Abstract
Objective:
To report the metabotropic glutamate receptor 5 (mGluR5) as the autoantigen of antibodies from 2 patients with Hodgkin lymphoma (HL) and limbic encephalopathy (Ophelia syndrome).
Methods:
Immunohistochemistry with brain tissue and cultures of rat hippocampal neurons were used to demonstrate antibodies. Immunoprecipitation, mass spectrometry, and mGluR5-null mice served to identify the antigen. HEK293 cells transfected with mGluR5 or mGluR1 were used to determine immunologic crossreactivity.
Results:
Both patients developed symptoms consistent with limbic encephalopathy; one had MRI findings typical of this disorder and the other had more extensive radiologic involvement, including parietal and occipital cortex. Patients' sera had antibodies that predominantly reacted with the neuropil of hippocampus and cell surface of live hippocampal neurons. Immunoprecipitation from cultured neurons and mass spectrometry demonstrated that the antigen was mGluR5, a receptor involved in processes of learning and memory. The reactivity of patients' sera was abrogated in brain of mGluR5-null mice, further confirming the antibody specificity. Studies with a large number of controls including 2 patients with cerebellar ataxia and mGluR1 antibodies showed that mGluR5 was only identified by sera of the 2 patients with the Ophelia syndrome, and that despite the homology of this receptor with mGluR1 each autoantigen was specific for a distinct syndrome.
Conclusions:
Antibodies to mGluR5 should be considered in patients with symptoms of limbic encephalitis and HL (Ophelia syndrome). Recognition of this disorder is important because it can affect young individuals and is reversible.
In 1982, Ian Carr1 described an unusual neuropsychiatric disorder in his daughter, who was then 15.5 years old. He noted that one day “a little of the sparkling precision of her conversation had gone.” This was followed by progressive loss of memory, depression, hallucinations, and bizarre behavior. A Hodgkin lymphoma (HL) was detected and successfully treated resulting in neurologic recovery except for “a neatly excised piece of memory for about eighteen months.” Dr. Carr postulated that a humorally mediated mechanism, “perhaps a circulating neurotransmitter-like molecule secreted by the tumor,” caused the disorder, which he named “Ophelia syndrome.” We report 2 patients with a similar syndrome and the characterization of the metabotropic glutamate receptor 5 (mGluR5) as the target antigen of patients' antibodies.
PATIENTS
Patient 1.
In April 2006, a 46-year-old woman was evaluated for depression and personality change. Examination showed enlarged cervical lymph nodes, and a CT scan revealed a right lung mass and cervical lymphadenopathy, which biopsy and tumor staging demonstrated HL (IIIA). In May 2006, she had a seizure, and brain MRI showed subtle increase in T2 signal in the right mesial temporal area. CSF analysis had normal results. She was treated with doxorubicin, vinblastine, bleomycin, and dacarbazine (AVBD) with improvement of the cervical lymphadenopathy. In June 2006, she developed more seizures and her mental status worsened. On examination she was alert but fearful and tremulous; she had short-term memory deficit and delusions, and was emotionally labile. Frequent myoclonic jerks were noted. Cranial nerves, strength, sensation, and coordination were normal. Repeated brain MRI showed increased T2 signal in the mesial temporal lobes, cingulate gyrus, insular regions, and right thalamus. Repeat CSF analysis showed 23 white blood cells/μL (90% lymphocytes), total protein 55 mg/dL, glucose 57 mg/dL, and negative cytology. Antibodies to onconeuronal antigens and glutamic acid decarboxylase (GAD) were negative. The patient continued with AVBD chemotherapy and she also received a course of IV methylprednisolone followed by a slow steroid taper. Her seizures remitted and her mental status improved to normal over several months. A follow-up 4 years after presentation showed that her mental status was normal. Analysis of archived serum (CSF not available) demonstrated mGluR5 antibodies.
Patient 2.
In 2009, a 15-year-old boy developed headache and nausea followed 5 days later by confusion, anxiety, extreme agitation, and auditory and visual hallucinations. He frequently expressed the idea that death will soon arrive. On hospital day 1, CSF revealed 114 leukocytes/μL (90% lymphocytes) and protein concentration of 0.40 g/L. Because of generalized seizures, he required nasotracheal intubation and received IV midazolam, fentanyl, and acyclovir. On day 4, CSF showed 64 leukocytes/μL and protein concentration of 0.40 g/L. Studies for varicella zoster, herpes and parvovirus B19, onconeuronal, and NMDA receptor antibodies were negative. On day 9, MRI diffusion sequences showed bilateral hyperintensities in the posterior parietal-occipital cortex. On day 12, sedation was discontinued and although his neurologic status was improving he required diazepam and risperidone. On day 25, the CSF showed 2 leukocytes/μL and protein concentration of 0.39 g/L along with intrathecal synthesis of immunoglobulin G. On day 32, he was transferred to a rehabilitation center, where he adapted well with other children and performed school tasks normally. He appeared distant and unconcerned by the hospital admission. Memory tests had normal results, but attention and verbal fluency were decreased. On day 35, a fluorodeoxyglucose (FDG) PET scan showed mediastinal lymph node uptake; biopsy and staging revealed HL (IIA). Mediastinal radiotherapy resulted in complete oncologic and neurologic recovery. Two years later, the patient remains neurologically normal. Analysis of archived serum (CSF not available) demonstrated mGluR5 antibodies.
Antibody and antigen characterization.
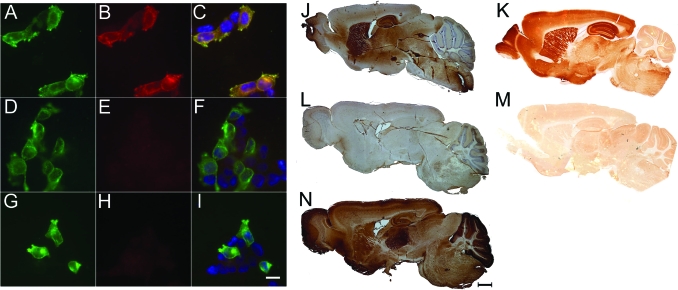
The characterization of patients' antibodies and the identification of mGluR5 as the target antigen were performed combining immunohistochemistry of rat brain, cultured rat hippocampal neurons, immunoprecipitation, and mass spectrometry, as reported (appendix e-1 and figure e-1 on the Neurology® Web site at www.neurology.org).2 HEK293 cells transfected with a plasmid coding for rat mGluR5 were specifically labeled by the 2 patients' sera (figure 1) but not by sera of 104 controls that included 2 patients with mGluR1antibodies (one of these patients has not been previously reported and is described in appendix e-1). Despite the high homology between mGluR5 and mGluR1, the sera of the 2 patients with the Ophelia syndrome did not react with mGluR1 (figure e-2B), and vice versa, the sera of 2 patients with mGluR1 antibodies (both with cerebellar ataxia) did not react with mGluR5 (figure 1E). Further demonstration that mGluR5 was the only antigen related to the Ophelia syndrome was provided by abrogation of patients' sera reactivity with brain of mGluR5-null mice.3
Figure 1. Patients' antibodies recognize metabotropic glutamate receptor 5 (mGluR5) in transfected cells and rodent brain.
Left panels show HEK cells transfected to express mGluR5, reacted with a commercial antibody to mGluR5 (A, D, G), serum from a patient with the Ophelia syndrome (B), serum from a patient with cerebellar ataxia and mGluR1 antibodies (E), and serum from a normal individual (H). Merged reactivities are shown in the column of the right where the nuclei of the cells are labeled blue with DAPI (C, F, I). Note that mGluR5 is only recognized by serum of the patient with Ophelia syndrome. Scale is 10 μm. Right panels show that the serum of a patient with Ophelia syndrome and mGluR5 antibodies intensively reacts with wild-type mouse brain, especially the hippocampus (J), but the reactivity is abrogated in mGluR5-null mouse brain (L). In contrast, the serum of a patient with ataxia and mGluR1 antibodies intensively reacts with mGluR5-null mouse brain, mainly the cerebellum (N; an identical reactivity was noted with the wild-type brain, not shown). A predominant reactivity with the hippocampus of wild-type brain (K) is also demonstrated with a polyclonal rabbit antibody to mGluR5 (06–541, Milipore, Billerica, MA; 1:500). The reactivity of this antibody is abrogated in the brain of mGluR5-null mouse (M). Scale is 1 mm.
Standard protocol approvals, registrations, and patient consents.
Studies were approved by the Institutional Review Board of the University of Pennsylvania. Written consent for studies was provided by guardians of patients.
DISCUSSION
This study identifies mGluR5 as a target antigen of antibodies from 2 patients with the Ophelia syndrome. Three features characterize this disorder: 1) the epitopes are in the extracellular domain of the receptor, as shown by patients' antibody binding to the surface of live neurons; 2) the antibodies specifically react with mGluR5, as demonstrated by the abrogation of reactivity with brain of mGluR5-null mice and lack of cross-reactivity with mGluR1; and 3) the neurologic disorder is reversible, as occurred in our 2 patients.
There are 8 mammalian mGluR receptors classified in 3 groups: group 1 (mGluR1 and mGluR5), group 2 (mGluR2 and mGluR3), and group 3 (mGluR4, mGluR6, mGluR7, and mGluR8). All these receptors modulate neuronal activity by the activation of intracellular signaling pathways. The mGluR5 shares 85% amino acid sequence homology with mGluR1 and both act via calcium/IP3 signaling to modulate synaptic functions including long-term depression (LTD).4 While mGluR5 is more important for LTD in the hippocampus,5 mGluR1 is necessary for rapid dendritic signaling in Purkinje cells.6 Genetic disruption of mGlur5 impairs certain types of behavioral learning, particularly those involving extinction of nonreinforced behaviors.7 In contrast, disruption of mGluR1 alters cerebellar development, synaptic plasticity, and motor coordination.8
Despite the homology between mGluR1 and mGluR5, each receptor has a different brain distribution and associates with a clinically and immunologically distinct disorder. For example, in this study we identified a new patient with cerebellar ataxia and mGluR1 antibodies, representing the fourth case reported with this disorder, and the second without HL.9 These antibodies are important because they alter LTD in cerebellar synapses, and cause ataxia when intrathecally injected to animals.10 While sera of patients with mGluR1 antibodies shows robust staining of the cerebellum, and this autoimmunity associates with cerebellar ataxia, the sera of patients with mGluR5 antibodies shows intense immunolabeling of the hippocampus and other brain regions (with the lowest reactivity in brainstem and cerebellum), consistent with the predominant limbic dysfunction that characterizes the Ophelia syndrome. Therefore, these 2 immune-mediated phenotypes are consistent with the distribution, function, and models of genetic disruption of the corresponding receptor.
Given that HL has a bimodal distribution of age incidence (peaking at 15–34 years, and >55 years),e1 and the Ophelia syndrome usually precedes the identification of HL, mGluR5 autoimmunity should be considered in patients of all ages, including children and teenagers with symptoms of limbic encephalopathy of unclear etiology. In contrast with classic paraneoplastic syndromes, but in line with other autoimmune synaptic encephalitis,e2 patients with mGluR5 antibody-associated encephalitis may start to improve before the tumor is treated. Although one of our patients did not receive immunotherapy and the other only corticosteroids, both patients had prompt and successful tumor treatment, suggesting that the main concern of the physician should be to recognize this disorder and search for HL. Future studies should clarify the role of immunotherapy.
Supplementary Material
ACKNOWLEDGMENT
The mGluR5 and pEGFP-mGluR1 plasmids were a gift of Dr. Steven R. Ikeda (NIH, Bethesda, MD). Serum of a patient with cerebellar ataxia and mGluR1 antibodies was provided by Dr. Peter Sillevis-Smitt (Erasmus University Medical Center, Rotterdam, the Netherlands).
GLOSSARY
- AVBD
doxorubicin, vinblastine, bleomycin, and dacarbazine
- FDG
fluorodeoxyglucose
- GAD
glutamic acid decarboxylase
- HL
Hodgkin lymphoma
- LTD
long-term depression
- mGluR5
metabotropic glutamate receptor 5.
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Lancaster: drafted manuscript, performed experiments, analyzed data. Dr. Martinez-Hernandez: performed experiments, analyzed data. Dr. Titulaer: performed experiments, analyzed data. Dr. Boulos: provided information, serum samples, and analyzed data. Dr. Weaver: provided information, serum samples, and analyzed data. Dr. Antoine: provided information, serum samples, and analyzed data. Dr. Leibers: provided patient information, analyzed data. Dr. Kornblum: provided information, serum samples, and analyzed data. Dr. Bein: provided information, serum samples, and analyzed data. Dr. Honnorat: provided patients' samples, analyzed data. Dr. Wong: performed experiments, analyzed data. Dr. Contractor: provided mGluR-null mouse, analyzed data. Dr. Xu: provided mGluR-null mouse, analyzed data. Dr. Balice-Gordon: interpreted data, revised manuscript. Dr. Dalmau: designed study, analyzed data, drafted and revised manuscript.
DISCLOSURE
Dr. Lancaster has received research support from Talecris Biotherapeutics, Lundbeck Inc., and the Dana Foundation. Dr. Martinez-Hernandez receives research support from Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias, Spain. Dr. Titulaer has served as a consultant for BioMarin Pharmaceutical Inc. and has received research support from the Dutch Cancer Society. Dr. Boulos reports no disclosures. Dr. Weaver serves on speakers' bureaus for Merck Serono and Schering-Plough Corp. Dr. Antoine serves on the editorial board of Revue Neurologique. Dr. Liebers has reviewed records for defendants in medical malpractice cases. Dr. Kornblum has received funding for travel and/or speaker honoraria from Genzyme Corporation, MEDA Pharmaceuticals Inc., Pfizer Inc, and Association Francaise Contre Les Myopathies; and receives research support from BMBF (German Federal Ministry of Education and Research). Dr. Bien has received speaker honoraria from UCB, Desitin Pharmaceuticals, GmbH, Eisai Inc., and Biogen Idec; receives research support from Hagedorn Foundation Bielefeld; and the Krankenhaus Mara gGmbH receives payment for antibody tests performed in his laboratory. Dr. Honnorat, Dr. Wong, and Dr. Xu report no disclosures. Dr. Contractor serves on the editorial board of Neuropharmacology and receives research support from the NIH/NINDS and the McKnight Foundation. Dr. Balice-Gordon receives research support from the NIH and the McKnight Foundation. Dr. Dalmau is Professor at Institució Catalana de Recerca i Estudis Avançats (ICREA) in IDIBAPS/Hospital Clínic, Barcelona, and serves on the editorial board of Neurology®; receives royalties from the editorial board of Up-To-Date; has filed a patent application for the use of LGI1 as a diagnostic test; has received royalties from Athena Diagnostics, Inc. for a patent re: Ma2 autoantibody test and has patents pending re: NMDA and GABAB receptor autoantibody tests (license fee payments received from EUROIMMUN AG); and receives research support from funding from EUROIMMUN AG, the NIH/NCI, and a McKnight Neuroscience of Brain Disorders award.
REFERENCES
- 1. Carr I. The Ophelia syndrome: memory loss in Hodgkin's disease. Lancet 1982;1:844–845 [DOI] [PubMed] [Google Scholar]
- 2. Lancaster E, Lai M, Peng X, Hughes E, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen.Lancet Neurol 2010;9:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning.J Neurosci 2009;29:3676–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicoletti F, Bockaert J, Collingridge GL, et al. Metabotropic glutamate receptors: from the workbench to the bedside.Neuropharmacology 2011;60:1017–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faas GC, Adwanikar H, Gereau RW, Saggau P. Modulation of presynaptic calcium transients by metabotropic glutamate receptor activation: a differential role in acute depression of synaptic transmission and long-term depression.J Neurosci 2002;22:6885–6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hildebrand ME, Isope P, Miyazaki T, et al. Functional coupling between mGluR1 and Cav3.1 T-type calcium channels contributes to parallel fiber-induced fast calcium signaling within Purkinje cell dendritic spines.J Neurosci 2009;29:9668–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simonyi A, Schachtman TR, Christoffersen GR. Metabotropic glutamate receptor subtype 5 antagonism in learning and memory.Eur J Pharmacol 2010;639:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ichise T, Kano M, Hashimoto K, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination.Science 2000;288:1832–1835 [DOI] [PubMed] [Google Scholar]
- 9. Marignier R, Chenevier F, Rogemond V, et al. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol 2010;67:627–630 [DOI] [PubMed] [Google Scholar]
- 10. Coesmans M, Smitt PA, Linden DJ, et al. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies.Ann Neurol 2003;53:325–336 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.