Abstract
OBJECTIVES:
To estimate the impact of gestational and childhood bisphenol A (BPA) exposures on behavior and executive function at 3 years of age and to determine whether child gender modified those associations.
METHODS:
We used a prospective birth cohort of 244 mothers and their 3-year-old children from the greater Cincinnati, Ohio, area. We characterized gestational and childhood BPA exposures by using the mean BPA concentrations in maternal (16 and 26 weeks of gestation and birth) and child (1, 2, and 3 years of age) urine samples, respectively. Behavior and executive function were measured by using the Behavior Assessment System for Children 2 (BASC-2) and the Behavior Rating Inventory of Executive Function-Preschool (BRIEF-P).
RESULTS:
BPA was detected in >97% of the gestational (median: 2.0 μg/L) and childhood (median: 4.1 μg/L) urine samples. With adjustment for confounders, each 10-fold increase in gestational BPA concentrations was associated with more anxious and depressed behavior on the BASC-2 and poorer emotional control and inhibition on the BRIEF-P. The magnitude of the gestational BPA associations differed according to child gender; BASC-2 and BRIEF-P scores increased 9 to 12 points among girls, but changes were null or negative among boys. Associations between childhood BPA exposure and neurobehavior were largely null and not modified by child gender.
CONCLUSIONS:
In this study, gestational BPA exposure affected behavioral and emotional regulation domains at 3 years of age, especially among girls. Clinicians may advise concerned patients to reduce their exposure to certain consumer products, but the benefits of such reductions are unclear.
Keywords: attention-deficit/hyperactivity disorder, child behavior, endocrine disruptors, prospective study
WHAT'S KNOWN ON THIS SUBJECT:
Virtually all persons in industrialized countries are exposed to bisphenol A (BPA), and early-life BPA exposure might be associated with behavior problems. Few human studies have been conducted, and the impact of gestational versus childhood BPA exposures is unclear.
WHAT THIS STUDY ADDS:
BPA exposure during pregnancy, but not childhood, was associated with worse behavior at 3 years of age, especially among girls. Domains related to behavioral and emotional regulation were most affected by gestational BPA exposure.
Bisphenol A (BPA) is used in a variety of consumer products, including dental sealants, food/beverage containers and linings, medical equipment, and thermal receipts.1 The use of BPA-containing products in daily life makes exposure ubiquitous in industrialized and industrializing countries.2–5 The predominant source of BPA exposure for most people is diet, although exposure also might occur through inhalation or dermal absorption, which results in substantial exposure among persons involved in the manufacture or handling of BPA-containing products.6–10
BPA might disrupt the endocrine system.11 Experimental studies with animals indicated that gestational BPA exposure disrupts normal neurodevelopment, affecting sexually dimorphic behaviors such as aggression, anxiety, exploration, and spatial memory.12–14 Sexually dimorphic clinical disorders such as attention-deficit/hyperactivity disorder, autism, and depression might be clinical correlates of these animal behaviors and might be related to early-life disruption of the endocrine system.15,16 These observations suggest that sexually dimorphic behavioral traits may serve as sensitive end points in epidemiological studies.
We reported previously that gestational BPA exposure was associated with increased hyperactivity and aggression scores for 2-year-old girls in a prospective birth cohort from Cincinnati, Ohio.2 However, important questions remain about these findings. The children were young, and the observed associations might not persist with continued development. Furthermore, our previous study was unable to examine the relationship between BPA exposure and executive function, which is a set of processes involved in inhibiting behavior, modulating emotions, and shifting between activities. Deficits in executive function are a feature of disorders such as attention-deficit/hyperactivity disorder.17 Finally, we did not examine the impact of infant/childhood BPA exposures on neurobehavior.
Additional research examining the neurotoxicity of BPA is needed, given the pervasiveness of exposure and the potential for even small effects to have substantial public health consequences.1,18 The purpose of this study was to determine whether previously observed associations remained at 3 years of age, whether executive functions were affected by BPA exposure, and whether gestational or childhood BPA exposures had greater effects on neurobehavior.
METHODS
Data Source
Data for this study were collected from mothers and their children who were participating in the Health Outcomes and Measures of the Environment Study, a prospective birth cohort in the Cincinnati, Ohio, metropolitan area designed for the study of low-level environmental toxicant exposures.19 Eligibility criteria and enrollment of this cohort were described previously.2
Urinary BPA Concentrations
Three maternal spot urine samples were collected between March 2003 and January 2006, twice during pregnancy, at ∼16 and ∼26 weeks of gestation, and within 24 hours after birth. Children's spot urine samples were collected at 1, 2, and 3 years of age, during clinic or home visits, between 2004 and 2009. Preference was given to samples collected during home visits because collection conditions were more standardized. Urine was collected directly into polypropylene specimen cups or, for non–toilet-trained infants, first into Kendall abdominal pads placed inside the diaper. Urine was stored at or below −20°C until analysis. Diaper inserts contaminated with stool were not analyzed.
The concentrations of total (free plus conjugated) species of BPA were measured at the Centers for Disease Control and Prevention (CDC), by using modified analytical chemistry methods described previously.20 The limit of detection (LOD) was 0.4 μg/L; concentrations below the LOD were given a value of LOD/√2.21
We characterized gestational and childhood BPA exposures by using the mean of ≥2 urine samples from the respective periods (ie, gestation or childhood), because individual urinary BPA concentrations vary and a single measure may misclassify exposure.22 We corrected for urine dilution by using urinary creatinine concentrations and calculated creatinine-standardized BPA concentrations (versus nonstandardized) by dividing individual urinary BPA concentrations by creatinine concentrations before calculating the mean.
Childhood Behavior and Executive Function
Children's behavior was assessed at 3 years of age by using the Behavior Assessment System for Children 2 (BASC-2) Parent Rating Scale for preschoolers. The BASC-2 is a valid, reliable, 134-item, parent-report assessment of a child's problem behaviors in community and home settings.23 We focused on clinical subscales because they might be more relevant to human behaviors and more comparable to behavioral end points used in animal studies.24 The analyzed subscales included aggression, attention, hyperactivity, depression, anxiety, and somatization.
Children's executive functions at 3 years of age were assessed by using the Behavior Rating Inventory of Executive Function-Preschool (BRIEF-P), a reliable, valid, 63-question, parent-report inventory.25 Our analyses focused on 5 clinical scales from the BRIEF-P; emotional control scores assess the ability to modulate emotions, inhibit scores reflect the capacity to control behavioral responses, plan/organize scores assess the ability to anticipate and to plan for future events, to set goals, and to grasp main ideas, shift scores measure the capacity to transition to and from events, and working memory scores measure the ability to hold information in mind for completing a task.
Parents were unaware of their own or their children's urinary BPA concentrations when they completed the 2 behavioral measures. Scores on the BASC-2 and BRIEF-P were normalized to a mean of 50 (SD: 10) by the test publishers. For both instruments, higher scores indicate more impairment. Subscales of the BASC-2 and BRIEF-P share a moderate degree of correlation (Pearson R = 0.5–0.7), and each test has been used to validate the other.23,26 We also used 2 summary scores from the BASC-2 (Behavioral Symptom Index) and BRIEF-P (Global Executive Composite) to describe the study sample and how behavior varied according to child and family characteristics.
Covariates
We included the following potential confounding variables in our exposure-outcome statistical models.22,27 Demographic variables (mother's race, education, marital status, and household income) were measured by trained interviewers during pregnancy. Perinatal variables included maternal depressive symptoms measured at 20 weeks of gestation by using the Beck Depression Inventory II.28 The caregiving environment was measured through administration of the Home Observation for Measurement of the Environment during the 1-year home visit.29
We controlled for gestational exposure to low molecular weight phthalates (compounds used in personal care products and other consumer products) and tobacco smoke by using metabolite concentrations from maternal urine and serum measurements, respectively. We calculated the mean concentrations of cotinine (a metabolite of nicotine) or low molecular weight phthalates from ≥2 samples collected during pregnancy or at birth. We used the summed molar concentrations of 3 low molecular weight phthalate metabolites, on the basis of their previous associations with childhood behavior and executive function.30 Metabolite concentrations were quantified at the CDC by using previously described methods.31,32
Statistical Analyses
We began by describing Global Executive Composite and Behavioral Symptom Index scores according to sociodemographic factors. We also examined univariate characteristics of urinary BPA concentrations. We calculated Pearson correlation coefficients between pairs of log10-transformed urinary BPA concentrations, to gauge their variability.
We fit 3-knot, restricted, cubic splines to examine the dose-response relationship and to examine model linearity assumptions for urinary BPA concentrations and neurobehavior.33 Restricted cubic polynomial splines allow the shape of the relationship between the exposure and outcome to be flexible and not inherently linear. We used multivariate linear regression to estimate the unadjusted and adjusted changes in BASC-2 and BRIEF-P scores with each 10-fold increase in urinary BPA concentrations during gestation or childhood. We also included the child's gender and a product interaction term between BPA variables and child gender because our previous findings suggested that child gender modified the association between gestational BPA concentrations and neurobehavior.2 We examined the P values for this interaction term and considered values of <.10 to be indicative of modification, because our statistical power was limited by sample size.34
Secondary Analyses
First, we examined whether the mutual adjustment for gestational and childhood urinary BPA concentrations changed the pattern of our results. Second, we examined whether additional adjustment for maternal IQ (Wechsler Abbreviated Scales of Intelligence), parity, or duration of breastfeeding (postnatal models only) changed the pattern of observed results.35 Third, we examined whether missing data biased our results by analyzing data for the subsets of women and children who had all 3 urine samples during gestation and childhood, respectively. Finally, we determined whether the exclusion of dilute or concentrated urine samples changed our results.3 Dilute urine was defined as that with creatinine concentrations of <20 mg/dL for mothers and <2 mg/dL for children. Concentrated urine was defined as that with creatinine concentrations of >200 mg/dL for mothers, >128 mg/dL for 1- and 2-year-old children, and >150 mg/dL for 3-year old children.36
Ethical Considerations
The institutional review boards of Cincinnati Children's Hospital Medical Center, the cooperating delivery hospitals, and the CDC approved this study. All mothers provided written informed consent for themselves and their children before enrollment in the study.
RESULTS
Descriptive Statistics
Of the 468 enrolled women, 67 dropped out before delivery, 9 delivered twins, and 3 experienced stillbirths. We also excluded 1 woman with a urinary BPA concentration of 1250 μg/L.37 Among the remaining 388 mother-child pairs, 262 (67%) completed follow-up assessments at 3 years of age, and 245 of those mother-child pairs had gestational or childhood BPA exposure measurements and complete covariate data. Among those women, 239 completed the BASC-2 and 237 completed the BRIEF-P.
Dyads with complete follow-up data were more likely to be white, married, 25 to 34 years of age, more educated, and wealthier, compared with dyads with incomplete data (results not shown). Gestational urinary BPA concentrations were lower among women who completed the follow-up assessments, compared with those who did not (geometric mean: 2.0 vs 2.5 μg/L). However, creatinine-standardized concentrations were similar for women with complete (geometric mean: 2.4 μg/g) and incomplete (geometric mean: 2.5 μg/g) follow-up data. Children from families with lower maternal education or household income had higher BASC-2 and BRIEF-P scores at 3 years of age (Table 1).
TABLE 1.
BASC-2 and BRIEF-P Scores According to Sociodemographic, Caregiving, and Environmental Factors
| n | Behavioral Symptom Index, Mean ± SD | Global Executive Composite, Mean ± SD | |
|---|---|---|---|
| All participants | 240 | 51 ± 9 | 53 ± 12 |
| Mother's race | |||
| Non-Hispanic white | 173 | 50 ± 8 | 51 ± 12 |
| Non-Hispanic black | 52 | 54 ± 12 | 57 ± 12 |
| Other | 15 | 49 ± 8 | 53 ± 10 |
| Mother's education | |||
| Graduate/professional school | 53 | 49 ± 6 | 50 ± 11 |
| Bachelor's degree | 89 | 49 ± 8 | 51 ± 11 |
| Some college | 57 | 53 ± 10 | 54 ± 14 |
| High school | 24 | 52 ± 11 | 57 ± 10 |
| Less than high school | 17 | 55 ± 13 | 59 ± 10 |
| Mother's marital status | |||
| Married or living with partner | 176 | 50 ± 7 | 51 ± 11 |
| Unmarried or living alone | 64 | 54 ± 11 | 57 ± 13 |
| Annual household income | |||
| $80 000 or more | 75 | 49 ± 7 | 50 ± 10 |
| $40 000 to less than $80 000 | 93 | 50 ± 8 | 51 ± 11 |
| $20 000 to less than $40 000 | 31 | 51 ± 8 | 54 ± 14 |
| Less than $20 000 | 41 | 56 ± 13 | 59 ± 12 |
| Maternal depression during pregnancy | |||
| Minimal | 190 | 49 ± 8 | 50 ± 11 |
| Mild | 35 | 56 ± 11 | 60 ± 12 |
| Moderate or severe | 15 | 62 ± 8 | 63 ± 13 |
| Child's gender | |||
| Female | 128 | 51 ± 9 | 52 ± 13 |
| Male | 112 | 51 ± 8 | 53 ± 11 |
| Home Observation for Measurement of the Environment score | |||
| <35 | 29 | 54 ± 10 | 57 ± 12 |
| 35–39 | 38 | 54 ± 11 | 57 ± 12 |
| ≥40 | 173 | 50 ± 8 | 51 ± 11 |
| Gestational tobacco smoke exposure | |||
| None | 103 | 50 ± 8 | 52 ± 11 |
| Secondhand smoke | 117 | 51 ± 10 | 53 ± 13 |
| Active | 20 | 53 ± 8 | 55 ± 11 |
| Gestational urinary low molecular weight phthalate concentrations | |||
| First quartile (0–0.6 μmol/g creatinine) | 59 | 50 ± 8 | 51 ± 12 |
| Second quartile (0.6–1.2 μmol/g creatinine) | 60 | 51 ± 9 | 53 ± 10 |
| Third quartile (1.2–2.5 μmol/g creatinine) | 60 | 51 ± 9 | 52 ± 12 |
| Fourth quartile (>2.5 μmol/g creatinine) | 61 | 51 ± 9 | 54 ± 13 |
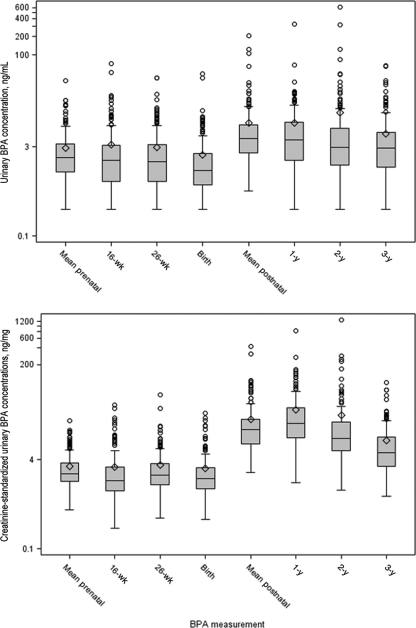
Maternal BPA concentrations were relatively stable between the first sample and birth (Fig 1 and Table 2). Children's urinary BPA concentrations decreased from 1 to 3 years of age, and this decrease became more apparent after creatinine standardization. Children's urinary BPA concentrations were higher and more variable than maternal concentrations. We observed weak correlations between pairs of time-specific maternal-maternal, child-child, and maternal-child urinary BPA concentrations (Pearson R < 0.25), which became weaker after creatinine standardization (Pearson R < 0.18).
FIGURE 1.
Box plots of nonstandardized and creatinine-standardized urinary BPA concentrations. Creatinine-standardized urinary BPA concentrations were calculated by dividing urinary BPA concentrations by urinary creatinine concentrations, to control for urine dilution, whereas nonstandardized concentrations were not. Whiskers represent the minimum and 1.5 times the interquartile range. The tops and bottoms of the boxes represent the 75th and 25th percentiles, respectively. The lines in the boxes represent the medians, and the diamonds represent the means. Circles represent extreme observations (>1.5 times the interquartile range).
TABLE 2.
BPA, Creatinine-Standardized BPA, and Creatinine Concentrations in Urine During Gestation and Childhood
| n | Proportion Detected, % | Minimum | 5th Percentile | 25th Percentile | Median | 75th Percentile | 95th Percentile | Maximum | |
|---|---|---|---|---|---|---|---|---|---|
| BPA level, μg/L of urine | |||||||||
| Gestational mean | 244 | 97 | <LOD | 0.5 | 1.1 | 2.0 | 3.3 | 7.6 | 37 |
| 16 wk | 244 | 89 | <LOD | <LOD | 0.8 | 1.8 | 3.2 | 9.7 | 72 |
| 26 wk | 240 | 90 | <LOD | <LOD | 0.8 | 1.7 | 3.3 | 9.4 | 42 |
| Birth | 223 | 86 | <LOD | <LOD | 0.7 | 1.2 | 2.3 | 6.8 | 49 |
| Childhood mean | 229 | 100 | 0.6 | 1.2 | 2.4 | 4.1 | 7.0 | 18 | 206 |
| 1 y | 213 | 97 | <LOD | 0.5 | 1.8 | 3.9 | 7.6 | 22 | 325 |
| 2 y | 195 | 99 | <LOD | 0.7 | 1.5 | 2.9 | 6.1 | 24 | 616 |
| 3 y | 222 | 96 | <LOD | 0.5 | 1.4 | 2.9 | 5.2 | 15 | 67 |
| Creatinine-standardized BPA level, μg/g creatininea | |||||||||
| Gestational mean | 244 | 97 | 0.5 | 1.1 | 1.6 | 2.2 | 3.5 | 8.0 | 20 |
| 16 wk | 243 | 89 | <LOD | 0.6 | 1.1 | 1.7 | 3.0 | 10 | 38 |
| 26 wk | 240 | 89 | 0.4 | 0.8 | 1.4 | 2.1 | 3.4 | 7.9 | 58 |
| Birth | 223 | 86 | <LOD | 0.6 | 1.2 | 1.8 | 2.8 | 9.4 | 27 |
| Childhood mean | 229 | 100 | 2.3 | 4.6 | 7.6 | 14 | 21 | 62 | 431 |
| 1 y | 212 | 97 | 1.5 | 4.7 | 9.9 | 18 | 34 | 91 | 812 |
| 2 y | 195 | 99 | 1.1 | 3.0 | 5.7 | 9.6 | 19 | 63 | 1273 |
| 3 y | 222 | 96 | 0.9 | 1.5 | 3.0 | 5.3 | 10 | 24 | 95 |
| Creatinine level, mg/dL urine | |||||||||
| 16 wk | 243 | 100 | 7.7 | 20 | 53 | 104 | 160 | 284 | 487 |
| 26 wk | 240 | 100 | 9.2 | 14 | 42 | 79 | 139 | 264 | 428 |
| Birth | 223 | 100 | 9.3 | 18 | 37 | 72 | 109 | 190 | 285 |
| 1 y | 212 | 100 | 3.83 | 5.8 | 12 | 19 | 34 | 75 | 144 |
| 2 y | 195 | 100 | 5.51 | 6.9 | 19 | 35 | 60 | 83 | 201 |
| 3 y | 222 | 100 | 6.9 | 12 | 34 | 54 | 73 | 119 | 269 |
Creatinine-standardized urinary BPA concentrations were calculated by dividing urinary BPA concentrations by urinary creatinine concentrations, to control for urine dilution, whereas nonstandardized concentrations were not.
The mean creatinine-standardized gestational and childhood BPA concentrations exhibited a weak correlation (Pearson R = 0.11). Mean BPA concentrations from gestation or childhood were similar in magnitude to individual measurements but were less variable.
Urinary BPA Concentrations and Behavior/Executive Function
The 3-knot, restricted, cubic, polynomial splines revealed approximately linear relationships between log10-transformed urinary BPA concentrations and BASC-2/BRIEF-P scores (Fig 2; childhood data not shown). Therefore, we chose to characterize BPA concentrations as continuous log10-transformed variables.
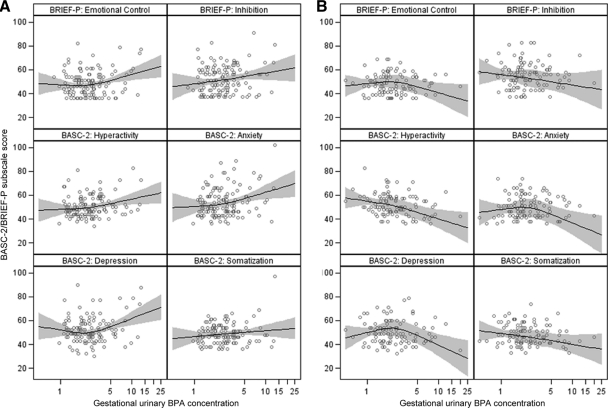
FIGURE 2.
Scatterplot and adjusted smoothed regression of gestational urinary BPA concentrations and BASC-2/BRIEF-P subscale scores at 3 years of age: A, girls; B, boys. Models were adjusted for race (white, black, or other), household income (continuous, in thousands of dollars), education (graduate/professional school, bachelor's degree, some college, high school, or less than high school), marital status (married or unmarried), depressive symptoms during pregnancy (continuous), Home Observation for Measurement of the Environment scores (continuous), log10-transformed mean gestational serum cotinine concentrations, and log10-transformed mean gestational low molecular weight urinary phthalate concentrations. The relationship was smoothed by using a restricted, cubic, polynomial spline with knots at the 10th, 50th, and 90th percentiles of the gestational urinary BPA concentrations. Black lines represent mean regression lines, gray bands are 95% CIs, and dots are raw data.
With adjustment for confounders, gestational BPA concentrations were positively associated with BASC-2 anxiety, hyperactivity, and depression scale scores (Table 3). The magnitude of these associations was greater among girls, compared with boys (P < .10). Anxiety and depression associations for girls were almost twice as large as associations for the whole sample, whereas associations for boys were close to the null. Notably, gestational BPA concentrations were associated with increases in BASC-2 hyperactivity scores among girls (β = 9.1 [95% confidence interval [CI]: 3.1–15]) but decreases among boys (β = −6.3 [95% CI: −12 to −0.6]). In general, associations between childhood urinary BPA concentrations and BASC-2 scores were positive in direction, and 95% CIs straddled the null value. We did not observe evidence that childhood BPA concentrations were modified by child gender.
TABLE 3.
Unadjusted and Adjusted Changes in BASC-2 Scores at 3 Years of Age With 10-Fold Increases in Creatinine-Standardized Gestational and Childhood Urinary BPA Concentrations
| Change in BASC-2 Score, Estimate (95% CI) |
||||
|---|---|---|---|---|
| All, Unadjusted | All, Adjusted | Girls, Adjusteda | Boys, Adjusteda | |
| Mean gestational BPA level (n = 239) | ||||
| Aggression scale | 1.6 (−3.4 to 6.6) | 2.1 (−2.7 to 6.9) | 5.0 (−2.1 to 12) | −0.2 (−6.8 to 6.4) |
| Hyperactivity scale | −0.6 (−5.0 to 3.8) | 0.5 (−3.8 to 4.8) | 9.1 (3.1 to 15) | −6.3 (−12 to −0.6)b |
| Anxiety scale | 6.8 (1.6 to 12) | 7.0 (1.7 to 12) | 12 (4.7 to 20) | 1.3 (−5.8 to 8.4)b |
| Depression scale | 2.7 (−2.3 to 7.7) | 4.9 (0.0 to 9.9) | 11 (3.6 to 18) | −0.5 (−7.2 to 6.2)b |
| Somatization scale | 0.4 (−3.7 to 4.6) | 0.3 (−3.9 to 4.5) | 5.0 (−1.1 to 11) | −4.0 (−9.6 to 1.7)b |
| Attention scale | −2.2 (−6.0 to 1.6) | −1.7 (−5.5 to 2.1) | −0.5 (−6.0 to 5.1) | −2.2 (−7.4 to 3.0) |
| Mean childhood BPA level (n = 225) | ||||
| Aggression scale | 2.7 (−1.3 to 6.6) | 1.6 (−2.4 to 5.7) | 0.7 (−4.8 to 6.2) | 3.1 (−2.8 to 9.1) |
| Hyperactivity scale | 0.3 (−3.0 to 3.7) | −1.0 (−4.4 to 2.4) | −1.2 (−5.8 to 3.3) | 0.5 (−4.4 to 5.4) |
| Anxiety scale | 2.7 (−1.6 to 6.9) | 2.9 (−1.5 to 7.4) | 1.3 (−4.7 to 7.4) | 3.3 (−3.1 to 9.8) |
| Depression scale | 4.3 (0.4 to 8.2) | 3.0 (−1.1 to 7.1) | 2.8 (−2.8 to 8.4) | 2.9 (−3.1 to 8.9) |
| Somatization scale | 3.3 (0.0 to 6.6) | 1.6 (−1.8 to 5.1) | 2.0 (−2.7 to 6.8) | 1.1 (−4.0 to 6.2) |
| Attention scale | 1.5 (−1.5 to 4.4) | 1.1 (−2.0 to 4.3) | 1.1 (−3.2 to 5.4) | 2.3 (−2.3 to 6.9) |
Models were adjusted for race (white, black, or other), household income (continuous, in thousands of dollars), education (graduate/professional school, bachelor's degree, some college, high school, or less than high school), marital status (married or unmarried), depressive symptoms during pregnancy (continuous), Home Observation for Measurement of the Environment scores (continuous), log10-transformed mean gestational serum cotinine concentrations, and log10-transformed mean gestational low-molecular weight urinary phthalate concentrations.
Model includes child gender and a BPA level–child gender interaction term.
For gender–BPA level interaction, P < .10.
Gestational BPA concentrations were positively associated with emotional control and inhibition scores on the BRIEF-P with adjustment for confounders (Table 4). The magnitudes of associations were similar to those observed for the BASC-2. Associations between gestational BPA concentrations and emotional control and inhibition scale scores were larger among girls, compared with boys (interaction P ≤ .10). Associations between childhood urinary BPA concentrations and BRIEF-P scores were null, and child gender did not modify these associations.
TABLE 4.
Unadjusted and Adjusted Changes in BRIEF-P Scores at 3 Years of Age With 10-Fold Increases in Creatinine-Standardized Gestational and Childhood Urinary BPA Concentrations
| Emotional Control Scale | Change in BRIEF-P Score, Estimate (95% CI) |
|||
|---|---|---|---|---|
| All, Unadjusted | All, Adjusted | Girls, Adjusteda | Boys, Adjusteda | |
| Mean gestational BPA level (n = 237) | ||||
| Emotional control scale | 2.9 (−1.7 to 7.4) | 4.9 (0.5 to 9.3) | 9.1 (2.8 to 15) | 1.1 (−5.1 to 7.2)b |
| Inhibit scale | 2.3 (−3.2 to 7.8) | 4.1 (−1.3 to 9.5) | 9.3 (1.8 to 17) | 0.4 (−7.0 to 7.8)b |
| Plan/organize scale | 0.2 (−5.4 to 5.8) | 1.1 (−4.4 to 6.7) | 5.4 (−2.5 to 13) | −2.2 (−10 to 5.5) |
| Shift scale | 1.4 (−3.6 to 6.4) | 3.1 (−2.0 to 8.2) | 5.5 (−1.7 to 13) | 1.4 (−5.7 to 8.5) |
| Working memory scale | −0.6 (−6.6 to 5.5) | 0.6 (−5.4 to 6.5) | 4.2 (−4.3 to 12) | −2.4 (−11 to 5.9) |
| Mean childhood BPA level (n = 223) | ||||
| Emotional control scale | 3.9 (0.3 to 7.5) | 2.5 (−1.2 to 6.2) | 1.6 (−3.4 to 6.7) | 4.1 (−1.3 to 9.5) |
| Inhibit scale | 1.8 (−2.5 to 6.0) | −0.7 (−5.1 to 3.8) | −1.2 (−7.1 to 4.7) | 2.2 (−4.2 to 8.5) |
| Plan/organize scale | 3.7 (−0.7 to 8.0) | 1.7 (−2.9 to 6.3) | 1.4 (−4.8 to 7.7) | 3.3 (−3.3 to 10) |
| Shift scale | 1.7 (−2.2 to 5.6) | 0.0 (−4.2 to 4.2) | 0.2 (−5.5 to 5.9) | 1.0 (−5.1 to 7.1) |
| Working memory scale | 3.3 (−1.4 to 8.0) | 1.7 (−3.3 to 6.6) | 1.2 (−5.4 to 7.9) | 3.5 (−3.7 to 11) |
Models were adjusted for race (white, black, or other), household income (continuous, in thousands of dollars), education (graduate/professional school, bachelor's degree, some college, high school, or less than high school), marital status (married or unmarried), depressive symptoms during pregnancy (continuous), Home Observation for Measurement of the Environment scores (continuous), log10-transformed mean gestational serum cotinine concentrations, and log10-transformed mean gestational low-molecular weight urinary phthalate concentrations.
Model includes child gender and a BPA-child gender interaction term.
For gender–BPA level interaction, P < .10.
Adjustment for confounders shifted most point estimates for associations between gestational BPA exposure and BASC-2/BRIEF-P scores up, and in some cases through the null. Conversely, adjustment for confounders shifted most estimated associations between childhood BPA exposure and neurobehavioral scores down, and sometimes through the null. BPA estimates from unadjusted and adjusted models had similar precision, as evidenced by the 95% CI width.
Secondary Analyses
The inclusion of both gestational and childhood urinary BPA concentrations in the same model did not alter the pattern of observed results, and neither did adjustment for maternal IQ, parity, or duration of breastfeeding (results not shown). We observed a similar pattern of results when we limited our analyses to women (n = 215) and children (n = 154) with all 3 urine samples during gestation and childhood, respectively. The exclusion of women (n = 32) or children (n = 13) with dilute or concentrated urine did not change the pattern of results.
DISCUSSION
Gestational urinary BPA concentrations were associated with some neurobehavioral measures at 3 years of age in this cohort. In particular, gestational BPA exposure was associated with higher scores for measures of anxiety, hyperactivity, emotional control, and behavioral inhibition. Similar to our previous findings, the effects of gestational BPA exposure on these behavioral domains were larger among girls than boys.2 The different responses to gestational BPA exposure were especially pronounced for hyperactivity; girls exhibited increases in hyperactivity, and boys exhibited decreases in hyperactivity. In contrast, childhood urinary BPA concentrations were less important predictors of behavior or executive functions in this study.
The findings presented are consistent with numerous studies demonstrating altered neurobehavior among BPA-exposed animals.14,38 Gestational BPA exposures might affect endocrine or other neurotransmitter pathways and disrupt sexual differentiation of the brain, to alter behavior in a gender-dependent manner.39,40 However, the exposures and behavioral end points used in some animal studies might not be relevant or comparable to human cases.41 A recent epidemiological study suggests that gestational BPA exposure may be associated with impaired social behaviors in children.42 However, the authors did not find that their associations were modified by child gender.
The association of anxious, hyperactive, and depressive behaviors with gestational BPA exposure seems paradoxical at first; however, there is substantial comorbidity between attention-deficit/hyperactivity disorder, depression, and anxiety disorders.43 The pattern of observed associations suggests that gestational BPA exposure may affect neurobehavioral domains associated with behavioral regulation. Additional research using neuropsychologically based measures of these domains would enhance our understanding of BPA-neurobehavior relationships.
Our results suggested that girls in this cohort were more sensitive to gestational BPA exposures than were boys. This pattern should be interpreted cautiously, given the imprecision of the observed associations among girls and the low statistical power for interactions between gender and BPA exposures. This finding is intriguing, however, given the endocrine-disrupting nature of BPA. Future studies should examine other sexually dimorphic behaviors and should address whether boys and girls have different levels of susceptibility to BPA at different periods of development.
The results from analyses using the BRIEF-P corroborated our findings with the BASC-2 and suggest that associations between gestational BPA exposure and behavior might be related to poor behavioral regulation. Alternatively, this result might reflect the shared correlation between these 2 measures, rather than deficits in performance-based measures of executive function.25,44 The BRIEF-P may merely serve as an additional measure of problem behaviors.
The generalizability of our findings might vary according to predictors of neurobehavior and levels of BPA exposure in selected target populations. Consistent with findings in the United States, children from lower socioeconomic backgrounds had scores indicative of more behavioral and executive function impairment. Our observed urinary BPA concentrations were similar to those measured in other studies with pregnant women.3,45,46 Children's urinary BPA concentrations at 1, 2, and 3 years of age were higher than concentrations reported for adults but were slightly lower than concentrations observed among 6- to 11-year-old children in previous studies.47,48 Higher BPA concentrations in children could be attributable to pharmacokinetic factors or increased food consumption per unit of body mass.24
Accurate assessment of BPA exposure during the correct period of susceptibility is difficult. One of the primary strengths of this study is that we collected 6 spot urine samples from mothers and their children and averaged ≥2 urinary BPA concentrations during gestation or childhood, to reduce exposure variability. BPA concentrations in multiple spot urine samples still may exhibit substantial within-person variability but may classify BPA exposure accurately over time scales of days to weeks.22,47,49,50 With the assumption of nondifferential exposure misclassification, this error would result in null-biased estimates.51 Integrated exposure measures such as mean BPA concentrations might reduce misclassification rates, but they would decrease the ability to identify short time-sensitive windows of development. Future studies should consider the importance of collecting multiple or integrated urinary concentration measurements, to improve exposure classification during critical windows of neurodevelopment.
We adjusted for a variety of confounders, including factors that are difficult to measure, such as the caregiving environment and biomarkers of other environmental toxicants. Adjustment did not greatly affect the magnitude of most estimates, which suggests that confounding by these factors was not an important source of bias. However, additional, unidentified, confounding factors, including other hormonally active chemicals that vary with BPA or heritable personality traits that influence BPA exposure and childhood behavior, might explain some of the observed associations.
Our sample size was modest, which reduced our statistical power to test for gender modification and led to wide CIs. In addition, we examined many exposure-outcome associations, which increased the likelihood that our results might include the null value through chance alone. Instead of applying mathematical corrections for multiple comparisons, such as the Dunn-Bonferroni correction, we avoided strict application and interpretation of statistical significance thresholds (such as the 95% CI excluding the null value).52 Instead, we focused on the patterns, magnitudes, and consistency of our results and compared those factors with findings from our previous studies and experimental studies with animals.38,42
The clinical relevance of these findings is unclear at this point. Despite this uncertainty, clinicians can advise concerned patients to reduce their exposure, as well as cautioning that it is difficult to avoid all sources of exposure and the health consequences of BPA exposure are not fully understood. BPA exposure can be reduced by avoiding canned and packaged foods, receipts, and polycarbonate bottles with the recycling symbol 7.22,38,53,54
CONCLUSIONS
The results of this study suggest that gestational BPA exposure might be associated with anxious, depressive, and hyperactive behaviors related to impaired behavioral regulation at 3 years of age. This pattern was more pronounced for girls, which suggests that they might be more vulnerable to gestational BPA exposure than boys. In contrast, childhood BPA exposure did not exhibit associations with behavior and executive function at 3 years of age. There is considerable debate regarding the toxicity of low-level BPA exposure, and the findings presented here warrant additional research.
ACKNOWLEDGMENTS
This study was funded in part by a Children's Environmental Health Center Grant from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (grant PO1 ES11261). Additional funding came from National Institute of Environmental Health Sciences training grants T32 ES007018 and T32ES007069. The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
We acknowledge the technical assistance of A. Bishop, X. Zhou, R. Hennings, and T. Jia (CDC, Atlanta, GA) in measuring the urinary concentrations of BPA.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- BASC-2
- Behavior Assessment System for Children 2
- BRIEF-P
- Behavior Rating Inventory of Executive Function-Preschool
- BPA
- bisphenol A
- CDC
- Centers for Disease Control and Prevention
- CI
- confidence interval
- LOD
- limit of detection
REFERENCES
- 1. Vandenberg LN, Chauhoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118(8):1055–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braun JM, Yolton K, Dietrich KN, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117(12):1945–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ye X, Pierik FH, Hauser R, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108(2):260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cantonwine D, Meeker JD, Hu H, et al. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ Health. 2010;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ Res. 2007;103(1):9–20 [DOI] [PubMed] [Google Scholar]
- 7. von Goetz N, Wormuth M, Scheringer M, Hungerbühler K. Bisphenol a: how the most relevant exposure sources contribute to total consumer exposure. Risk Anal. 2010;30(3):473–487 [DOI] [PubMed] [Google Scholar]
- 8. Li D, Zhou Z, Qing D, et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod. 2010;25(2):519–527 [DOI] [PubMed] [Google Scholar]
- 9. Fu P, Kawamura K. Ubiquity of bisphenol A in the atmosphere. Environ Pollut. 2010;158(10):3138–3143 [DOI] [PubMed] [Google Scholar]
- 10. Kaddar N, Harthe C, Dechaud H, Mappus E, Pugeat M. Cutaneous penetration of bisphenol A in pig skin. J Toxicol Environ Health. 2008;71(8):471–473 [DOI] [PubMed] [Google Scholar]
- 11. Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures, part III: endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147(6 suppl):S56–S69 [DOI] [PubMed] [Google Scholar]
- 12. vom Saal FS, Akingbemi BT, Belcher SM, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24(2):131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ Res. 2008;108(2):150–157 [DOI] [PubMed] [Google Scholar]
- 14. Tian YH, Baek JH, Lee SY, Jang CG. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse. 2010;64(6):432–439 [DOI] [PubMed] [Google Scholar]
- 15. Weiss B. Sexually dimorphic nonreproductive behaviors as indicators of endocrine disruption. Environ Health Perspect. 2002;110(suppl 3):387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martel MM, Klump K, Nigg JT, Breedlove SM, Sisk CL. Potential hormonal mechanisms of attention-deficit/hyperactivity disorder and major depressive disorder: a new perspective. Horm Behav. 2009;55(4):465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aguiar A, Eubig PA, Schantz SL. Attention deficit/hyperactivity disorder: a focused overview for children's environmental health researchers. Environ Health Perpsect. 2010;118(12):1646–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bellinger DC. What is an adverse effect? A possible resolution of clinical and epidemiological perspectives on neurobehavioral toxicity. Environ Res. 2004;95(3):394–405 [DOI] [PubMed] [Google Scholar]
- 19. Braun JM, Daniels JL, Poole C, Olshan AF, Hornung R, Bernert JT, et al. Prenatal environmental tobacco smoke exposure and early childhood body mass index. Paediatr Perinat Epidemiol. 2010;24(6):524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–5413 [DOI] [PubMed] [Google Scholar]
- 21. Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51 [Google Scholar]
- 22. Braun JM, Kalkbrenner AE, Calafat AM, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119(1):131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reynolds CR, Kamphaus RW. The Clinician's Guide to the Behavior Assessment System for Children (BASC). New York, NY: Guilford; 2002 [Google Scholar]
- 24. World Health Organization; Food and Agriculture Organization Joint FAO/WHO Expert Meeting to Review Toxicological and Health Aspects of Bisphenol A Summary Report. New York, NY: United Nations Food and Agriculture Organization; 2010 [Google Scholar]
- 25. Gioia GA, Andrews Espy K, Isquith PK. Behavior Rating Inventory of Executive Function-Preschool Version (BRIEF-P). Lutz, FL: Psychological Assessment Resources; 2003 [Google Scholar]
- 26. Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Rev. 2002;3(8):617–628 [DOI] [PubMed] [Google Scholar]
- 27. Greenland S, Brumback B. An overview of relations among causal modelling methods. Int J Epidemiol. 2002;31(5):1030–1037 [DOI] [PubMed] [Google Scholar]
- 28. Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35(8):785–791 [DOI] [PubMed] [Google Scholar]
- 29. Caldwell B, Bradley R. HOME Inventory Administration Manual. Little Rock, AK: University of Arkansas at Little Rock; 2003 [Google Scholar]
- 30. Engel SM, Miodovnik A, Canfield RL, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118(4):565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernert JT, Jacob P, III, Holiday DB, et al. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine Tob Res. 2009;11(12):1458–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr. 2007;860(1):106–112 [DOI] [PubMed] [Google Scholar]
- 33. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–1057 [DOI] [PubMed] [Google Scholar]
- 34. Selvin S. Statistical Analysis of Epidemiologic Data. New York, NY: Oxford University Press; 1996 [Google Scholar]
- 35. Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corp; 1999 [Google Scholar]
- 36. Carrieri M, Trevisan A, Bartolucci GB. Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine. Int Arch Occup Environ Health. 2001;74(1):63–67 [DOI] [PubMed] [Google Scholar]
- 37. Sathyanarayana S, Braun JM, Yolton K, Liddy S, Lanphear BP. Case report: high prenatal bisphenol A exposure and infant neonatal neurobehavior. Environ Health Perspect. 2011;119(8):1170–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chapin RE, Adams J, Boekelheide K, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83(3):157–395 [DOI] [PubMed] [Google Scholar]
- 39. Manson JE. Prenatal exposure to sex steroid hormones and behavioral/cognitive outcomes. Metabolism. 2008;57(suppl 2):S16–S21 [DOI] [PubMed] [Google Scholar]
- 40. Collaer ML, Hines M. Human behavioral sex differences: a role for gonadal hormones during early development? Psychol Bull. 1995;118(1):55–107 [DOI] [PubMed] [Google Scholar]
- 41. Li AA, Baum MJ, McIntosh LJ, Day M, Liu F, Gray LE., Jr Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology. 2008;29(3):504–519 [DOI] [PubMed] [Google Scholar]
- 42. Miodovnik A, Engel SM, Zhu C, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844 [DOI] [PubMed] [Google Scholar]
- 44. McAuley T, Chen S, Goos L, Schachar R, Crosbie J. Is the Behavior Rating Inventory of Executive Function more strongly associated with measures of impairment or executive function? J Int Neuropsychol Soc. 2010;16(3):495–505 [DOI] [PubMed] [Google Scholar]
- 45. Ye X, Pierik FH, Angerer J, et al. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa). Int J Hyg Environ Health. 2009;212(5):481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Teitelbaum SL, Britton JA, Calafat AM, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106(2):257–269 [DOI] [PubMed] [Google Scholar]
- 48. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mahalingaiah S, Meeker JD, Pearson KR, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect. 2008;116(2):173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect. 2011;119(7):983–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jurek AM, Greenland S, Maldonado G. How far from non-differential does exposure or disease misclassification have to be to bias measures of association away from the null? Int J Epidemiol. 2008;37(2):382–385 [DOI] [PubMed] [Google Scholar]
- 52. Poole C. Low P-values or narrow confidence intervals: which are more durable? Epidemiology. 2001;12(3):291–294 [DOI] [PubMed] [Google Scholar]
- 53. Rudel RA, Gray JM, Engel CL, et al. Food packaging and bisphenol A and bis(2-ethylhexyl)phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carwile JL, Luu HT, Bassett LS, et al. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect. 2009;117(9):1368–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]