Abstract
An 8-year-old girl complained for 4 months of right arm pain, weakness in both legs, difficulty in arising from a seated or squatting position, and 1 month of pain in her hips, ankles, and knees. On physical examination, she had weak neck flexors, weak proximal and abdominal muscles, and an assisted Gower maneuver; both knees and ankles were painful. Erythematous macules on her elbows, knees, and medial ankles were present without heliotrope rash or dilated eyelid capillaries. She had nail-fold erythema and decreased numbers of nail-fold capillary end-row loops (ERLs) (5.42 ERLs per mm [normal: ≥6.8 ERLs per mm]) without digital ulcers or tight skin. Laboratory testing revealed slightly elevated creatine phosphokinase (440 IU/L [normal: ≤199 IU/L]) and aldolase (11.7 U/L [normal: ≤8.6 U/L]) levels. Her eosinophilia (7.2%) was not characteristic of juvenile dermatomyositis. Rheumatologic evaluation included a positive antinuclear antibody test result (1:5120 titer), speckled pattern (normal: <80 titer), myositis-associated and -specific antibodies that showed indeterminate Mi-2, with the others negative, including p155/140, elevated immunoglobulin G (IgG) (1440 mg/dL [normal range: 608–1229]) and IgE (409 kU/L [normal: <160 kU/L]) levels, and normal levels of IgM and IgA. She had an increased neopterin level (20 nm/L [normal: <10 nm/L]) and decreased absolute count of CD3-CD56/16+ natural killer cells (89 [lower normal limit: 138]). MRI of her thigh muscles revealed serpiginous increased T-2 signals consistent with inflammation and a complex round mass in the left pelvis. A muscle biopsy did not indicate juvenile dermatomyositis. Pelvic ultrasound confirmed a solid mass of the left ovary consistent with a mature teratoma. After surgical removal of the teratoma, the myositis, synovitis, and cutaneous findings resolved over 4 months without further therapy.
Keywords: myositis, teratoma, immune modulation, paraneoplastic syndrome
Juvenile dermatomyositis (JDM) is a rare, often chronic, immune-mediated, pediatric idiopathic inflammatory myopathy characterized by muscle and skin inflammation.1 This uncommon systemic vasculopathy has an incidence of 0.32 per 100 000 children,1 and adult dermatomyositis occurs in 0.74 per 100 000 adults.2 Dermatomyositis is characterized by symmetrical proximal muscle weakness and pathognomonic cutaneous erythema that includes a heliotrope rash over the eyelids and Gottron papules over extensor joint surfaces; the diagnostic criteria for adults and children are similar.3 In adult patients, the onset of dermatomyositis may be part of a paraneoplastic syndrome secondary to a malignant tumor.4 This phenomenon has rarely been noted in pediatric patients with JDM, and an extensive workup for malignancy is not indicated.5 The purpose of this report is twofold: (1) to present the case of an 8-year-old girl with severe proximal muscle weakness, arthritis, a mild nonspecific rash, and muscle biopsy that did not meet the diagnostic criteria for JDM but who, in fact, had a teratoma and (2) to reinforce the requirement to document the type/extent of muscle involvement to confirm the accurate diagnosis of JDM.
CASE REPORT
An 8-year-old girl came to the pediatric myositis clinic with a 4-month history of right arm pain and weakness in both legs; she could not easily rise from a seated or squatting position. The pain was rated 5 of 10 and described as achy without radiation, tingling, or numbness. There was no history of trauma or infection preceding the pain. Previously independent, she now required help dressing. Her mother denied a change in voice, acquisition of a nasal tone, and dysphagia. One month earlier, she had developed pain in her hips, ankles, and knees that was exacerbated by activity but had no joint swelling or fevers. A history of infections and surgeries was denied. She was born after 35 weeks' gestation to nonconsanguineous parents. Family history was positive for celiac disease in her father and thyroid problems in her paternal grandfather and paternal aunt. In addition, the maternal grandmother had both bipolar disease and colon cancer. Her father was Hispanic, and her mother was Taiwanese. She lived with both parents and a 12-year-old brother. Her immunizations were up to date. Review of systems revealed only that she had always had frigid fingers, which turned purple with cold exposure. She denied weight loss or decreased appetite.
Her temperature was 36.6°C, respiratory rate was 24 breaths per minute, blood pressure was 101/56 mm Hg, pulse was 95 beats per minute, height was 145.6 cm (>97th percentile), and weight was 30.7 kg (90th percentile). She was alert and in no acute distress. Her ears, nose, eyes, and throat were normal except for mild erythema in the medial canthus of the right eye without vessel dilation. There was no lymphadenopathy. Cardiac examination was notable for mild systolic 2/6 murmur at the left upper sternal boarder, which changed with change in position. Her lungs were clear bilaterally with good aeration. Her abdomen was soft with dullness to percussion in the right upper quadrant, and she had mild pain on deep palpation of both the lower right and left quadrants. Genitourinary evaluation was normal. Her skin had erythematous papulomacular lesions on the elbows, knees, and medial aspect of the ankles (Fig 1A). The malar or heliotrope characteristic of JDM was absent, but she did have erythema of the distal digits, most pronounced around the nail beds of the fingers, as well as the proximal and distal interphalangeal joints (Fig 1B). Neither tight skin nor digital ulcers were present. On musculoskeletal examination, she had flexion contractures of her elbows and limited extension of both knees and ankles with pain on movement but no joint swelling. Compression of the proximal lower muscles produced complaints of tenderness. Neurologic examination was notable for muscle weakness (neck flexors [3 of 5], upper proximal [4 of 5], and lower extremity [3 of 5]) and core weakness (she was unable to perform a sit-up unassisted). She had hip and thigh weakness, using her hands and arms to ″walk″ up her body from a squatting position (positive Gower maneuver).
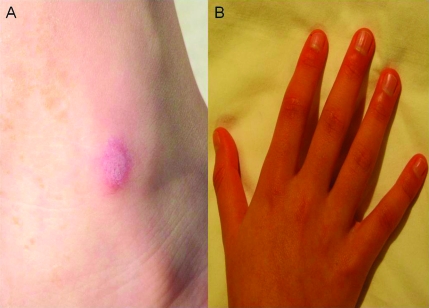
FIGURE 1.
A, Medial aspect of the left ankle, showing a papular, circular area of mild erythema (∼3 × 2 cm) on a more erythematous base. B, Both hands at diagnosis with erythema of the distal digits, most pronounced around the nail beds of the fingers, as well as the proximal and distal interphalangeal joints. Raised, thickened areas (Gottron papules) were not present.
Laboratory evaluation with a complete blood and white blood cell count revealed 5580/μL (normal range: 4500–13 500/μL) (53.5% polymorphonuclear cells, 31.8% lymphocytes, 6.8% eosinophils, 7.2% mononuclear cells [which was not characteristic for JDM], and 0.7% basophilic cells), a hemoglobin level of 12.7 g/dL (normal range: 12–16 g/dL), hematocrit at 37.1% (normal range: 36%–46%), and a platelet count of 298 000/μL (normal range: 150 000–450 000/μL). Her creatine kinase (440 IU/L [normal range: 49–199 IU/L]) and aldolase (11.7 U/L [normal range: 3.4–8.6 U/L]) levels were mildly elevated, but she had normal levels of aspartate aminotransferase (38 IU/L), alanine aminotransferase (9 IU/L), and lactate dehydrogenase (274 IU/L). Rheumatologic evaluation included a positive antinuclear antibody test result (1:5120 titer), speckled pattern (normal: <80 titer), myositis-associated and -specific antibodies that showed indeterminate Mi-2, with the others negative, including p155/140 elevated immunoglobulin G (IgG) (1440 mg/dL [normal range: 608–1229]) and IgE (409 kU/L [normal: <160 kU/L]) levels, and normal levels of IgM and IgA. Her neopterin level was elevated at 20 nm/L (normal: <10 nm/L). Complement concentrations were normal (C3: 97.4 mg/dL; C4: 21.3 mg/dL). Peripheral blood flow cytometry revealed decreased numbers of natural killer cells (CD3−CD16CD56+) (absolute count: 89 [normal range: 138–1027]), a decreased number of CD3 cells, and normal numbers of B cells. Nail-fold capillary end-row loops (ERLs) were decreased at 5.42 ERLs per mm (normal: ≥6.8 ERLs per mm).6
MRI revealed abnormally increased T2-weighted signal compatible with inflammatory infiltration within nearly all of the muscles of the thighs bilaterally (Fig 2), consistent with dermatomyositis, and a complex round mass in the left side of the pelvis. Ultrasound findings indicated a teratoma (a solid avascular left ovarian mass with increased echogenicity), which suggested a significant fat component. The ovarian mass was surgically removed, and the biopsy confirmed the presence of a mature ovarian teratoma without immature components. It should be noted that the muscle biopsy did not indicate JDM, because it only displayed mild variability in fiber size but no perifascicular atrophy, perivascular inflammation, or loss of intramuscular capillaries. Her symptoms and abnormal laboratory results resolved without immunosuppressive therapy by 4 months after surgical removal of the teratoma.
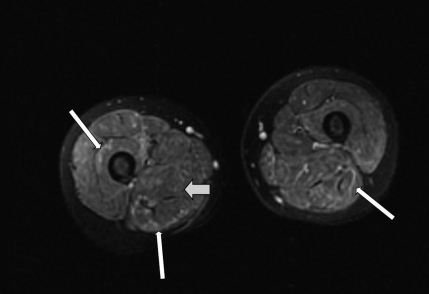
FIGURE 2.
MRI with fat suppression of the patient's proximal lower extremities, which revealed an increased T2-weighted signal in many of the muscles of the thighs bilaterally. The wide arrow points to the darker, more normal muscle tissue, and the thin arrows point to the ribbons of irregular light areas of muscle tissue, which indicate inflammation.
DISCUSSION
Ovarian neoplasms constitute 1% of all childhood malignancies. Although two-thirds of affected girls present with abdominal pain/distention as their primary symptom, this patient had no abdominal complaints or symptoms suggestive of malignancy.7 However, she did have lower abdominal tenderness on palpation, which is usually not present in children with JDM at diagnosis.1 Although ovarian cancers are extremely uncommon in children, teratomas are the most common neoplasm in adolescents. Mature teratomas are benign and usually composed of 3 germ-cell layers (ectoderm, mesoderm, and endoderm).7 Benign teratomas have been associated with a variety of paraneoplastic syndromes including limbic encephalitis,8,9 opsoclonus-myoclonus syndrome,10 seronegative polyarthritis, tenosynovitis,11 and autoimmune hemolytic anemia,12 but the association with JDM reported here is, to our knowledge, a novel observation. The clinical features of these syndromes are thought to be mediated by an autoimmune response to the contents of the teratoma. Antibodies to tissue antigens have been identified in patients with ovarian tumors.7,8,13 Our patient had high-titer antinuclear antibody, an elevated serum neopterin level, a decreased number of CD3− natural killer cells, and hypergammaglobulinemia (IgG and IgE), which suggested immune activation similar to that found with JDM,1 but at diagnosis, the eosinophilia was a differentiating factor, given the absence of an allergy history. The muscle biopsy results were also not compatible with the diagnosis of JDM.
In a report of 12 children with JDM/polymyositis and malignancy, ranging from lymphoproliferative disorders, neuroblastoma, and sarcoma to dysgerminoma, 9 children had unexpected findings such as splenomegaly, lymphadenopathy, and an atypical rash.14 In contrast, in adults, reports of malignancy in association with adult dermatomyositis and, to a much lesser extent, polymyositis, have ranged from 10% to 42% of cases.5,8,14,15 In 618 adults with adult dermatomyositis, there was a strong association with ovarian tumors (most of them adenocarcinomas) followed by lung cancer, gastrointestinal cancers, and non-Hodgkin lymphoma.8 In adults with myositis, but not children (as in this case), myositis-specific antibody to p155/140 seemed to be a prognostic factor for malignancy, and ethnicity strongly influenced the type of malignancy found.15
CONCLUSIONS
This report provides evidence that myositis, arthritis, and skin manifestations can be associated with benign ovarian teratoma in a child despite the absence of severe abdominal symptoms. We suggest that the findings of an atypical rash, splenomegaly, lymphadenopathy, and/or a muscle biopsy that does not show the characteristic changes of perifascicular atrophy and inflammation in a child with prolonged symptoms of an inflammatory myopathy merit further investigation.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin R01 AR48289, the Cure JM Foundation, and Macy's Miracle Foundation.
Dr Ibarra made substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data, and drafting the article or revising it critically for important intellectual content and gave final approval of the version to be published; Dr Chou made substantial contributions to acquisition of data and analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and gave final approval of the version to be published; and Dr Pachman identified the problem in the patient, made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, and drafting of the article or revising it critically for important intellectual content and gave final approval of the version to be published.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- JDM
- juvenile dermatomyositis
- Ig
- immunoglobulin
REFERENCES
- 1. Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371(9631):2201–2212 [DOI] [PubMed] [Google Scholar]
- 2. Koler RA, Montemarano A. Dermatomyositis. Am Fam Physician. 2001;64(9):1565–1572 [PubMed] [Google Scholar]
- 3. Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292(7):344–347 [DOI] [PubMed] [Google Scholar]
- 4. Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy proven inflammatory myopathy. Ann Intern Med. 2001;134(12):1087–1095 [DOI] [PubMed] [Google Scholar]
- 5. Stockton D, Doherty VR, Brewster DH. Risk of cancer in patients with dermatomyositis or polymyositis, and follow up implications: a Scottish population-based cohort study. Br J Cancer. 2001;85(1):41–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christen-Zaech S, Seshadri R, Sundberg J, Paller AS, Pachman LM. Persistent association of nailfold capillaroscopy changes and skin involvement over thirty-six months with duration of untreated disease in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;58(2):571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanfilippo JS. Gynecological problems of childhood. In: Kliegman R, Behrman R, Jenson H, Stanton B. eds. Nelson Textbook of Pediatrics. 18th ed Philadelphia, PA: Saunders; 2007:1837–1838 [Google Scholar]
- 8. Yang YW, Tsai CH, Chang FC, Lu MK, Chiu PY. Reversible paraneoplastic limbic encephalitis caused by a benign teratoma: report of a case and review of literature. J Neurooncol. 2006;80(3):309–312 [DOI] [PubMed] [Google Scholar]
- 9. Tonomura Y, Kataoka H, Hara Y, et al. Clinical analysis of paraneoplastic encephalitis associated with ovarian teratoma. J Neurooncol. 2007;84(3):287–292 [DOI] [PubMed] [Google Scholar]
- 10. Fitzpatrick AS, Gray OM, McConville J, McDonnell GV. Opsoclonus myoclonus syndrome associated with benign ovarian teratoma. Neurology. 2008;70(15):1292–1293 [DOI] [PubMed] [Google Scholar]
- 11. Wiese W, Alansari H, Tranchida P, Madrid FF. Paraneoplastic polyarthritis in an ovarian teratoma. J Rheumatol. 2004;31(9):1854–1857 [PubMed] [Google Scholar]
- 12. Kim I, Lee JY, Kwon JH, et al. A case of autoimmune hemolytic anemia associated with an ovarian teratoma. J Korean Med Sci. 2006;21(2):365–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96–100 [DOI] [PubMed] [Google Scholar]
- 14. Morris P, Dare J. Juvenile dermatomyositis as a paraneoplastic phenomenon: an update. J Pediatr Hematol Oncol. 2010;32(3):189–191 [DOI] [PubMed] [Google Scholar]
- 15. Selva-O'Callaghan A, Trallero-Araguas E, Grau-Junyent JM, Labrador-Horrillo M. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol. 2010;22(6):627–632 [DOI] [PubMed] [Google Scholar]