oldest old were more likely to have neurofibrillary tangles without concomitant neuritic plaques—in our sample 25% of the oldest old had high levels of neurofibrillary tangles but low levels of neuritic plaques compared to 10% of those 70 to 74 years (p < 0.001). This corroborates previous observations that the oldest old are more likely to have a condition coined as senile dementia with tangles, which is characterized by massive deposition of neurofibrillary tangles in limbic areas without concomitant neuritic plaques.24 It is possible that neurofibrillary tangles may be less likely to cause sufficient cognitive impairment to be diagnosed with clinical AD without the additional contribution of neuritic plaques. Finally, coexisting microvascular disease, which contributes to cognitive impairment, may have been more severe among older age groups, where severity may be important for predicting clinical outcomes.25 In the NACC database, microvascular disease was only reported as present or absent so the reports were insufficient to capture an effect of severity.
Unlike the relationship with neurofibrillary tangles, clinical AD was similarly associated with other neuropathologic features across age groups when we controlled for coexisting neuropathologic features. Even though the prevalence of vascular pathologies increased with age, the odds of AD based on vascular pathologies did not because people with normal cognition also had an increase in the frequency of vascular pathologies with age, though this was not statistically significant. Furthermore, a trend for diminished association between clinical AD and neuritic plaques and Lewy bodies, which was observed in other studies,6,7,13,26 was attenuated when coexisting neuropathologic features were considered. This suggests that the preliminary observation of a trend toward weaker association between clinical AD and Lewy bodies and neuritic plaques in older age groups in this and other studies may be partially due to differing levels of coexisting neuropathologic features that also contribute to cognitive impairment across age groups (such as neurofibrillary tangles and microvascular disease).8,27 In this study, the average number of coexisting neuropathologic features did not differ by age, in contrast to previous studies,28 so it is likely the combinations of neuropathologic features that were of primary importance.
In this study, neuropathologic features differentiated people with clinical AD from those with normal cognition less accurately among the oldest old relative to the young old in ROC analyses, though non-AD pathology significantly improved the differentiation accuracy across all age groups. Why the precision by which neuropathologic features differentiated those with from those without a clinical diagnosis of AD among the oldest old was worse than among the young old is unclear. It is possible that the clinical diagnosis is less accurate in this group because neuropsychological assessments often do not have norms that are specific to the oldest old. Alternatively it may be due to survival bias, where people who survive to very old ages are also more likely to employ successful neurocompensation techniques, whether genetically predetermined or behaviorally mediated, to maintain cognition despite significant pathologic changes. It is also possible that there are additional neuropathologic features that are important to consider among the oldest old that are not accounted for here. For example, cerebral atrophy and synaptic protein loss, not recorded in the NACC database, are understood to be associated with cognition in the oldest old and the young-old.13,29 In particular, the frequency of cerebral atrophy increases with age.28
Importantly, though worse among the oldest old, the neuropathologic features differentiated those with from those without a clinical diagnosis of AD very well in all age groups. Even among the oldest old, neuropathologic features could determine the likelihood of a clinical cognitive diagnosis to 81% accuracy. That is, in any pairing, there was 81% chance that an individual with a clinical diagnosis of AD had greater neuropathologic load than someone with normal cognition.
Our study has several strengths. We had a large sample with standardized neuropathologic reports. In addition, all participants had clinical cognitive diagnosis determined at an ADC. However, our study also has some limitations. Although all ADCs reported common neuropathologic measures, the methods to determine each measure may have differed between sites and by year. In addition, the rating of vascular pathology was imprecise, a common problem faced in neuropathologic examinations,30 and was marked only as present or absent. We also did not have a measure of cerebral atrophy. In addition, although we had clinical cognitive diagnosis, we did not have a continuous measure of cognition close to death for the entire sample. Finally, those included in the NACC database are unlikely to represent the general AD population31; the database includes fewer people with severe AD than would be found in the general AD population. In addition, participants are primary white and highly educated.
The oldest old accounted for approximately 40% of dementia cases by the mid-1990s. Since the oldest old are the fastest growing segment of the population, this proportion is expected to rise in the coming decades. The relationship between clinical diagnosis of AD and neurofibrillary tangles is attenuated among the oldest old and neuropathologic features do not differentiate people with from people without a clinical AD diagnosis as well in this age group compared to the young old. The explanation is not clear but it is possible that additional neuropathologic features need to be taken into account or that these oldest old survivors are better able to cope with neuropathology to maintain cognition despite pathologic changes in the brain. Future studies should further investigate the impact of additional coexisting pathology on cognition in the oldest old.
GLOSSARY
- %AD
Alzheimer disease
- ADC
Alzheimer Disease Center
- CERAD
Consortium to Establish a Registry of Alzheimer's Disease
- MDS
minimum dataset
- NACC
National Alzheimer's Coordinating Center
- NIA
National Institute of Aging
- ROC
receiver operator characteristic
AUTHOR CONTRIBUTIONS
Dr. Middleton contributed to study concept and design, drafting and editing of the manuscript for content, analysis and interpretation of data, and statistical analysis. Dr. Grinberg contributed to study concept and design, editing of the manuscript for content, and interpretation of data. Dr. Miller contributed to editing of the manuscript for content. Dr. Kawas contributed study concept and editing of the manuscript for content. Dr. Yaffe contributed to study concept and design, editing of the manuscript for content, and interpretation of data.
DISCLOSURE
Dr. Middleton serves on the editorial board of the Journal of Alzheimer's Disease and receives fellowship support from the Canadian Institute of Health Research fellowship. Dr. Grinberg serves as an Associate Editor for Frontiers in Dementia and Cell and Tissue Banking; and receives research support from the Alzheimer's Association and the John Douglas French Alzheimer's Foundation. Dr. Miller serves on a scientific advisory board for the Alzheimer's Disease Clinical Study; serves as an Editor for Neurocase and as an Associate Editor of ADAD; receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge, 2009), Handbook of Neurology (Elsevier, 2009), and The Human Frontal Lobes (Guilford, 2008); serves as a consultant for Lundbeck Inc., Elan Corporation, and Allon Therapeutics, Inc.; serves on speakers' bureaus for Novartis and Pfizer Inc.; and receives research support from Novartis and the NIH/NIA and the State of California Alzheimer's Center. Dr. Kawas serves on a scientific advisory board for Quintiles and receives research support from the NIH/ Alzheimer's Disease Research Centre. Dr. Yaffe has served on data safety monitoring boards for Pfizer Inc, Medivation, Inc., and the NIH (NIMH and NIA trials); and has received research support from the NIH (NIA, NIDDK, NIMH), the Department of Defense, American Health Assistance Foundation, Anonymous Foundation, and the Alzheimer Association.
REFERENCES
- 1. McDowell I. Alzheimer's disease: insights from epidemiology. Aging 2001;13:143–162 [DOI] [PubMed] [Google Scholar]
- 2. Canadian Study of Health and Aging: study methods and prevalence of dementia. CMAJ 1994;150:899–913 [PMC free article] [PubMed] [Google Scholar]
- 3. US Census Bureau. Resident Population Estimates of the United States by Age and Sex: April 1, 1990 to July 1, 1999, with Short-Term Projection to November 1, 2000. Washington, DC: US Census Bureau; 2001 [Google Scholar]
- 4. Kawas CH. The oldest old and the 90+ Study. Alzheimers Dement 2008;4:S56–S59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brayne C, Richardson K, Matthews FE, et al. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge City over-75s Cohort (CC75C) Study. J Alzheimers Dis 2009;18:645–658 [DOI] [PubMed] [Google Scholar]
- 6. Haroutunian V, Schnaider-Beeri M, Schmeidler J, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol 2008;65:1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Imhof A, Kovari E, von GA, et al. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci 2007;257:72–79 [DOI] [PubMed] [Google Scholar]
- 8. Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol 2010;119:421–433 [DOI] [PubMed] [Google Scholar]
- 9. Kawas CH, Corrada MM. Alzheimer's and dementia in the oldest-old: a century of challenges. Curr Alzheimer Res 2006;3:411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oinas M, Polvikoski T, Sulkava R, et al. Neuropathologic findings of dementia with Lewy bodies (DLB) in a population-based Vantaa 85+ Study. J Alzheimers Dis 2009;18:677–689 [DOI] [PubMed] [Google Scholar]
- 11. Perls T. Dementia-free centenarians. Exp Gerontol 2004;39:1587–1593 [DOI] [PubMed] [Google Scholar]
- 12. Polvikoski T, Sulkava R, Myllykangas L, et al. Prevalence of Alzheimer's disease in very elderly people: a prospective neuropathological study. Neurology 2001;56:1690–1696 [DOI] [PubMed] [Google Scholar]
- 13. Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med 2009;360:2302–2309 [DOI] [PubMed] [Google Scholar]
- 14. Silver MH, Newell K, Brady C, Hedley-White ET, Perls TT. Distinguishing between neurodegenerative disease and disease-free aging: correlating neuropsychological evaluations and neuropathological studies in centenarians. Psychosom Med 2002;64:493–501 [DOI] [PubMed] [Google Scholar]
- 15. Sinka L, Kovari E, Gold G, et al. Small vascular and Alzheimer disease-related pathologic determinants of dementia in the oldest-old. J Neuropathol Exp Neurol 2010;69:1247–1255 [DOI] [PubMed] [Google Scholar]
- 16. Beekly DL, Ramos EM, van BG, et al. The National Alzheimer's Coordinating Center (NACC) database: an Alzheimer disease database. Alzheimer Dis Assoc Disord 2004;18:270–277 [PubMed] [Google Scholar]
- 17. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259 [DOI] [PubMed] [Google Scholar]
- 18. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathological assessment of Alzheimer's disease. Neurology 1991;41:479–486 [DOI] [PubMed] [Google Scholar]
- 19. Nelson PT, Jicha GA, Kryscio RJ, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol 2010;257:359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 21. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 2001;357:169–175 [DOI] [PubMed] [Google Scholar]
- 22. Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE. Cognition and neuropathology in aging: multidimensional perspectives from the Rush Religious Orders Study and Rush Memory and Aging Project. Curr Alzheimer Res. doi: 10.2174/156720511795745302. Epub 2011 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50 in nonagenarians. Arch Neurol 2000;57:713–719 [DOI] [PubMed] [Google Scholar]
- 24. Jellinger KA, Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia). Brain Pathol 1998;8:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giannakopoulos P, Gold G, Kovari E, et al. Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol 2007;113:1–12 [DOI] [PubMed] [Google Scholar]
- 26. Jicha GA, Parisi JE, Dickson DW, et al. Age and apoE associations with complex pathologic features in Alzheimer's disease. J Neurol Sci 2008;273:34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nelson PT, Jicha GA, Schmitt FA, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 2007;66:1136–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia Aging Study. J Alzheimers Dis 2009;18:713–725 [DOI] [PubMed] [Google Scholar]
- 29. Head E, Corrada MM, Kahle-Wrobleski K, et al. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol Aging 2009;30:1125–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grinberg LT, Heinsen H. Toward a pathological definition of vascular dementia. J Neurol Sci 2010;299:136–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord 2007;21:249–258 [DOI] [PubMed] [Google Scholar]