Abstract
OBJECTIVE:
GM2 gangliosidoses are caused by an inherited deficiency of lysosomal β-hexosaminidase and result in ganglioside accumulation in the brain. Onset during infancy leads to rapid neurodegeneration and death before 4 years of age. We set out to quantify the rate of functional decline in infantile GM2 gangliosidosis on the basis of patient surveys and a comprehensive review of existing literature.
METHODS:
Patients with infantile GM2 gangliosidosis (N = 237) were surveyed via questionnaire by the National Tay Sachs & Allied Diseases Association (NTSAD). These data were supplemented by survival data from the NTSAD database and a literature survey. Detailed retrospective surveys from 97 patients were available. Five patients who had received hematopoietic stem cell transplantation were evaluated separately. The mortality rate of the remaining 92 patients was comparable to that of the 103 patients from the NTSAD database and 121 patients reported in the literature.
RESULTS:
Common symptoms at onset were developmental arrest (83%), startling (65%), and hypotonia (60%). All 55 patients who had learned to sit without support lost that ability within 1 year. Individual functional measures correlated with each other but not with survival. Gastric tube placement was associated with prolonged survival. Tay Sachs and Sandhoff variants did not differ. Hematopoietic stem cell transplantation was not associated with prolonged survival.
CONCLUSIONS:
We studied the timing of regression in 97 cases of infantile GM2 gangliosidosis and conclude that clinical disease progression does not correlate with survival, likely because of the impact of improved supportive care over time. However, functional measures are quantifiable and can inform power calculations and study design of future interventions.
Keywords: infantile GM2 gangliosidosis, natural history, Tay Sachs, Sandhoff, survival, lysosomal disorders, neurodegeneration
WHAT'S KNOWN ON THIS SUBJECT:
GM2 gangliosidosis is an autosomal recessive inherited condition of the nervous system with a heterogeneous clinical spectrum. Infants are affected most severely, but the pace and characteristics of neurologic decline and factors that affect survival have not been systematically described.
WHAT THIS STUDY ADDS:
This is the largest cohort of infantile GM2 gangliosidosis studied to date. This article presents the first survival estimates and quantifies the gain and loss of specific developmental milestones in these patients.
Monosialoganglioside 2 (GM2) gangliosidoses represent a heterogeneous autosomal recessive group of disorders caused by deficiency of the lysosomal enzyme β hexosaminidase.1 The resulting accumulation of ganglioside GM2 occurs primarily in neuronal cells and coincides with a progressive broad spectrum of neurologic deterioration. The classic infantile form is known to lead to death between 3 and 5 years of life, but the rate of functional decline remains poorly defined. The juvenile- and adult-onset variants of the hexosaminidase deficiencies have later onset, slower progression, and more variable neurologic findings.2,3
β hexosaminidase consists of 2 major isoenzymes: β-hexosaminidase A (HEXA) and β-hexosaminidase B (HEXB); it needs a noncatalytic GM2 activator for GM2 ganglioside hydrolysis. Isoenzyme HEXA has the structure αβ, and isoenzyme HEXB consists of a ββ structure. Subunit α is encoded by the gene HEXA, subunit β is encoded by HEXB, and GM2A encodes the GM2 activator. Mutations in these 3 genes result in the 3 major forms of GM2 gangliosidoses: (1) Tay Sachs disease, caused by mutations of HEXA that result in a deficiency of HEXA but normal HEXB activity; (2) Sandhoff disease, caused by mutations of HEXB that result in a deficiency of both isoenzymes HEXA and HEXB; and (3) the AB variant, caused by mutations in GM2A that result in detectable activities of HEXA and HEXB but an inability to form a functional ganglioside GM2/GM2A complex. The incidence of the Tay Sachs variant in the general population is estimated to be 1 in 222 000 live births4; Sandhoff-variant incidence has been reported at 1 in 422 000.
Recently, correction of the gene and enzyme has been achieved in animal models of GM gangliosidoses with gene delivery of the lysosomal enzymes using adeno-associated viral vectors.5–7 The observed benefits are encouraging and support the development of human clinical trials. We set out to quantify functional status and its variability over time and establish a scoring system that could be applied to future clinical trials. The study was facilitated by access to patients through the National Tay Sachs & Allied Diseases Association (NTSAD), an advocacy organization.
METHODS
Identification and Recruitment of Patients
A survey was developed at Massachusetts General Hospital (MGH) and the National Institutes of Health (Drs Eichler and Tifft) (see Supplemental Appendix). Patients were identified and recruited through the NTSAD database. This database included contact information for families of 237 patients with infantile GM2 gangliosidosis. Surveys were distributed to these families and returned to the NTSAD, where they were deidentified. Anonymized surveys were received and analyzed at MGH (by Drs Bley, Giannikopoulos, and Eichler). The study was approved by the MGH institutional review board, which granted a waiver of written consent (because replying to the NTSAD and completing questionnaires provided implied consent). Patients who had received an experimental treatment such as a hematopoietic stem cell transplant (HSCT) were evaluated separately. Data from patients whose families did not respond but for whom life-span data were available were also analyzed. In addition, we searched PubMed with key words “Tay Sachs” (1597 resulting articles) and “GM2 gangliosidosis not Tay Sachs” (412 resulting articles) for articles from 1946 onward, and additional references of review chapters provided literature dating back to 1881. Articles in English, German, Spanish, French, Italian, and Portuguese were screened for case reports with a description of the life span of children with infantile GM2 gangliosidosis. Inclusion criteria were a confirmed diagnosis of Tay-Sachs disease/Sandhoff disease/GM2 gangliosidosis with a compatible clinical description of the clinical course of the disease and/or description of a cherry red spot within the older literature.
Statistics
We performed descriptive statistical calculations to summarize the clinical findings from our survey patients. Patients who had received an HSCT were analyzed separately. The median survival rate was calculated by using the Kaplan-Meier method. Survival curves among survey patients, patients from the NTSAD database, and patients from the literature were compared by using the log-rank test.
From the clinical information in the surveys, the life span of patients with symptoms in the first 6 months of life was compared with that of the patients with later symptom onset. We also compared the life span of patients who had received a gastric tube (GT) to that of the patients who had not. GM2 variants and gender were compared with a Student's t test.
A Spearman rank correlation was performed for the most commonly reported symptoms. For the correlation analysis, missing data were mean-imputed, and the month of functional loss was set to 0 (the worst possible outcome) for patients who never attained the function. A Cox proportional hazards model was used to determine independent predictors of survival.
RESULTS
Patient Population
Survey data were available for 97 patients (41% of contacted families). The majority (85%) of the surveys were completed by the patient's mother. Life-span data on an additional 103 patients (88 deceased, 15 alive) were obtained from the NTSAD database. Life-span data on 121 patients (all deceased) was extracted from the literature.8–75 Information from the survey, the NTSAD database, and the literature was used to report on survival. Information from surveys alone was used to report on symptoms and functional domains.
Our survey included 49 boys (50%) and 48 girls (50%). Twenty-two patients (23%) had infantile Sandhoff disease, and 75 (77%) had infantile Tay-Sachs disease. All responding patients with the AB variant had onset later than in infancy and were excluded from our analysis. Of the 97 patients, 78 (80%) were deceased. Five of the 97 patients (5%) had undergone an HSCT and were evaluated separately. Hence, 92 patients formed the basis of our analysis (Tables 1–5).
TABLE 1.
Ethnic Background of the Patients With Infantile GM2 Gangliosidosis
| Ethnicity | Percentage of Survey Population |
|---|---|
| Mixed | 51 |
| Unknown | 23 |
| Jewish | 17 |
| British | 2 |
| Indian | 2 |
| Hispanic | 1 |
| German | 1 |
| Filipino | 1 |
| Black/African American | 1 |
| Cajun | 1 |
TABLE 2.
Characteristics of the Patients With Infantile GM2 Gangliosidosis
| Group | Total (N = 92) | Tay Sachs Disease (n = 72) | Sandhoff Disease (n = 20) |
|---|---|---|---|
| Variants | |||
| Age at disease onset, mean ± SD, mo | 5.0 ± 3.3 | 5.2 ± 3.2 | 4.4 ± 3.5 |
| Age at diagnosis, mean ± SD, mo | 13.3 ± 5.3 | 13.1 ± 4.7 | 14.0 ± 7.1 |
| Male/female, n/n | 46/46 | 36/36 | 10/10 |
| Clinical features at onset, n (%)a | |||
| Not making milestones | 76 (83) | 58 (81) | 18 (90) |
| Exaggerated startle response | 60 (65) | 46 (64) | 14 (70) |
| Low muscle tone | 55 (60) | 42 (58) | 13 (65) |
| Fine motor problems | 39 (42) | 28 (39) | 11 (55) |
| Visual problems | 38 (41) | 28 (39) | 10 (50) |
| Seizures | 38 (41) | 28 (39) | 10 (50) |
| Loss of motor skills | 36 (39) | 27 (38) | 9 (45) |
| Decreased responsiveness | 36 (39) | 27 (38) | 9 (45) |
| Failure to thrive | 24 (26) | 15 (21) | 9 (45) |
| Sleep disturbance | 19 (21) | 12 (17) | 7 (35) |
| Screaming episodes | 15 (16) | 12 (17) | 3 (15) |
| Irritability | 15 (16) | 12 (17) | 3 (15) |
| Abnormal movements | 14 (15) | 8 (11) | 6 (30) |
In decreasing order of prevalence.
TABLE 3.
Developmental Milestones in Infantile GM2 Gangliosidosis: Gain and Loss
| Milestones of Normal Development | Patients Who Gained Function, n (%) | Patients Who Lost Function, n/N (%) | All Patients (n = 92), Age of Gain, Mean ± SD, mo | All Patients (n = 92), Age of Loss, Mean ± SD, mo | Tay Sachs Disease (n = 72), Age of Gain, Mean ± SD, mo | Sandhoff Disease (n = 20), Age of Gain, Mean ± SD, mo | Normal Age of Gain (DDST II), Mean, mo |
|---|---|---|---|---|---|---|---|
| All study children | |||||||
| Head control | 82 (89) | 55/82 (67) | 4.1 ± 2.2 | 15.3 ± 9.7 | 4.2 ± 2.4 | 4.0 ± 1.6.0 | 3.5 |
| Ability to vocalize | 74 (80) | 63/74 (85) | 4.4 ± 3.6 | 14.2 ± 5.9 | 4.4 ± 3.8 | 4.4 ± 1.8 | 1.9/5a |
| Reach for object | 86 (93) | 79/86 (92) | 4.8 ± 1.8 | 16.0 ± 8.1 | 5.0 ± 1.9 | 4.3 ± 1.2 | 4.6 |
| Transfer from hand to hand | 66 (72) | 53/66 (80) | 6.5 ± 2.4 | 13.6 ± 8.1 | 6.2 ± 1.9 | 7.4 ± 3.3 | 6.0 |
| Sitting without propping | 51 (55) | 51/51 (100) | 6.8 ± 1.5 | 13.1 ± 6.7 | 7.0 ± 1.5 | 6.1 ± 1.4 | 5.9 |
| 5 study children with HSCTb | |||||||
| Head control | 5 (100) | 4/5 (80) | 4.0 | 24.0 ± 17.0 | — | 4.0 | 3.5 |
| Ability to vocalize | 4 (80) | 3/4 (75) | 2.0 ± 1.0 | 9.0 ± 4.2 | 1.0 | 2.5 ± 0.7 | 1.9/5.0a |
| Reach for object | 4 (80) | 79/86 (92) | 4.0 ± 2.0 | 13.3 ± 9.5 | 6.0 | 3.0 ± 1.4 | 4.6 |
| Transfer from hand to hand | 66 (72) | 53/66 (80) | 4.5 ± 2.1 | 15.0 ± 12.7 | — | 4.5 ± 2.1 | 6.0 |
| Sitting without propping | 0 (0) | — | — | — | — | — | — |
DDST II indicates Denver Developmental Screening Test II.
Average age for vocalizing is listed for both squealing (1.9 months) and imitating sounds (5 months).
The 5 patients (3 with Tay Sachs disease, 2 with Sandhoff disease) who had undergone an HSCT showed no significant differences, and no milestones were gained after the procedure.
TABLE 4.
Caregivers Reporting on Developmental Milestones
| Milestones of Normal Development | Caregivers Reporting on Gain, n/N (%) | Caregivers Reporting on Loss, n/N (%) | Caregivers Reporting on Age of Gain, n/N (%) | Caregivers Reporting on Age of Loss, n/N (%) |
|---|---|---|---|---|
| Head control | 88/92 (96) | 60/82 (73) | 48/88 (55) | 33/60 (55) |
| Ability to vocalize | 90/92 (98) | 74/74 (100) | 48/92 (52) | 47/74 (64) |
| Reach for object | 92/92 (100) | 86/86 (100) | 59/92 (64) | 46/86 (53) |
| Transfer from hand to hand | 90/92 (98) | 65/66 (98) | 36/90 (40) | 24/65 (37) |
| Sitting without propping | 89/92 (97) | 51/51 (100) | 44/89 (49) | 41/51 (80) |
TABLE 5.
Prevalence and Timing of Neurologic Signs and Symptoms of Infantile GM2 Gangliosidosis
| Symptomsa | Patients, n (%) | All Patients (n = 92), Age at Onset, Mean ± SD, mo | Tay Sachs Disease (n = 72), Age at Onset, Mean ± SD, mo | Sandhoff Disease (n = 20), Age at Onset, Mean ± SD, mo |
|---|---|---|---|---|
| Exaggerated startle response | 90 (98) | 7.9 ± 6.6 | 8.1 ± 6.4 | 7.2 ± 7.4 |
| Seizures | 90 (98) | 17.4 ± 5.9 | 16.8 ± 5.5 | 19.5 ± 7.0 |
| Diminished eyesight | 78 (85) | 17.9 ± 11.3 | 18 ± 11.6 | 17.6 ± 11.0 |
| Hypotonia | 63 (68) | 10.9 ± 3.9 | 10.8 ± 3.6 | 11.3 ± 5.0 |
| No eyesight | 63 (68) | 28.4 ± 14.7 | 30 ± 14.8 | 16 ± 7.2 |
| Spasticity | 56 (61) | 16.3 ± 11.4 | 17.4 ± 13.2 | 13.4 ± 4.5 |
| Decreased hearing | 45 (49) | 22.8 ± 15.1 | 24.1 ± 13.7 | 19 ± 21.9 |
| Loss of hearing | 31 (34) | 23.8 ± 17.3 | 23.8 ± 17.3 | — |
| Screaming episodes | 31 (34) | 13.9 ± 8.6 | 13.7 ± 8.9 | 14.6 ± 8.4 |
| Dyskinesia | 21 (23) | 18.6 ± 11.5 | 18.3 ± 10.0 | 19.5 ± 17.2 |
| Ataxia | 11 (12) | 16.3 ± 10.1 | 13.8 ± 6.9 | 37 |
In decreasing order of prevalence.
Ethnicity
Details of ethnicity as determined by the ethnic background of the 4 grandparents are shown in Table 1. Only 17% were of 100% Jewish ancestry, and another 10% had some Jewish ancestry. Patients of Jewish ancestry had no difference in survival rate or rate of functional decline.
Survival
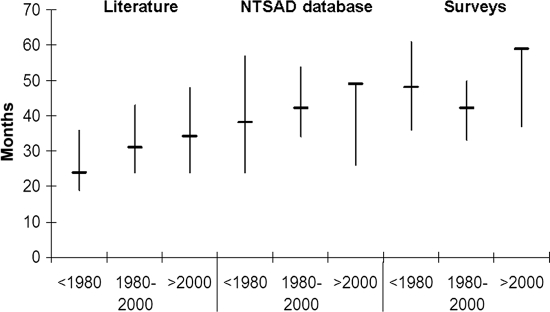
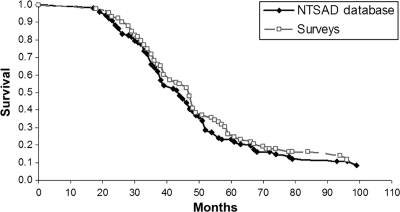
The median life span of patients increased over time in all 3 groups analyzed (surveys, NTSAD database, literature) (Fig 1). The literature included only deceased patients, and their life span was shorter than that in the survey data. Kaplan-Meier curves for patients in the surveys and the NTSAD database are shown in Fig 2. Patients with Tay Sachs disease had an identical median life span compared with that of patients with Sandhoff disease (47 months). There was a trend for improved survival rates in patients who had had a GT placed (hazard ratio: 0.597; P = .0687), which indicates that within any given month their probability of dying was ∼60% compared with those patients without a GT.
FIGURE 1.
Mortality data according to cohort and birth year for patients with infantile GM2 gangliosidosis. Shown are the median and interquartile range.
FIGURE 2.
Survival curves for patients with infantile GM2 gangliosidosis.
Onset of Disease
The mean age at onset of the earliest symptom was 5.0 ± 3.3 months (Table 2). The average age at diagnosis of our patients with GM2-gangliosidoses was 13.3 ± 5.3 months. Patients who had an onset of symptoms between 0 and 6 months of age had the same life span as patients who had their first symptoms thereafter. The most common initial symptoms were developmental arrest (83%), abnormal startle response (65%), and low muscle tone (60%). Data from 62 of the patients (67%) indicated that the finding of a cherry-red spot by an ophthalmologist triggered testing that led to the diagnosis.
Developmental Milestones and Neurologic Symptoms
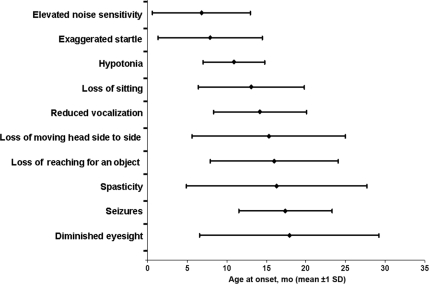
The majority of infants were able to acquire at least some developmental milestones. Those that gained milestones acquired them at the appropriate age according to the Denver Developmental Screening Test II (Fig 3, Tables 3–5). More caregivers answered the inquiries on gain and loss of milestones (∼90%–100%) than on exact timing of those gains and losses (∼40%–60%) (Table 4).
FIGURE 3.
Disease progression of infantile GM2 gangliosidosis.
More infants gained earlier milestones than later milestones. For example, 93% of those in our cohort learned to reach for an object. They gained this skill at an average age of 4.8 ± 1.8 months, which is slightly later than the normal range (2.5–4.0 months on the Denver Developmental Screening Test II [DDST II]). The mean time span for retaining this ability was 12.1 ± 8.7 months. Seventy-two percent of those in our cohort learned to transfer an object from one hand to the other. The skill was gained at an average age of 6.5 ± 2.4 months, which is within the normal range (5–8 months on the DDST II). There was good correlation between certain functions. Patients who gained the ability to sit retained the ability to transfer an object from one hand to the other longer (11.2 ± 9.5 months) than infants who were never able to sit without propping (3.6 months ± SD). Spearman Rank correlation coefficient between time of losing ability to sit and ability to transfer from one hand to the other was ρ = 0.464 [P < .0001]. It is interesting to note that there was an excellent correlation between caregiver-reported onset of increased startle response and increased auditory sensitivity (ρ = 0.79 [P < .0001]). There was little correlation between onset of startle and onset of seizures (ρ = 0.19 [P = .06]).
Most of the patients developed seizures (98%). Most patients required multiple anticonvulsant medications for seizure control (only 23 patients required only 1 anticonvulsant versus 43 patients who required ≥2 of them). Only a few caregivers reported the use of antispasmodic medications, whereas tranquilizers and pain medications were used more frequently. The medications used by these patients are listed in Supplemental Table 7.
Medical Management
Sixty-nine patients (75%) were partially or completely fed by a GT. Seventeen infants underwent gastric surgery with fundoplication. Poor handling of mucus and respiratory congestion are common problems in patients with GM2 gangliosidoses. Parents and guardians of 85 of the children (92%) reported that the infants choked on mucous or oral secretions. The average age of onset was 22 months. Eighty-one infants (88%) needed regular suctioning.
Hematopoietic Stem Cell Transplant.
Of the 5 patients who had undergone an HSCT, 2 had a diagnosis of Sandhoff disease and 3 had a diagnosis of Tay Sachs disease (Table 3). Their average age of symptom onset was 3.8 ± 2.6 months. The average age at diagnosis was 8.8 ± 5.0 months. None of the infants gained the ability to sit without propping. All of them suffered from excessive startle, diminished hearing and eyesight, and spasticity. The average age of HSCT was 10.4 ± 5.8 months. After HSCT, no milestones were gained.
Of the 5 patients, 4 had died and 1 was still alive at the time of the survey. The median life span of the deceased patients was 64 months, but that was not significantly different from that of the patients who had not undergone an HSCT. One patient reportedly died from complications of the HSCT procedure, 2 died from “primary disease,” and 1 died from aspiration pneumonia.
End of Life
Most of the children (60 of 74 [81%]) died at home, only 9 (12%) died in a hospital, and 5% were reported to have died in a hospice. The most common stated cause of death was primary disease (34 of 74 [46%]), followed by aspiration pneumonia (17 of 74 [23%]). Five patients (7%) died from seizures.
DISCUSSION
We report for the first time, to our knowledge, the results of a systematic analysis of milestones during the natural history of infantile GM2 gangliosidoses. In our comprehensive retrospective study we analyzed the neurologic symptoms of 92 patients and compared their survival rate to that of 121 cases from the literature and 103 patients from the NTSAD database.
Our study revealed that more than half of the infants with GM2 gangliosidoses attained initial motor developmental milestones. It was surprising that the infants with GM2 gangliosidoses who attained milestones did so within the standard range of normal development. The majority of infants achieved early motor milestones, such as head control, reaching, and transferring. Those who did not attain initial milestones tended not to gain that ability later in life. Hence, developmental delay is less a feature of infantile GM2 gangliosidosis than frank regression.
The average age at first symptom was 5.0 ± 3.3 months. However, despite the early and progressive symptoms, the diagnosis was, on average, not established until 13.3 months. Misdiagnoses listed in our surveys included cerebral palsy and mitochondrial disorders. Remediating this delay in diagnosis is crucial to therapeutic efforts, because the window for intervention might be brief and later efforts might be futile.
Hypotonia and acoustic hypersensitivity were among the most common early symptoms, followed by hypertonia/spasticity and subsequent loss of hearing. Other early signs and symptoms included a loss of manual dexterity and vocalization and diminished eyesight. Only 55% of the children in our cohort learned to sit without support, and most of those who gained this ability lost it within 1 year (see Table 3). If an infant did not gain the ability to sit by 10 months, he or she did not gain that skill thereafter. The combination of exaggerated startle response and low tone is relatively uncommon and should prompt the general practitioner and neurologist to consider GM2 gangliosidosis as a diagnosis.
Seizures were a late but common symptom. Early seizures seemed to be a marker of disease severity, or at least worse motor developmental outcomes. For example, those infants who had seizures within their first 12 months of life retained the ability to sit for an average of only 3.5 months, whereas some infants with seizures after 12 months retained the ability to sit for as long as 14 months. Once in an advanced stage, seizures were often the focus of clinical care, necessitating multiple anticonvulsant medications, the adverse effects of which were burdensome for the patients and caregivers. This highlights the importance of ongoing multidisciplinary care with a neurologist and palliative care specialist to help optimize symptom control and minimize drug-related morbidity.
On the basis of our findings in infants with GM2 gangliosidosis, we propose a clinical severity scoring system that rates disease severity according to the time at which abilities are lost or new abnormal symptoms arise (Fig 3, Tables 3–6). On our scale, earlier occurrence is scored higher than later occurrence. The less severe the disease, the longer the functions persist. The age cutoffs for loss of abilities or development of symptoms are based on the average age of symptom occurrence plus 1 SD in our population of patients with GM2 gangliosidosis. Patients who develop symptoms before the cutoff receive 2 points, and those who develop them at or after the cutoff receive 1 point. Because of the temporal aspect of the scoring system, some points might still be added up to 4 years of age. We are currently planning a prospective study for validation of this scoring system.
TABLE 6.
Clinical Severity Score of Infantile GM2 Gangliosidosis
| 2 Points | 1 Point | 0 Points |
|---|---|---|
| Increased noise sensitivity at <14 mo | Increased noise sensitivity at ≥14 mo | No increase in noise sensitivity |
| Muscle hypotonia at <16 mo | Muscle hypotonia at ≥16 mo | No muscle hypotonia |
| Startling at <16 mo | Startling at ≥16 mo | No startling |
| Loss of sitting at <21 mo | Loss of sitting at ≥21 mo | Able to sit |
| Reduced vocalization at <21 mo | Reduced vocalization at ≥21 mo | Able to vocalize |
| Seizures at <24 mo | Seizures at ≥24 mo | No seizures |
| Loss of reaching for an object at <25 mo | Loss of reaching for an object at ≥25 mo | Able to reach for an object |
| Loss of moving head side to side at <26 mo | Loss of moving head side to side at ≥26 mo | Able to move head side to side |
| Spasticity at <29 mo | Spasticity at ≥29 mo | No spasticity |
| Diminished eyesight at <30 mo | Diminished eyesight at ≥30 mo | Normal eyesight |
The higher the score, the more advanced the disease.
The Kaplan-Meier survival curves (Fig 2) (based on 92 survey patients and 103 patients from the NTSAD database) are, to our knowledge, the first published analysis results of survival in patients affected by infantile GM2 gangliosidosis. These survival curves confirm that this is a devastating disorder; only one-quarter of patients survive to the age of 5 years. Nearly half of the patients had died by the age of 3 years. The overall life span seems to have increased over the last 50 years (Fig 1), which likely reflects improved symptomatic management such as use of antibiotics and GT placement. This result is consistent with that of our Cox proportional hazard model in which both the influence of birth decade and GT placement on survival approached significance (P = .0544 and .0687, respectively). The fact that life span in the literature is shorter for all decades compared to the NTSAD database may indicate the tendency to publish the most severe cases.
Receipt of an HSCT did not significantly alter survival rates but our sample size was limited (n = 5). Although some of the survey patients received the transplant as early as 5 months of age, no survival advantage or retention of milestones was observed. No controlled clinical trials have been conducted regarding the efficacy of HSCT for infantile GM2 gangliosidosis, but our series data suggest that little or no benefit is to be expected.
It is interesting to note that there was no significant difference in life span between children with an onset of symptoms before and after 6 months. (Even 2 patients who preserved the ability to sit for >2 years longer than the average did not live significantly longer than the average.) We conclude that life span is not a good marker for disease severity in children with infantile GM2 gangliosidoses.
For many decades, GM2 gangliosidoses, especially Tay Sachs, were thought to only affect Jewish children. Our results reveal that a 100% Jewish background was only present in 17% of the patients, which likely reflects the impact of carrier screening within the Ashkenazi community that began in the early 1970s.76 As a result, the majority of patients with infantile Tay-Sachs disease currently born in North America are of non-Jewish ancestry. Therefore, we emphasize the importance of considering a diagnosis of GM2 gangliosidosis for any patient who presents with clinical symptoms, not just those with Jewish ancestry.
Our study revealed no gender differences and, in contrast with late-onset cases of GM2 gangliosidosis,2 few differences in the neurologic phenotype when comparing Tay Sachs and Sandhoff diseases. Among the 92 studied patients, we observed that there was a tendency for seizures, visual problems, and movement abnormalities to occur earlier in patients with Tay Sachs disease.
Limitations of our study lie in its retrospective nature and the self-reporting by parents or other family members. Although our survey data revealed that caregivers can provide a detailed recollection of distinct clinical findings, many of which are often recorded in infant books, milestones might be tainted by subjective impression and cannot be verified objectively. Clinical features that are harder to isolate and define, such as vocalizing, might be more prone to misrepresentation. Reported cases from the literature and life-span data from the NTSAD database allowed for a comparison of survival rates in our survey data to that of other patients with infantile GM2 gangliosidosis. However, we cannot exclude recall bias, ascertainment bias, and the bias of potentially missing data for individual questions.
Our retrospective cohort could serve as a historical control for future clinical trials with recognition of important caveats. The fact that life expectancy has improved over time might lead to false-positive results, because the standard of care will have evolved since the data were acquired. If, in assessing an intervention, the expected effect size of a drug is modest, the variability in the present data might be prohibitive. Yet, the onset of certain symptoms within a narrow time frame might allow for more rapid assessment of interventions than life-span data alone and needs to be prospectively assessed.
CONCLUSIONS
Infantile GM2 gangliosidosis remains one of the most devastating inherited neurologic disorders despite advances in supportive care. Loss of motor milestones and late recalcitrant seizures mark the relentless disease course that poses a challenge to caregivers and specialists alike. Details on the course of regression presented here are a valuable foundation from which to choose outcome measures and design trials for future interventions.
Supplementary Material
ACKNOWLEDGMENTS
This work was conducted with support from Harvard Catalyst/Harvard Clinical and Translational Science Center (National Institutes of Health award UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers), National Institutes of Health training grant K08NS52550 (to Dr Eichler), and National Institutes of Health “Lysosomal Disease Network” grant 5U54NS065768. Ms. Giannikopoulos, Mrs. Kubilus, and Dr. Eichler were supported by the NTSAD.
We thank the families who completed the surveys for their participation, without which this study could not have been performed. We thank Sue Kahn at the NTSAD for her generous support and Dr Alfried Kohlschuetter for supporting Dr Bley. We also thank Dr April Eichler for critical reading of the manuscript.
Drs Eichler and Tifft developed surveys at Massachusetts General Hospital and the National Institutes of Health; Mrs. Kubilus identified and recruited patients through the NTSAD database and distributed surveys, deidentified them, and forwarded them to Drs Bley and Eichler and Ms. Giannikopoulos; Drs Bley and Eichler and Ms. Giannikopoulos analyzed the data; and Mr. Hayden performed the statistical analyses of the data.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- GM2
- monosialoganglioside 2
- HEXA
- β-hexosaminidase A
- HEXB
- β-hexosaminidase B
- GM2A
- GM2 activator
- NTSAD
- National Tay Sachs Allied Diseases Association
- HSCT
- hematopoietic stem cell transplant
- GT
- gastric tube
REFERENCES
- 1. Gravel RA, Kaback MM, Proia RL, Sandhoff K, Suzuki K, Suzuki K. The GM2 gangliosidoses. In: Scriver C, Beaudet A, Sly W, Valle D. eds. Metabolic and Molecular Bases of Inherited Diseases. New York, NY: McGraw-Hill; 2001:3827–3876 [Google Scholar]
- 2. Maegawa GH, Stockley T, Tropak M, et al. The natural history of juvenile or subacute GM2 gangliosidosis: 21 new cases and literature review of 134 previously reported [published correction appears in Pediatrics. 2007;120(4):936]. Pediatrics. 2006;118(5). Available at: www.pediatrics.org/cgi/content/full/118/5/e1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neudorfer O, Pastores GM, Zeng BJ, et al. Late-onset Tay-Sachs disease: phenotypic characterization and genotypic correlations in 21 affected patients. Genet Med. 2005;7(2):119–123 [DOI] [PubMed] [Google Scholar]
- 4. Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281(3):249–254 [DOI] [PubMed] [Google Scholar]
- 5. Broekman ML, Tierney LA, Benn C, Chawla P, Cha JH, Sena-Esteves M. Mechanisms of distribution of mouse beta-galactosidase in the adult GM1-gangliosidosis brain. Gene Ther. 2009;16(2):303–308 [DOI] [PubMed] [Google Scholar]
- 6. Cachón-González MB, Wang SZ, Lynch A, Ziegler R, Cheng SH, Cox TM. Effective gene therapy in an authentic model of Tay-Sachs-related diseases. Proc Natl Acad Sci U S A. 2006;103(27):10373–10378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Broekman ML, Baek RC, Comer LA, Fernandez JL, Seyfried TN, Sena-Esteves M. Complete correction of enzymatic deficiency and neurochemistry in the GM1-gangliosidosis mouse brain by neonatal adeno-associated virus-mediated gene delivery. Mol Ther. 2007;15(1):30–37 [DOI] [PubMed] [Google Scholar]
- 8. Abe T, Ogawa K, Fuziwara H, Urayama K, Nagashima K. Spinal ganglia and peripheral nerves from a patent with Tay-Sachs disease: morphological and ganglioside studies. Acta Neuropathol (Berl). 1985;66(3):239–244 [DOI] [PubMed] [Google Scholar]
- 9. Aidar O, De Assis JL. Fatty degeneration of the neuraxon; anatoma-clinical considerations on an atypical form of familial amaurotic idiocy [in Portuguese]. Arq Neuropsiquiatr. 1951;9(3):276–281 [DOI] [PubMed] [Google Scholar]
- 10. Alvarez-Rodriguez A, Triggs-Raine B, Barros-Nunez P, Lozano CM. A novel HEXA mutation [1393G>A (D465N)] in a Mexican Tay-Sachs disease patient. Hum Mutat. 2001;17(5):437. [DOI] [PubMed] [Google Scholar]
- 11. Aronson SM, Volk BW, Epstein N. Morphologic evolution of amaurotic family idiocy: the protracted phase of the disease. Am J Pathol. 1955;31(4):609–631 [PMC free article] [PubMed] [Google Scholar]
- 12. Bach G, Navon R, Zeigler M, Beyth Y, Porter B, Cohen MM. Tay-Sachs disease in a Moroccan Jewish family: a possible new mutation. Isr J Med Sci. 1976;12(12):1432–1439 [PubMed] [Google Scholar]
- 13. Benninger C, Ullrich-Bot TB, Zhan SS, Schmitt HP. GM2D gangliosidosis B1 variant in a boy of German/Hungarian descent. Clin Neuropathol. 1993;12(4):196–200 [PubMed] [Google Scholar]
- 14. Bjelkhagen I. A case of infantile amaurotic family idiocy. Acta Paediatr. 1950;39(4–5):445–451 [DOI] [PubMed] [Google Scholar]
- 15. Borri PF, Hooghwinkel GJ. Splenic and hepatic gangliosides in amaurotic idiocy of the Tay-Sachs type [in Italian]. Riv Neurobiol. 1967;13(1):207–215 [PubMed] [Google Scholar]
- 16. Carlyll HB, Mott FW. Seven cases of amaurotic idiocy (Tay-Sachs disease). Proc R Soc Med. 1911;4(Pathol Sect):147–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clement R, Gruner J, Rameix P, Bretagne J. Tay-Sachs' amaurotic idiocy [in French]. Presse Med. 1953;61(13):253–255 [PubMed] [Google Scholar]
- 18. Cotlier E. Tay-Sachs' retina: deficiency of acetyl hexosaminidase A. Arch Ophthalmol. 1971;86(3):352–356 [DOI] [PubMed] [Google Scholar]
- 19. Dargeon HW, Barclay M, Calathes DN, Brownell MJ. Observations on the plasma lipoproteins in a case of Tay-Sachs Disease. J Pediatr. 1958;52(1):48–53 [DOI] [PubMed] [Google Scholar]
- 20. De Silva CC, Tennekoon GE. Tay-Sachs disease in two Sinhalese children. Br Med J. 1955;2(4942):768–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Der Kaloustian VM, Khoury MJ, Hallal R, et al. Sandhoff disease: a prevalent form of infantile GM2 gangliosidosis in Lebanon. Am J Hum Genet. 1981;33(1):85–89 [PMC free article] [PubMed] [Google Scholar]
- 22. Dolman CL, MacLeod PM, Chang E. Fine structure of cutaneous nerves in ganglioside storage disease. J Neurol Neurosurg Psychiatry. 1977;40(6):588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Driessen OA. Identity of Tay-Sachs' and Niemann-Pick's diseases [in Dutch]. Maandschr Kindergeneeskd. 1953;21(7):242–247 [PubMed] [Google Scholar]
- 24. Drucker L, Golan A, Boles DJ, el Bedour K, Proia RL, Navon R. Novel HEXA mutation in a Bedouin Tay-Sachs patient associated with exon skipping and reduced transcript level. Hum Mutat. 1997;9(3):260–264 [DOI] [PubMed] [Google Scholar]
- 25. Duke JR, Clark DB. Infantile amaurotic familial idiocy (Tay-Sachs disease) in the Negro race. Am J Ophthalmol. 1962;53:800–805 [DOI] [PubMed] [Google Scholar]
- 26. Esente I, D'Aprile V, Rossi R. Infantile amaurotic idiocy or Tay-Sachs disease: case report [in Italian]. Riv Clin Pediatr. 1957;59(2):204–212 [PubMed] [Google Scholar]
- 27. Franceschetti A, Wildi E, Klein D. Anatomo-clinical examination of a case of infantile amaurotic idiocy (Tay-Sachs) [in French]. Acta Genet Stat Med. 1955;5(4):343–357 [PubMed] [Google Scholar]
- 28. Garner A. Ocular pathology of GM2 gangliosidosis: type 2 (Sandhoff's disease). Br J Ophthalmol. 1973;57(7):514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gautron S, Poenaru L, Boue J, Puissant H, Lisman JJ, Dreyfus JC. Evidence for the presence of beta-subunit of hexosaminidase in a case of Sandhoff disease using a blotting technique. Hum Genet. 1983;63(3):258–261 [DOI] [PubMed] [Google Scholar]
- 30. Gordon BA, Gordon KE, Hinton GG, et al. Tay-Sachs disease: B1 variant. Pediatr Neurol. 1988;4(1):54–57 [DOI] [PubMed] [Google Scholar]
- 31. Gourley IM, Wiglesworth FW. Amaurotic familial idiocy (Tay-Sachs disease) in non-Hebrew siblings. Can Med Assoc J. 1955;72(7):521–524 [PMC free article] [PubMed] [Google Scholar]
- 32. Grosso S, Farnetani MA, Berardi R, et al. GM2 gangliosidosis variant B1 neuroradiological findings. J Neurol. 2003;250(1):17–21 [DOI] [PubMed] [Google Scholar]
- 33. Hadfield MG, Mamunes P, David RB. The pathology of Sandhoff's disease. J Pathol. 1977;123(3):137–144 [DOI] [PubMed] [Google Scholar]
- 34. Haridas C. Waren Tay-Sachs disease in a Chinese infant. Br J Ophthalmol. 1947;31(7):428–436 [PubMed] [Google Scholar]
- 35. Hertzberg R. Tay-Sachs disease: report of three cases in one family. Med J Aust. 1956;43(16):664–665 [PubMed] [Google Scholar]
- 36. Huang JQ, Trasler JM, Igdoura S, Michaud J, Hanal N, Gravel RA. Apoptotic cell death in mouse models of GM2 gangliosidosis and observations on human Tay-Sachs and Sandhoff diseases. Hum Mol Genet. 1997;6(11):1879–1885 [DOI] [PubMed] [Google Scholar]
- 37. Inui K, Wenger DA. Concentrations of an activator protein for sphingolipid hydrolysis in liver and brain samples from patients with lysosomal storage diseases. J Clin Invest. 1983;72(5):1622–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Itoh H, Tanaka J, Morihana Y, Tamaki T. The fine structure of cytoplasmic inclusions in brain and other visceral organs in Sandhoff disease. Brain Dev. 1984;6(5):467–474 [DOI] [PubMed] [Google Scholar]
- 39. Jadhav MV, Landge MP, Sawaimoon SK, Harke AB, Deshmukh SD. Tay Sachs disease: an autopsy case report. Indian J Pathol Microbiol. 2005;48(4):479–480 [PubMed] [Google Scholar]
- 40. Jampel RS, Quaglio ND. Eye movements in Tay-Sachs disease. Neurology. 1964;14:1013–1019 [DOI] [PubMed] [Google Scholar]
- 41. Kelemen G. Tay-Sachs disease and the organ of hearing [in German]. Z Laryngol Rhinol Otol. 1965;44(11):728–738 [PubMed] [Google Scholar]
- 42. Korey SR, Terry RD. Studies in Tay-Sachs disease. I. B. Clinical and pathologic descriptions. J Neuropathol Exp Neurol. 1963;22:10–17 [PubMed] [Google Scholar]
- 43. Krivit W, Desnick RJ, Lee J, et al. Generalized accumulation of neutral glycosphingolipids with GM2 ganglioside accumulation in the brain. Sandhoff's disease (variant of Tay-Sachs disease). Am J Med. 1972;52(6):763–770 [DOI] [PubMed] [Google Scholar]
- 44. Manchanda SS, Nirankari MS, Maudgal MC. Tay-Sachs's disease (amaurotic family idiocy). Br J Ophthalmol. 1957;41(3):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mandel L. Case of Tay-Sachs disease. Proc R Soc Med. 1923;16(Sect Study Dis Child):55–56 [PMC free article] [PubMed] [Google Scholar]
- 46. Maniar B, Arora VD. Amaurotic familial idiocy: case reports of Tay-Sachs' disease in two siblings and a review of twenty cases from the Indian literature. Indian Pediatr. 1966;3(7):241–251 [PubMed] [Google Scholar]
- 47. Marx P, Dhermy P, Langlois J, Tayot J, Brasseur G, Ducastelle T. Anatomo-clinical study of a case of Tay-Sachs disease [in French]. Bull Soc Ophtalmol Fr. 1980;80(2):119–123 [PubMed] [Google Scholar]
- 48. Mathur PS, Srivastava SP. Amaurotic family idiocy: Tay-Sachs disease. J Indian Med Assoc. 1958;31(1):25. [PubMed] [Google Scholar]
- 49. Myerowitz R, Lawson D, Mizukami H, Mi Y, Tifft CJ, Proia RL. Molecular pathophysiology in Tay-Sachs and Sandhoff diseases as revealed by gene expression profiling. Hum Mol Genet. 2002;11(11):1343–1350 [DOI] [PubMed] [Google Scholar]
- 50. Moriwaki S, Takashima S, Yoshida H, Kawano N, Goto M. Histological observation of the brain of Tay-Sachs disease with seizure and chronic DPH intoxication: report of an autopsy case. Acta Pathol Jpn. 1977;27(3):387–407 [DOI] [PubMed] [Google Scholar]
- 51. Morrell F, Torres F. Electrophysiological analysis of a case of Tay-Sachs disease. Brain. 1960;83:213–224 [DOI] [PubMed] [Google Scholar]
- 52. Nagashima K, Kikuchi F, Suzuki Y, Abe T. Retinal amacrine cell involvement in Tay-Sachs disease. Acta Neuropathol (Berl). 1981;53(4):333–336 [DOI] [PubMed] [Google Scholar]
- 53. Norman RM, Tingey AH, Newman CG, Ward SP. Tay-Sachs disease with visceral involvement and its relation to gargoylism. Arch Dis Child. 1964;39:634–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Novoa F, Colombo M, Clericus J. Tay-Sachs disease [in Spanish]. Rev Chil Pediatr. 1974;45(6):495–499 [PubMed] [Google Scholar]
- 55. Ohman R, Ekelund H, Svennerholm L. The diagnosis of Tay-Sachs disease. Acta Paediatr Scand. 1971;60(4):399–406 [DOI] [PubMed] [Google Scholar]
- 56. Ozkara HA, Sandhoff K. A new point mutation (G412 to A) at the last nucleotide of exon 3 of hexosaminidase alpha-subunit gene affects splicing. Brain Dev. 2003;25(3):203–206 [DOI] [PubMed] [Google Scholar]
- 57. Rand CW. Report of two cases of amaurotic family idiocy (Tay-Sachs' disease). Cal State J Med. 1917;15(12):503–505 [PMC free article] [PubMed] [Google Scholar]
- 58. Yoshikawa H, Yamada K, Sakuragawa N. MRI in the early stage of Tay-Sachs disease. Neuroradiology. 1992;34(5):394–395 [DOI] [PubMed] [Google Scholar]
- 59. Rosengren B, Månsson JE, Svennerholm L. Composition of gangliosides and neutral glycosphingolipids of brain in classical Tay-Sachs and Sandhoff disease: more lyso-GM2 in Sandhoff disease? J Neurochem. 1987;49(3):834–840 [DOI] [PubMed] [Google Scholar]
- 60. Sachs B. On arrested cerebral development with special reference to its cortical pathology. J Nerv Ment Dis. 1887;14:541 [Google Scholar]
- 61. Sachs B. A familial form of idiocy, generally fatal associated with early blindness. J Nerv Ment Dis. 1896;21:475 [Google Scholar]
- 62. Sandhoff K, Harzer K, Wassle W, Jatzkewitz H. Enzyme alterations and lipid storage in three variants of Tay-Sachs disease. J Neurochem. 1971;18(12):2469–2489 [DOI] [PubMed] [Google Scholar]
- 63. Schmitt HP, Berlet H, Volk B. Peripheral intraaxonal storage in Tay-Sachs' disease (GM2-gangliosidosis type 1). J Neurol Sci. 1979;44(1):115–124 [DOI] [PubMed] [Google Scholar]
- 64. Schneck L, Maisel J, Volk BW. The startle response and serum enzyme profile in early detection of Tay-Sachs' disease. J Pediatr. 1964;65:749–756 [DOI] [PubMed] [Google Scholar]
- 65. Sinici I, Ozkara HA, Topcu M, Ciliv G. Biochemical and molecular characterization of mutant hexosaminidase A in a Turkish family. Pediatr Int. 2003;45(1):16–22 [DOI] [PubMed] [Google Scholar]
- 66. Snyder PD, Jr, Krivit W, Sweeley CC. Generalized accumulation of neutral glycosphingolipids with GM2 ganglioside accumulation in the brain. J Lipid Res. 1972;13(1):128–136 [PubMed] [Google Scholar]
- 67. Svennerholm L. The chemical structure of normal human brain and Tay-Sachs gangliosides. Biochem Biophys Res Commun. 1962;9:436–441 [DOI] [PubMed] [Google Scholar]
- 68. Taketomi T, Kawamura N. Cerebral and visceral glycolipids in a case of Tay-Sachs disease. J Biochem. 1969;66(2):165–174 [DOI] [PubMed] [Google Scholar]
- 69. Tanaka A, Ohno K, Sandhoff K, et al. GM2-gangliosidosis B1 variant: analysis of beta-hexosaminidase alpha gene abnormalities in seven patients. Am J Hum Genet. 1990;46(2):329–339 [PMC free article] [PubMed] [Google Scholar]
- 70. Tanaka A, Sakuraba H, Isshiki G, Suzuki K. The major mutation among Japanese patients with infantile Tay-Sachs disease: a G-to-T transversion at the acceptor site of intron 5 of the beta-hexosaminidase alpha gene. Biochem Biophys Res Commun. 1993;192(2):539–546 [DOI] [PubMed] [Google Scholar]
- 71. Tay W. Symmetrical changes in the region of the yellow spot in each eye of an infant. Trans Ophtalmol Soc UK. 1881;1:155 [Google Scholar]
- 72. Toma L, Pinto W, Rodrigues VC, Dietrich CP, Nader HB. Impaired sulphated glycosaminoglycan metabolism in a patient with GM-2 gangliosidosis (Tay-Sachs disease). J Inherit Metab Dis. 1990;13(5):721–731 [DOI] [PubMed] [Google Scholar]
- 73. Yutani C, Miyaji T. An autopsy case of Tay-Sachs' disease. Acta Pathol Jpn. 1974;24(3):393–404 [DOI] [PubMed] [Google Scholar]
- 74. Zampieri S, Filocamo M, Buratti E, et al. Molecular and functional analysis of the HEXB gene in Italian patients affected with Sandhoff disease: identification of six novel alleles. Neurogenetics. 2009;10(1):49–58 [DOI] [PubMed] [Google Scholar]
- 75. Zokaeem G, Bayleran J, Kaplan P, Hechtman P, Neufeld EF. A shortened beta-hexosaminidase alpha-chain in an Italian patient with infantile Tay-Sachs disease. Am J Hum Genet. 1987;40(6):537–547 [PMC free article] [PubMed] [Google Scholar]
- 76. Kaback MM. Screening and prevention in Tay-Sachs disease: origins, update, and impact. Adv Genet. 2001;44:253–265 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.