Abstract
OBJECTIVE:
Methicillin-resistant Staphylococcus aureus (MRSA) colonization in NICUs increases the risk of nosocomial infection. Network analysis provides tools to examine the interactions among patients and staff members that put patients at risk of colonization.
METHODS:
Data from MRSA surveillance cultures were combined with patient room locations, nursing assignments, and sibship information to create patient- and unit-based networks. Multivariate models were constructed to quantify the risk of incident MRSA colonization as a function of exposure to MRSA-colonized infants in these networks.
RESULTS:
A MRSA-negative infant in the NICU simultaneously with a MRSA-positive infant had higher odds of becoming colonized when the colonized infant was a sibling, compared with an unrelated patient (odds ratio: 8.8 [95% confidence interval [CI]: 5.3–14.8]). Although knowing that a patient was MRSA-positive and was placed on contact precautions reduced the overall odds of another patient becoming colonized by 35% (95% CI: 20%–47%), having a nurse in common with that patient still increased the odds of colonization by 43% (95% CI: 14%–80%). Normalized group degree centrality, a unitwide network measure of connectedness between colonized and uncolonized patients, was a significant predictor of incident MRSA cases (odds ratio: 18.1 [95% CI: 3.6–90.0]).
CONCLUSIONS:
Despite current infection-control strategies, patients remain at significant risk of MRSA colonization from MRSA-positive siblings and from other patients with whom they share nursing care. Strategies that minimize the frequency of staff members caring for both colonized and uncolonized infants may be beneficial in reducing the spread of MRSA colonization.
Keywords: network analysis, infant, newborn, infection control, methicillin-resistant Staphylococcus aureus
WHAT'S KNOWN ON THIS SUBJECT:
Outbreaks of methicillin-resistant Staphylococcus aureus (MRSA) are a concern in NICUs. Efforts to decrease rates of both colonization and infection are commonly undertaken in such units.
WHAT THIS STUDY ADDS:
Although infection-control efforts seem effective in reducing the overall colonization risk, siblings remain at increased risk. Strategies addressing this issue and attempting to minimize episodes of staff members caring for colonized and uncolonized infants may help reduce MRSA spread.
Outbreaks of methicillin-resistant Staphylococcus aureus (MRSA) are a common concern in NICUs.1–3 In attempts to prevent and to control outbreaks, some hospitals have instituted active surveillance programs to test infants for MRSA colonization and to isolate infants with colonization or infection.3–8 Results from such screening programs provide an opportunity to characterize how MRSA colonization spreads within a unit. Although MRSA is thought to be transmitted horizontally among patients by colonized or infected health care workers or parents,7,9–12 few reports have identified and characterized specifically the role of adults in the transmission of MRSA among newborns. Such reports, which often stem from a particular MRSA case or cluster, have relied on costly methods for molecular typing of each MRSA isolate and, once a particular MRSA-positive adult has been identified, have reviewed retrospectively the medical records and screening results for other infants who might have been exposed.
Network analysis has been useful for studying health outcomes, including obesity,13 happiness,14 smoking,15 and sexually transmitted infections.16–18 It provides tools with which to characterize complex systems, their underlying components, and the connections between them.19 The current project uses this approach to evaluate how the ties between NICU patients and their interactions with providers influence the risk of MRSA colonization.
METHODS
Patients and Setting
The Beth Israel-Deaconess Medical Center NICU is a level III facility that contains 24 two-bed rooms. Standard precautions are practiced. Alcohol-based hand sanitizer and sinks are available at the entrance to each room and in the vicinity of each bedspace. No clinical outbreaks of MRSA infection occurred during the study period. Universal weekly screening for MRSA colonization was initiated in June 1999. Cultures were obtained initially from the nares, umbilicus, and rectum. Beginning in November 2004, rectal swabs were eliminated because they were found not to add information beyond that obtained from nasal and umbilical cultures. Infants who were found to have colonization on the basis of surveillance cultures or infection, on the basis of clinical isolates were separated from uncolonized infants, with the exception of siblings, who were allowed to remain together. Cobedding was available for uncolonized siblings. No other attempts were made to cohort MRSA-colonized patients in particular areas of the NICU during the study period. No attempts were made to decolonize patients during the study period, but patients with clinical infections were treated with appropriate antibiotics. Contact precautions (gowns and gloves) were used by health care workers for all infants who were found to be colonized or infected, as well as for uncolonized siblings who were roommates of colonized or infected infants.
We used the electronic health record (EHR) to identify patients who were present in the NICU between June 1, 2002 and December 31, 2007 and who underwent ≥1 screening culture for MRSA. Screening rates were calculated by comparing the weekly number of screening results with the number of patients who were in the NICU for ≥2 nursing shifts during each week. We excluded 16 weeks for which archived patient/nursing assignment data were largely incomplete because of technical problems with the EHR archiving system.
Data Sources
MRSA screening results were obtained from infection-control records. Room assignments and the timing of room transfers were determined from the hospital's information system. A nurse was considered to have cared for a patient if a progress note from the nurse was contained within the patient's EHR. During each nursing shift (generally 12 hours in length), ≥1 note is written by the nurse caring for each patient according to unit policy. Patient/nurse assignments were matched by using notes' time stamps. The hospital's committee on clinical investigation approved the study.
Model Conceptualization and Construction
In network analysis, the terms “ego” and “alter” are used to identify subjects of interest.20 Patients who were susceptible to the outcome of interest, namely, incident MRSA colonization, are termed “egos.” All other patients who were present in the NICU at the same time as the index patient are termed “alters.” Alters, specifically the alters' MRSA colonization status, represented the exposure of interest. Egos were censored after first becoming colonized because they were no longer at risk for the outcome of interest, that is, incident MRSA colonization. In line with infection-control practices in the study NICU, egos continued to be considered MRSA-colonized and thus remained censored even if they were treated with antibiotics because of clinical infections. They remained in the models as MRSA-positive alters for other patients. Patients who were found to be colonized at the initial screening after NICU entry were similarly censored as egos because it was not possible to determine whether their colonization was nosocomial or what their network connections were at the time of colonization. In accordance with clinical practice, patients who were found to be colonized with MRSA were assumed to be colonized for all subsequent epochs in which they remained hospitalized. We used this “once positive, always positive” approach because patients who ever screened positive for MRSA are cohorted separately from never-colonized patients. In addition, any bias attributable to falsely treating MRSA-uncolonized alters as colonized would be toward the null hypothesis; if MRSA colonization in an alter were associated with an ego becoming colonized, then assuming that a MRSA-negative alter were colonized would lessen the association, because that MRSA-“positive” alter would decrease the odds of an ego becoming colonized, compared with the situation if the alter were truly colonized.
Ego-alter pairings changed over time as patients were admitted and discharged. We divided the study period into epochs on the basis of MRSA screening dates. MRSA screening in the NICU usually was performed on a weekly basis; therefore, most epochs (99%) were 7 ± 1 days long. The remaining epochs were 14 days long. We determined all patient statuses on an epoch basis. The ties present between patients were held constant over the course of an epoch.
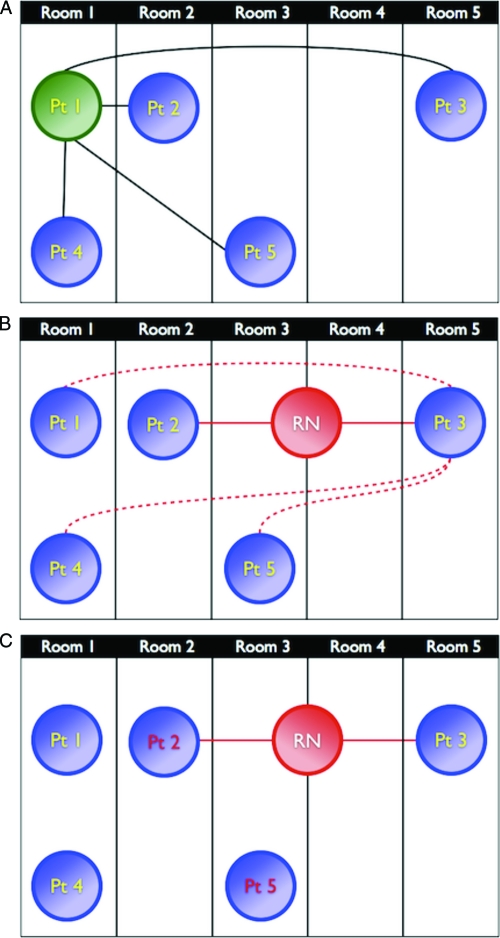
Ego-alter pairs may be connected through different types of connections or ties. The most basic tie considered in this study was being present in the NICU on the same day. We subdivided an ego-alter pair's physical proximity on the basis of the closest rooming assignment of the 2 patients in each epoch, which we categorized as roommates, 1 room apart, 2 rooms apart, or >2 rooms apart. Infants might be moved during an epoch for a variety of reasons that could not be determined in this retrospective study; only the closest rooming assignment during an epoch was considered. Next, we considered ego-alter ties through nurses. Nursing connections, as opposed to other provider connections, were analyzed for 2 reasons: (1) detailed data on which particular nurse interacted with which patients and when those interactions occurred were readily available in our EHR and (2) such information is not readily identifiable from existing sources for physicians because physician notes in this NICU, which has on service at any time no residents and only 1 fellow who comes into contact with all patients, are less likely to reflect the extent or timing of contact with a patient. A pair of patients was considered connected by a nurse if, on ≥1 shift during an epoch, they were cared for by the same nurse. We refer to this tie as a direct nursing connection. Alternatively, we hypothesized that an ego might be exposed to an alter's MRSA through incidental contact from the alter's nurse who was not taking care of the ego but was in his or her vicinity while caring for the ego's roommate or next-door neighbor. This might occur, for example, when the ego's primary nurse requires assistance or is unavailable. We considered such an incidental nursing connection only when an ego and an alter did not share a nurse during an epoch (to distinguish levels of exposure intensity on the basis of nursing connections). These different connections are diagramed schematically in Fig 1A and B. Finally, because siblings were treated differently in terms of infection-control policies in our NICU and might be particularly at risk of MRSA colonization,6 we specifically considered ego-alter pairs tied through sibship.
FIGURE 1.
Schematic diagrams of some of the patient ties (connections) analyzed. Room numbers correspond to physical locations of rooms relative to one another (that is, room 1 is immediately next to room 2, whereas there are 3 rooms between rooms 1 and 5). Details of tie definitions are presented in the text. Pt indicates patient; RN, nurse. A, Black lines represent ties between patient 1 and 4 other patients who were in the NICU with patient 1. From the perspective of patient 1, patient 4 is a roommate, patient 2 is 1 room away, patient 5 is 2 rooms away, and patient 3 is >2 rooms away. B, Patients 2 and 3 are connected through a direct nursing connection (red circle). As a result, indirect nursing connections (dotted lines) exist between patient 3 and patients 1, 4, and 5, because they are all within 1 room of patient 2. C, The normalized group degree centrality in this diagram is 0.33, because only 1 of 3 possible direct nursing connections exists between MRSA-negative infants (yellow text) and ≥1 MRSA-positive infant (red text).
To examine predictors of new MRSA colonization from a NICU-wide perspective, we compared epochs in which there was an incident case of MRSA colonization and epochs in which there were no newly colonized infants. For each epoch, we calculated the average daily census and patient/nurse ratio on the basis of data for all infants who were in the NICU during the study period, including patients with short admissions who were not present at the time of screening cultures. To examine patient care network topologic features, network diameter and density21 were determined for each epoch for the network of patients connected through direct nursing connections. Diameter represents the greatest number of ties, through nursing connections, that must be traversed to connect any 2 patients through the fewest possible nurses. Density describes the proportion of all possible connections between patients that actually exist. Finally, we calculated normalized group degree centrality on the basis of direct nursing connections.22 This measure reflects the proportion of possible connections that actually exist between MRSA-uncolonized infants and ≥1 colonized infant (Fig 1C). For example, a normalized group degree centrality of 1 indicates that every MRSA-uncolonized infant is connected through a direct nursing connection to ≥1 MRSA-colonized infant, whereas a normalized group degree centrality of 0 indicates that no uncolonized infant was cared for by a nurse who simultaneously was caring for a MRSA-colonized patient.
Statistical Analyses
We constructed longitudinal logistic regression models, with clustering with respect to ego and alter, in which ego incident MRSA colonization was predicted as a function of alters' MRSA colonization status and the ties between ego-alter pairs. All models controlled for ego gestational age (dichotomized as <32 vs ≥32 weeks) and postnatal age. Final models also controlled for alters' colonization status in the previous epoch. We assumed an unstructured working correlation structure and used Huber-White sandwich estimates of variance.23,24 After demonstrating that an ego-alter pair composed of siblings of whom 1 was MRSA-positive had significantly higher odds of the uncolonized sibling becoming colonized compared with nonsibling ego-alter pairs, we removed sibling ego-alter pairs from subsequent models. The individual patients remained in the model as egos and alters for other patients. Therefore, although 34% of births were products of multiple gestation, only 1% of data (in which an ego-alter pair was composed of siblings) was lost. We attempted to analyze sibling ego-alter pairs separately but, because of small numbers, we were unable to draw statistically meaningful conclusions from the data.
Univariate descriptors of NICU-wide characteristics were compared by using Student's t tests. Variables found to have P values of <0.2 were considered for inclusion in subsequent multivariate models. Logistic regression was used to construct multivariate models predicting an epoch having an incident case of MRSA colonization at the end of the epoch.
Analyses were performed using Stata 10.1 (Stata Corp, College Station, TX) and SAS 9.1 (SAS Institute, Cary, NC). P values of <.05 were considered statistically significant. Networks were visualized in Pajek,25 a freely available software package for drawing social networks by using the algorithm described by Kamada and Kawai.26
RESULTS
We identified 3488 patients who were treated in the NICU during >1 nursing shift during the study period. Of those, 2620 infants (75%) were screened for MRSA at least once on 272 distinct screening dates, for a total of 9678 screening results. One hundred thirty-five patients (5.2%) were found to be colonized during their hospitalization. Of these, 30 patients tested positive on initial screening and thus were censored as egos because it was not possible to determine their network connections at the time of their colonization; they remained in the model as alters. Patient characteristics are presented in Table 1. The median weekly prevalence of MRSA colonization was 5.9% (range: 0%–34%). The monthly median MRSA colonization incidence was 1.07 cases per 1000 patient-days (range: 0–15 cases per 1000 patient-days). Fifteen of the colonized patients (11.1%) had clinical isolates positive for MRSA before discharge (blood: n = 2; abscess/pustule: n = 2; eye: n = 6; endotracheal: n = 2; other: n = 3); colonization in surveillance cultures preceded or coincided with positive clinical isolates in all cases. Two additional patients had MRSA-positive clinical isolates but persistently negative surveillance cultures (blood: n =1; endotracheal: n = 1).
TABLE 1.
Characteristics of 2620 Patients Screened for MRSA at Least Once During the Study Period
| n (%) | |
|---|---|
| Gestational age | |
| ≤28 wk | 341 (13) |
| 29–32 wk | 743 (28) |
| 33–36 wk | 1160 (44) |
| ≥37 wk | 358 (14) |
| Data not available | 18 (0.7) |
| Birth weight | |
| <1000 g | 237 (9) |
| 1000–1499 g | 461 (18) |
| 1500–2499 g | 1274 (49) |
| ≥2500 g | 625 (24) |
| Data not available | 23 (0.9) |
| Length of stay | |
| ≤7 d | 922 (35) |
| 8–14 d | 543 (21) |
| 15–21 d | 270 (10) |
| 22–28 d | 163 (6) |
| 29–60 d | 448 (17) |
| >60 d | 274 (10) |
| Male | 1402 (54) |
| Multiple gestation | 896 (34) |
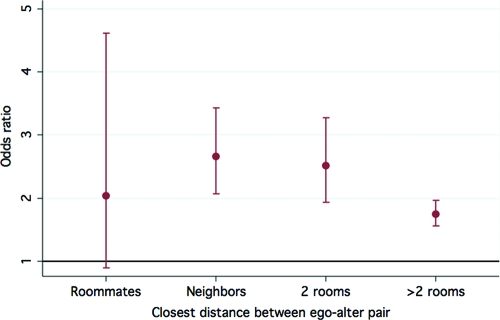
In multivariate models controlling for postnatal age and gestational age of <32 weeks, an ego was significantly more likely to become colonized when exposed (by virtue of being in the NICU simultaneously) to a colonized alter, as opposed to being in the NICU simultaneously with an uncolonized alter (odds ratio [OR]: 2.03 [95% confidence interval [CI]: 1.85–2.23]; P < .001). When a colonized alter was the ego's sibling, compared with a nonsibling colonized alter, the OR increased to 8.8 (95% CI: 5.3–14.8; P < .001) because of influences that could not be ascertained in the current study. Physical proximity between nonsibling patients was not associated with risk of MRSA colonization, although there was a suggestion of a trend of decreasing relative odds with increasing distance (Fig 2).
FIGURE 2.
Effect of physical distance between ego and alter on the risk of MRSA colonization. Bars represent 95% CIs around estimated ORs for an ego becoming MRSA-positive when an alter at a given distance is MRSA-positive instead of MRSA-negative.
When findings were graphed in exploratory analyses, MRSA-positive patients appeared to cluster together when connected through direct or indirect nursing connections (data not shown). When an alter was known to be colonized with MRSA from a previous epoch (and therefore was being treated with contact precautions), the relative odds of an ego becoming colonized decreased significantly (OR: 0.65 [95% CI: 0.53–0.80]; P < .001). However, patient connections through shared nursing care remained a significant risk factor for MRSA colonization. When an ego was connected to a MRSA-colonized alter through a direct nursing connection, the ego was significantly more likely to become colonized, compared with a situation in which the alter was in the NICU but did not share a nurse with the ego during that epoch (OR: 1.43 [95% CI: 1.14–1.80]; P = .002). Similarly, when the connection was through an indirect nursing connection, the OR was 1.21 (95% CI: 0.98–1.50; P = .07), although the results did not reach statistical significance. These results are summarized in Table 2.
TABLE 2.
Multivariate Model Predicting Patient's Incident MRSA Colonization
| OR (95% CI) | P | |
|---|---|---|
| Direct nursing | 1.43 (1.14–1.80) | .002 |
| Indirect nursing | 1.21 (0.98–1.50) | .07 |
| Alter known to be MRSA-positive in previous epoch | 0.65 (0.53–0.80) | <.001 |
| Ego gestational age of <32 wk | 2.13 (1.98–2.30) | <.001 |
Direct nursing and indirect nursing refer to having such connections to a MRSA-positive patient. The model was adjusted for postnatal age at the time of screening.
In univariate analyses, epochs with an incident case of MRSA colonization had higher average daily census counts and higher patient/nurse ratios, compared with epochs with no new MRSA cases, although absolute differences generally were small (Table 3). In addition, normalized group degree centrality was significantly higher in epochs in which there was a newly colonized infant. Network diameter and density did not differ between epochs with incident MRSA colonization and those without. In multivariate models, only normalized group degree centrality (OR: 18.1 [95% CI: 3.6–90.0]; P < .001) remained a significant predictor of a new patient becoming colonized in that epoch. In addition, the patient/nurse ratio showed a trend toward statistical significance (OR: 3.4 [95% CI: 0.89–13.1]; P = .07). The average daily census was not included in the final model because of colinearity with the patient/nurse ratio.
TABLE 3.
Univariate Comparison of NICU-wide Statistics in Epochs With ≥1 Incident Case of MRSA Colonization and Those Without Any and Multivariate Model Predicting That Epoch Would Have ≥1 Incident Case of MRSA Colonization
| Incident MRSA | No Incident MRSA | OR (95% CI) | P | |
|---|---|---|---|---|
| Univariate | — | |||
| Daily census, mean ± SD, No. of patients | 33.8 ± 0.56 | 33.0 ± 0.46 | — | .17 |
| Patient/nurse ratio, mean ± SD | 2.9 ± 0.03 | 2.8 ± 0.02 | — | .004 |
| Network diameter, mean ± SD, No. of ties | 4.5 ± 0.11 | 4.4 ± 0.08 | — | .45 |
| Network density, mean ± SD, % | 0.21 ± 0.004 | 0.21 ± 0.003 | — | .96 |
| Normalized GDC, mean ± SD | 0.39 ± 0.03 | 0.28 ± 0.02 | — | <.0001 |
| Multivariate | ||||
| Patient/nurse ratio | — | — | 3.4 (0.89–13.1) | .07 |
| Normalized GDC | — | — | 18.1 (3.6–90.0) | .0004 |
GDC indicates group degree centrality.
DISCUSSION
The results of this study indicate that specific connections between colonized and uncolonized patients have a significant impact on the likelihood that MRSA-negative patients will become colonized. To our knowledge, this study represents the first application of social network theory to infection-control data in an inpatient setting. Despite current infection-control strategies, including contact precautions and physical separation of MRSA-positive infants, we found that persistent connections to these infants through sibship and nursing care significantly increased uncolonized patients' odds of becoming colonized. Of note, these risk factors are both potentially modifiable through changes in staffing patterns, cohorting practices, improvements in hand hygiene, and infection-control practices focused on multiple gestations for parents and staff members.
Network analysis conceptualizes a system as being composed of objects and the connections that exist between them.27,28 Networks can be examined by using graphical visualization or assessment of quantitative metrics.19 Although a wide variety of analytic measures and methods are available to examine network characteristics at single points in time, methods to analyze quantitatively longitudinal network changes are only beginning to be widely used.21,29
It is not surprising that we found that a MRSA-positive infant would be significantly more likely to put his or her sibling at risk of colonization, compared with a nonsibling infant. Several mechanisms may underlie this finding. Khoury et al6 found that multiple gestation was a significant independent risk factor for MRSA colonization, even with controlling for lower birth weight and gestational age. Those authors proposed that 2 factors may contribute to the risk, that is, inherent vulnerability of products of multiple gestation and siblings sometimes sharing the same bed, thus facilitating transmission among them. Of note, in our NICU, siblings were not moved to separate rooms when 1 was found to be MRSA-positive, unlike nonsibling roommates. Although cobedding was not practiced when 1 sibling was known to be colonized and the other was not, a sibling pair might have cobedded before their colonization status was known (given that screening was performed on a weekly basis). Parents also may have a particular role in spreading colonization among siblings, and health care workers may practice infection-control procedures differently when moving among siblings.
We found that a MRSA-negative patient had significantly increased odds of colonization when that patient was connected to a MRSA-positive infant through a nurse, compared with a situation in which the 2 patients had no nursing care in common. Moreover, when the common nurse was caring for the MRSA-negative infant directly, compared with caring only for that infant's roommates or neighbors, and the exposure was presumably more intense, the relative odds of MRSA colonization were higher. The increased risk of shared nursing assignments for the development of new MRSA colonization remained even for connections to infants who were already designated for contact precautions on the basis of a previous week's MRSA screening culture results (although it was not possible to determine compliance with this infection-control measure for any particular patient in this retrospective study). The increased risk observed with a shared nursing connection is not simply a reflection of cohorting practices, because our outcome of interest, incident MRSA colonization, affects only infants not previously known to be MRSA-positive.
From a NICU-wide perspective, we found that a larger proportion of connections between colonized and uncolonized infants through shared nursing connections was an independent risk factor (indeed, the only risk factor that remained statistically significant in the multivariate model) for an incident case of MRSA in that epoch. Therefore, cohorting of nurses and other health care workers such that they care only for colonized or uncolonized infants may be beneficial, particularly during MRSA outbreaks. Furthermore, when MRSA colonization seems to be increasing in a NICU, cohorting nursing assignments to separate areas of the unit may be beneficial. This notion is supported by the mathematical modeling of the spread of Mycoplasma pneumoniae in an inpatient setting described by Ancel Meyers et al,30 which suggested that limiting the diversity of interactions between different caregivers and patients may be the most effective infection-control intervention.
This investigation carries the inherent limitations of a retrospective approach. Although our models controlled for lower gestational age, we did not have more precise measures of illness severity.31,32 Other unmeasured confounders, such as adherence to hand hygiene guidelines, also may exist. To bias our results, however, such confounders would need to be associated not only with becoming colonized with MRSA but also with being linked to a MRSA-positive infant through the specific tie of interest. Severing such a tie (eg, a nursing connection) between 2 infants still represents a potential point of intervention for curbing the spread of MRSA colonization. As suggested by our results, even a 10% reduction in the proportion of potential connections between the colonized and uncolonized groups of infants would be predicted to be associated with a 25% (95% CI: 12%–36%) reduction in the odds of developing new cases of MRSA colonization. These improvements could be expected without the need to decrease average daily census counts or to increase staffing levels through increased attention to the design of nursing care assignments on the basis of patients' MRSA status. Our model's central estimate of the effect of the patient/nurse ratio on MRSA spread would suggest a need to increase nurse staffing by 9% (eg, an additional 1 or 2 nurses per shift) to achieve a similar result. This study was conducted in a single NICU, and the results may not be generalizable to other settings. Finally, this study examined only 1 type of connection among patients through health care workers, namely, nurses. We chose to examine connections through nursing care because nursing documentation translates directly into contact with patients at particular times and data on nursing assignments were readily available in our EHR. Future studies may use expanded data sources, such as notes from bedside clinician groups and computerized provider order entry, to identify other health care workers involved in patient care.
CONCLUSIONS
In agreement with other studies,3 our analyses revealed the generally low incidence of MRSA colonization and progression to clinical infection that can be associated with routine surveillance programs and isolation of colonized infants as part of an overall infection-control strategy. We have also demonstrated the importance of considering an infant's ties to other colonized patients, in addition to individual patient characteristics, when examining the spread of MRSA colonization. Despite current infection-control policies, MRSA-negative infants are at significant risk of colonization through connections to colonized siblings and to any other colonized patient with whom they share a nursing connection. Use of neglected metadata contained in the EHR and network analytic methods allowed us to identify modifiable risk factors for MRSA colonization. These methods, which are not traditionally used in health care research, are likely to prove useful in future quality improvement and epidemiological endeavors.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the National Library of Medicine (grant 1 G08 LM 010703-01) and by the Center for Integration of Medicine and Innovative Technology (US Army Medical Research Acquisition Activity Cooperative Agreements DAMD17-02-2-0006, W81XWH-07-2-0011, and W81XWH-09-2-0001).
We thank Elise Robinson, PhD, for invaluable statistical advice.
The information contained herein does not necessarily reflect the position or policy of the US government, and no official endorsement should be inferred.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- MRSA
- methicillin-resistant Staphylococcus aureus
- EHR
- electronic health record
- OR
- odds ratio
- CI
- confidence interval
REFERENCES
- 1. Carey AJ, Duchon J, Della-Latta P, Saiman L. The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit, 2000–2007. J Perinatol. 2010;30(2):135–139 [DOI] [PubMed] [Google Scholar]
- 2. Gerber SI, Jones RC, Scott MV, et al. Management of outbreaks of methicillin-resistant Staphylococcus aureus infection in the neonatal intensive care unit: a consensus statement. Infect Control Hosp Epidemiol. 2006;27(2):139–145 [DOI] [PubMed] [Google Scholar]
- 3. Gregory ML, Eichenwald EC, Puopolo KM. Seven-year experience with a surveillance program to reduce methicillin-resistant Staphylococcus aureus colonization in a neonatal intensive care unit. Pediatrics. 2009;123(5). Available at: www.pediatrics.org/cgi/content/full/123/5/e790 [DOI] [PubMed] [Google Scholar]
- 4. Back NA, Linnemann CC, Jr, Staneck JL, Kotagal UR. Control of methicillin-resistant Staphylococcus aureus in a neonatal intensive-care unit: use of intensive microbiologic surveillance and mupirocin. Infect Control Hosp Epidemiol. 1996;17(4):227–231 [DOI] [PubMed] [Google Scholar]
- 5. Jernigan JA, Titus MG, Gröschel DH, Getchell-White S, Farr BM. Effectiveness of contact isolation during a hospital outbreak of methicillin-resistant Staphylococcus aureus. Am J Epidemiol. 1996;143(5):496–504 [DOI] [PubMed] [Google Scholar]
- 6. Khoury J, Jones M, Grim A, Dunne WM, Jr, Fraser V. Eradication of methicillin-resistant Staphylococcus aureus from a neonatal intensive care unit by active surveillance and aggressive infection control measures. Infect Control Hosp Epidemiol. 2005;26(7):616–621 [DOI] [PubMed] [Google Scholar]
- 7. Saiman L, Cronquist A, Wu F, et al. An outbreak of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2003;24(5):317–321 [DOI] [PubMed] [Google Scholar]
- 8. Sax H, Posfay-Barbe K, Harbarth S, et al. Control of a cluster of community-associated, methicillin-resistant Staphylococcus aureus in neonatology. J Hosp Infect. 2006;63(1):93–100 [DOI] [PubMed] [Google Scholar]
- 9. Al-Tawfiq JA. Father-to-infant transmission of community-acquired methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2006;27(6):636–637 [DOI] [PubMed] [Google Scholar]
- 10. Bertin ML, Vinski J, Schmitt S, et al. Outbreak of methicillin-resistant Staphylococcus aureus colonization and infection in a neonatal intensive care unit epidemiologically linked to a healthcare worker with chronic otitis. Infect Control Hosp Epidemiol. 2006;27(6):581–585 [DOI] [PubMed] [Google Scholar]
- 11. Hollis RJ, Barr JL, Doebbeling BN, Pfaller MA, Wenzel RP. Familial carriage of methicillin-resistant Staphylococcus aureus and subsequent infection in a premature neonate. Clin Infect Dis. 1995;21(2):328–332 [DOI] [PubMed] [Google Scholar]
- 12. Morel AS, Wu F, Della-Latta P, Cronquist A, Rubenstein D, Saiman L. Nosocomial transmission of methicillin-resistant Staphylococcus aureus from a mother to her preterm quadruplet infants. Am J Infect Control. 2002;30(3):170–173 [DOI] [PubMed] [Google Scholar]
- 13. Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370–379 [DOI] [PubMed] [Google Scholar]
- 14. Fowler JH, Christakis NA. Dynamic spread of happiness in a large social network: longitudinal analysis over 20 years in the Framingham Heart Study. BMJ. 2008;337:a2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358(21):2249–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fichtenberg CM, Muth SQ, Brown B, Padian NS, Glass TA, Ellen JM. Sexual network structure among a household sample of urban African American adolescents in an endemic sexually transmitted infection setting. Sex Transm Dis. 2009;36(1):41–48 [DOI] [PubMed] [Google Scholar]
- 17. Lee SS, Tam DK, Tan Y, et al. An exploratory study on the social and genotypic clustering of HIV infection in men having sex with men. AIDS. 2009;23(13):1755–1764 [DOI] [PubMed] [Google Scholar]
- 18. Rothenberg R, Muth SQ, Malone S, Potterat JJ, Woodhouse DE. Social and geographic distance in HIV risk. Sex Transm Dis. 2005;32(8):506–512 [DOI] [PubMed] [Google Scholar]
- 19. Lazer D, Pentland A, Adamic L, et al. Computational social science. Science. 2009;323(5915):721–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iacobucci D. Graphs and matrices. In: Wasserman S, Faust K. eds. Social Network Analysis: Methods and Applications. Cambridge, England: Cambridge University Press; 1994:92–166 [Google Scholar]
- 21. Wasserman S, Faust K. Social Network Analysis: Methods and Applications. Cambridge, England: Cambridge University Press; 1994 [Google Scholar]
- 22. Everett MG, Borgatti SP. Extending centrality. In: Carrington PJ, Scott J, Wasserman S. eds. Models and Methods in Social Network Analysis. Cambridge, England: Cambridge University Press; 2005:57–76 [Google Scholar]
- 23. Froot KA. Consistent covariance matrix estimation with cross-sectional dependence and heteroskedasticity in financial data. J Financ Quant Anal. 1989;24(3):333–355 [Google Scholar]
- 24. Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–646 [DOI] [PubMed] [Google Scholar]
- 25. Batagelj V, Mrvar A. Pajek: analysis and visualization of large networks. In: Junger M, Mutzel P. eds. Graph Drawing Software. Berlin, Germany: Springer; 2003:77–103 [Google Scholar]
- 26. Kamada T, Kawai S. An algorithm for drawing general undirected graphs. Inf Process Lett. 1989;31(1):7–15 [Google Scholar]
- 27. Barabasi AL. Linked: How Everything Is Connected to Everything Else and What It Means for Business, Science, and Everyday Life. New York, NY: Plume; 2003 [Google Scholar]
- 28. Barabási AL. Network medicine: from obesity to the “diseasome.” N Engl J Med. 2007;357(4):404–407 [DOI] [PubMed] [Google Scholar]
- 29. Luke DA, Harris JK. Network analysis in public health: history, methods, and applications. Annu Rev Public Health. 2007;28:69–93 [DOI] [PubMed] [Google Scholar]
- 30. Ancel Meyers L, Newman ME, Martin M, Schrag S. Applying network theory to epidemics: control measures for Mycoplasma pneumoniae outbreaks. Emerg Infect Dis. 2003;9(2):204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gray JE, Richardson DK, McCormick MC, Workman-Daniels K, Goldmann DA. Neonatal therapeutic intervention scoring system: a therapy-based severity-of-illness index. Pediatrics. 1992;90(4):561–567 [PubMed] [Google Scholar]
- 32. Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics. 1993;91(3):617–623 [PubMed] [Google Scholar]