Abstract
Salmonellosis is a frequently diagnosed infectious disease of passerine birds in garden habitats within Great Britain with potential implications for human and domestic animal health. Postmortem examinations were performed on 1,477 garden bird carcasses of circa 50 species from England and Wales, 1999 to 2007 inclusive. Salmonellosis was confirmed in 263 adult birds of 10 passerine species in this 11-year longitudinal study. A subset of 124 fully biotyped Salmonella enterica subsp. enterica serovar Typhimurium isolates was examined using pulsed-field gel electrophoresis to investigate the hypothesis that these strains are host adapted and to determine whether this molecular technique offers greater resolution in understanding the epidemiology of Salmonella Typhimurium infection than phage typing alone. For the two most common phage types, definitive type (DT) 40 and DT56v, which together accounted for 97% (120/124) of isolates, pulsed-field gel electrophoresis groupings closely correlated with phage type with remarkably few exceptions. A high degree of genetic similarity (>90%) was observed within and between the two most common pulsed-field gel electrophoresis groups. No clustering or variation was found in the pulsed-field gel electrophoresis groupings by bird species, year, or geographical region beyond that revealed by phage typing. These findings support the hypothesis that there are currently two host-adapted Salmonella phage types, S. Typhimurium DT40 and DT56v, circulating widely in British garden birds and that the reservoir of infection is maintained within wild bird populations. Large-scale multilocus sequence typing studies are required to further investigate the epidemiology of this infection.
INTRODUCTION
Salmonellosis is a common disease of garden birds in Great Britain, chiefly affecting highly social and seed-eating species, such as the greenfinch (Carduelis chloris), chaffinch (Fringilla coelebs), and house sparrow (Passer domesticus), during the winter months (10, 14). The bacterium Salmonella enterica subsp. enterica serotype Typhimurium (S. Typhimurium) (8, 15) is the causative agent, with the definitive phage types (DT) 40 and DT56variant (DT56v) having been most frequently isolated since the 1990s (14, 21).
While S. Typhimurium phage types typically isolated from “wild birds” (i.e., DT40 and DT56v from passerines, DT2 and DT99 from pigeons, DT41 and DT195 from gulls) have previously been isolated from a variety of domesticated species, including cattle, pigs, sheep, and poultry, they have been shown to account for only a small proportion of Salmonella sp. isolates detected through livestock surveillance schemes (20). Opportunities for cross-infection from wild birds exist, especially when they are feeding in farms or stables or when cats predate infected birds (22, 23, 30). There also is potential for zoonotic infection with S. Typhimurium from passerines (12, 23, 29).
In England and Wales, there was variation in the temporal and spatial distributions of phage types involved in passerine salmonellosis incidents during the period 1993 to 2003 inclusive (14). Mortality due to DT40 and DT56v tended to occur in central and western England and Wales; DT40 was detected throughout the period, while DT56v was first isolated in the mid-nineties, with an apparent increase in incidence from 2000 (14). The spatial distributions of salmonellosis incidents due to phage types DT40 and DT56v were similar for both greenfinches and house sparrows during this period (14). A recent longitudinal study of passerine salmonellosis incidents in Scotland, 1995 to 2008 inclusive, found evidence of regional variation, with DT40 incidents predominant in northern Scotland and both DT40 and DT56v observed from incidents in southern Scotland. In addition, some evidence for regional variation in the phage type according to the species affected was found (21).
Macrorestriction pulsed-field gel electrophoresis (PFGE) is a technique that permits fine-scale strain discrimination, which has been widely used to complement traditional biotyping studies (phage typing and serology) for epidemiological investigations (18, 27). A pilot study to further characterize S. Typhimurium isolates from passerine mortality incidents collected from northern England, 2005 to 2006, using PFGE found minimal variation among isolates, some of which appeared to be clonal, indicating that the bacterial strains may be host adapted to wild bird populations (10). Here we present a comprehensive survey of isolates collected from a wide range of passerine species across England and Wales (1999 to 2007 inclusive) using PFGE to further investigate this hypothesis. The purpose of this study was to determine whether the PFGE groupings might identify any temporo-spatial epidemiological trends, or clustering by species, that were not revealed through phage typing alone.
MATERIALS AND METHODS
Although there is no routine monitoring of Salmonella in wild birds in England and Wales or any uniform intensity of surveillance, garden bird mortality incidents have been investigated since 1993 using a combination of opportunistic only (1993 to 2003 inclusive) (14), and opportunistic and systematic (2005 to 2007 inclusive) (28), surveillance methodologies in collaboration with the general public and a citizen science network. Specifically, ad hoc reports of garden bird mortality were received from the general public, who reported their observations to nongovernmental bird conservation organizations or directly to participating diagnostic laboratories (14). Systematic surveillance was achieved through a citizen science network of ca. 750 participants, recruited from the British Trust for Ornithology's Garden BirdWatch scheme, and stratified across Great Britain to ensure good geographical and habitat-type coverage (28). Participants in this stratified network reported whether or not they observed any evidence of sick or dead birds in their gardens, regardless of the perceived cause (e.g., window collision, cat predation, infectious disease). This was done on a weekly basis from 1 April 2005 until the end of the study. This structured surveillance minimized the potential for submission biases by region, season, or species, for example following local media attention about garden bird mortality events (28). When available, garden bird carcasses were submitted to a network of regional diagnostic centers for postmortem examination following a standardized protocol (14, 28). Details of the species, age, date found, and geographical location were recorded for each carcass. Birds were classified as adult when beyond their postjuvenile molt.
The liver and small intestine, with or without the crop/esophagus, in addition to any lesions found, were routinely sampled aseptically and examined for the presence of pathogenic bacteria using a standard protocol (14). Briefly, tissue samples were plated directly onto (i) Columbia blood agar supplemented with 5% horse blood (QCM Laboratories, London, United Kingdom) in triplicate, and incubated under each of aerobic, anaerobic, and CO2 conditions; (ii) xylose-lysine deoxycholate (XLD) agar (QCM Laboratories) and incubated under aerobic conditions; or (iii) chocolate blood agar (QCM Laboratories) and incubated under 5 to 10% CO2 conditions, or were (iv) immersed in selenite Salmonella-selective enrichment broth (QCM Laboratories) under aerobic conditions for 24 h followed by subculture onto XLD agar aerobically. All samples were incubated at 37°C with inspection of the agar plates at 24, 48, and 120 h postinoculation. Bacterial isolates were identified using colony morphology and Gram staining, followed by biochemical properties as determined using the API biochemical test strip method (API-bioMérieux, Marcy l'Etoile, France). Slide agglutination tests were performed for the identification of suspected Salmonella sp. isolates using poly-O antisera (Pro-Lab Diagnostics, Neston, United Kingdom).
Since 1999, a subset of Salmonella isolates were placed onto Microbank beads (Pro-Lab Diagnostics) and stored at both −25°C and −70°C; all archived isolates were grown in pure culture from a single colony. Isolates were archived on an ad hoc basis as time and resources permitted until 2005, when they were retained as routine. Batches of isolates were subsequently submitted to the UK Veterinary Laboratories Agency or to the Salmonella Reference Unit of the Health Protection Agency (England, Wales, and Northern Ireland) for biotyping (serotype and phage type) according to standardized international protocols (3, 8).
In cases where a Salmonella sp. was isolated from a lesion, or lesions, characteristic for salmonellosis (14, 19) in the absence of any other obvious cause of death, a diagnosis of salmonellosis was made. A salmonellosis incident was defined as a garden site from which one or more birds were diagnosed with salmonellosis.
All available S. Typhimurium isolates from the period 1999 to 2004 were processed for PFGE along with a subset of archived isolates collected over the period 2005 to 2007. From these later years we selected a wide and representative sample of isolates which we considered to be most likely to encompass any variation present. This included a similar proportion (circa 50 to 60%) of isolates from each of the garden bird species in which salmonellosis was commonly diagnosed and isolates from the majority of cases in less-frequently examined bird species, from a wide geographical region across England and Wales, from a similar proportion of isolates from each year, from a similar proportion of the commonly isolated phage types, and including all of the less-frequently isolated phage types.
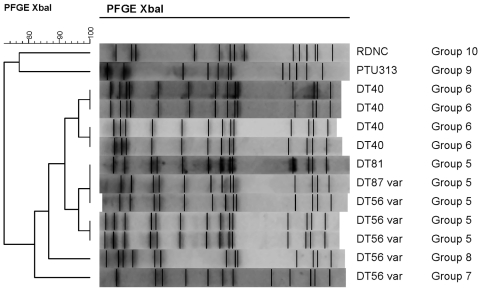
Block preparation and PFGE were performed according to the standardized PulseNet Rapid Escherichia coli PFGE method with slight modifications (4) following the protocol of Hughes et al. (10). All PFGE in this study was performed in the same laboratory with the same equipment used by Hughes et al. (10), enabling robust, direct comparison between isolates. In summary, genomic DNA from Salmonella isolates was digested for 2 h using 50 U per sample XbaI (Promega, Southampton, United Kingdom). Macrorestriction-digested fragments were separated on a 1% agarose gel (pulsed-field certified; Bio-Rad Laboratories, Hertfordshire, United Kingdom), at 210 V for 19 h at 14°C on a contour-clamped homogeneous electric field (CHEF) DRIII system (Bio-Rad Laboratories). Pulse times were ramped from 2.2 to 54.2 s, and a reorientation angle of 120° was applied. Bacteriophage λ DNA concatemers (Bio-Rad Laboratories) embedded in 1% low-melting-point (LMP) agarose were used as molecular weight markers, and S. Typhimurium SL1344 and S. Typhimurium F98 were used as controls. Gels were stained for 20 min with 1% ethidium bromide solution and visualized using UV light. BioNumerics version 4.0 (Applied Maths BVBA, Sint-Martens-Latem, Belgium) software was used for image analysis. A percent similarity between pulsed-field banding patterns was computed according to the Dice similarity coefficient method with a 2% tolerance window, and a dendrogram was constructed using the unweighted-pair group method with averages. PFGE profiles were compared with findings from the pilot study (10), and the same PFGE groupings (1 to 6) were used for consistency to facilitate comparison of findings between the studies.
Spatial data were presented using ArcView 3.0 GIS software (ESRI GIS and Mapping Software, Redlands, CA).
RESULTS
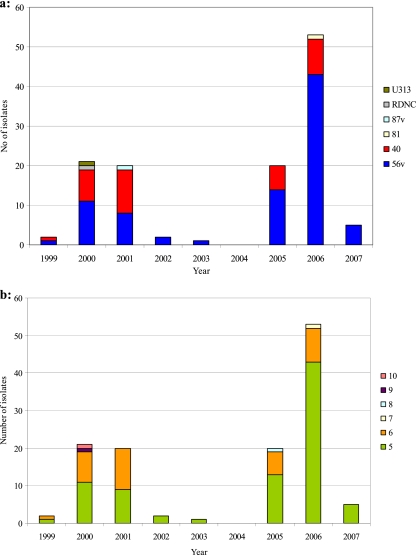
Postmortem examinations were performed on 1,477 garden bird carcasses of circa 50 species from England and Wales between 1999 and 2007 inclusive. Salmonellosis was confirmed in 263 adult birds of 10 species (Fringillidae, including 161 greenfinch, 19 siskin Carduelis spinus, 13 goldfinch Carduelis carduelis, 7 bullfinch Pyrrhula pyrrhula, 6 chaffinch, 2 brambling Fringilla montifringilla, and 1 lesser redpoll Carduelis flammea; Passeridae, including 53 house sparrow and 1 tree sparrow Passer montanus; Emberizidae, including 1 reed bunting Emberiza schoenicurius) from 204 sites. The S. Typhimurium isolates comprised two common definitive phage types, DT56v (n = 156) and DT40 (n = 76), single isolates of DT81, DT87v, DT160, DT193, and provisional types U277 and U313, 6 isolates of the phage type known as “reacts but does not conform” (RDNC), and 20 isolates that were not submitted for full biotyping.
Fully biotyped S. Typhimurium isolates were included in the PFGE survey from 124 of these birds, comprising 87 cases of Fringillidae species and 37 cases of Passeridae species (Table 1). The S. Typhimurium isolates were from salmonellosis incidents obtained throughout the study period, although the number varied markedly between years (Fig. 1). The S. Typhimurium isolates comprised two common definitive phage types, DT56v (n = 85) and DT40 (n = 35), and single isolates of DT81, DT87v, U313, and RDNC (Table 1).
Table 1.
Phage type and host origin of the S. Typhimurium isolates used in this study
| Species | No. of isolates of phage type |
Total | |||||
|---|---|---|---|---|---|---|---|
| 40 | 56v | 81 | 87v | RDNCa | U313 | ||
| Bullfinch | 1 | 3 | 0 | 0 | 0 | 0 | 4 |
| Chaffinch | 1 | 4 | 0 | 0 | 0 | 0 | 5 |
| Goldfinch | 0 | 3 | 1 | 0 | 0 | 0 | 4 |
| Greenfinch | 20 | 46 | 0 | 1 | 0 | 0 | 67 |
| House sparrow | 12 | 22 | 0 | 0 | 1 | 1 | 36 |
| Siskin | 1 | 6 | 0 | 0 | 0 | 0 | 7 |
| Tree sparrow | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Total | 35 | 85 | 1 | 1 | 1 | 1 | 124 |
Isolates that did not conform to any of the recognized phage typing patterns were classified as “reacts but does not conform” (RDNC).
Fig. 1.
Phage type (a) and PFGE group (b) for all S. Typhimurium isolates by year, 1999–2007 inclusive.
Using PFGE, we identified six groupings of XbaI profiles (Fig. 2). PFGE group 5 included 98% (83/85) of the S. Typhimurium DT56v isolates, from a range of species and locations (Table 2; Fig. 3b). In addition, PFGE group 5 included the single DT87v isolate (from a greenfinch found dead in England in 2001) and the single DT81 isolate (from a goldfinch found dead in Wales in 2006). Within PFGE group 5, two profiles consisting of 12 or 13 DNA fragments clustered together with >95% overall genetic similarity. The fragment sizes ranged from approximately 40 kb to 825 kb. Variation was by the addition of an extra band with a size of 1,000 kb in some isolates.
Fig. 2.
Dendrogram showing the percent similarity between Salmonella Typhimurium isolates digested with XbaI restriction enzyme. PFGE band profiles are shown, and the scale indicates percent similarity.
Table 2.
Pulsed-field gel electrophoresis group and host origin of S. Typhimurium isolates
| Species | No. of isolates of PFGE group |
Total | |||||
|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | 10 | ||
| Bullfinch | 3 | 1 | 0 | 0 | 0 | 0 | 4 |
| Chaffinch | 4 | 1 | 0 | 0 | 0 | 0 | 5 |
| Goldfinch | 4 | 0 | 0 | 0 | 0 | 0 | 4 |
| Greenfinch | 46 | 20 | 0 | 1 | 0 | 0 | 67 |
| House sparrow | 21 | 12 | 1 | 0 | 1 | 1 | 36 |
| Siskin | 6 | 1 | 0 | 0 | 0 | 0 | 7 |
| Tree sparrow | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total | 85 | 35 | 1 | 1 | 1 | 1 | 124 |
Fig. 3.
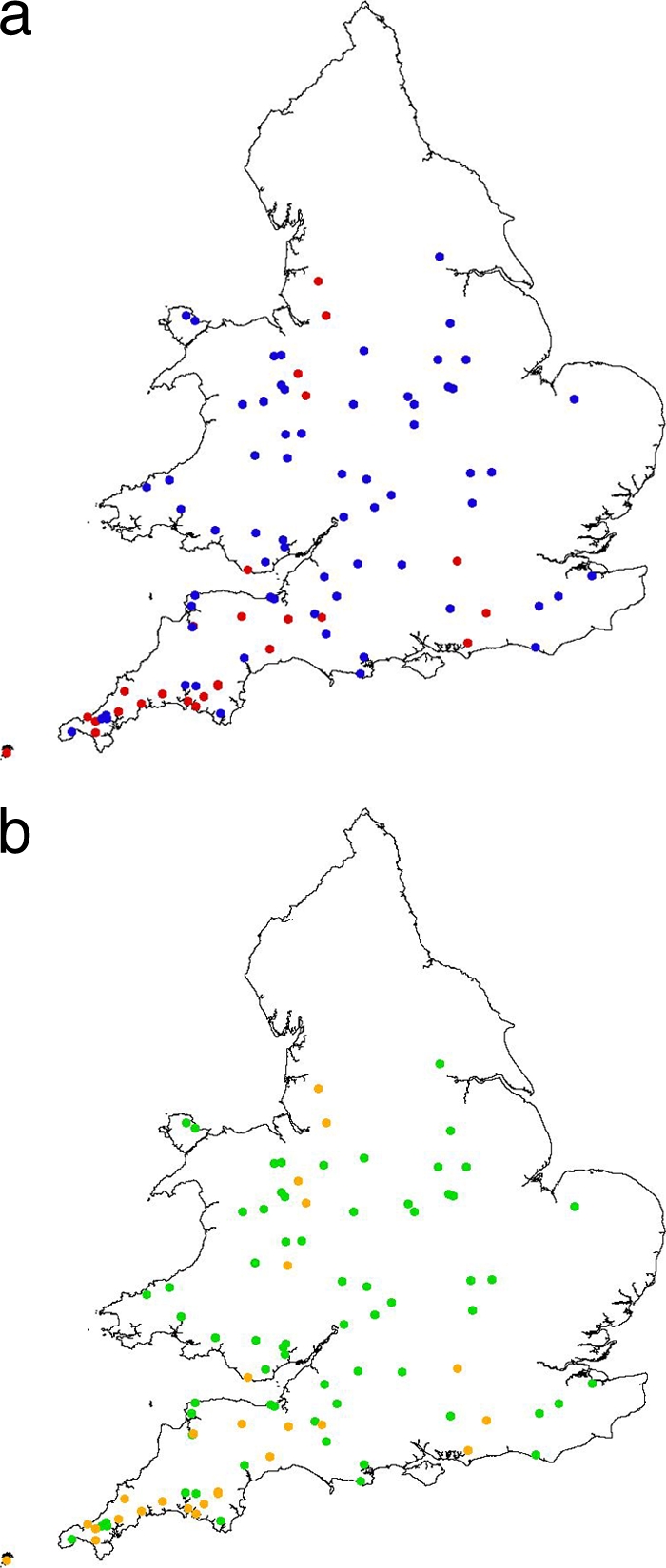
Distribution of S. Typhimurium isolates by phage type (DT40 in red, DT56v in blue) (a) and PFGE group (PFGE 5 in green, PFGE 6 in orange) (b), 1999–2007 inclusive. Contains Ordnance Survey data © Crown copyright and database right (2011).
Similarly, all of the 35 DT40 isolates were assigned to PFGE group 6 and consisted of two profiles that clustered together with >95% overall genetic similarity. Either 12 or 13 DNA fragments were present, with sizes ranging from approximately 40 kb to 825 kb, and some isolates varied by the addition of an extra band with a size of 600 kb. These isolates were from a range of species (Table 2).
A high degree of genetic similarity (>90%) was observed between PFGE groups 5 and 6, which collectively accounted for 97% (120 of 124) of the passerine S. Typhimurium isolates in this study.
Four isolates produced unique PFGE profiles that were designated PFGE groups 7 to 10. PFGE group 7 was a DT56v isolate from a house sparrow (England, 2006) and PFGE group 8 was a DT56v isolate from a greenfinch (Wales, 2005). These two DT56v isolates gave anomalous PFGE profiles divergent from those of the other isolates of this phage type.
PFGE group 9 was a U313 isolate and PFGE group 10 was a RDNC isolate, and both showed less than 77% similarity to each other or to the other PFGE profiles. These two divergent isolates were both cultured from house sparrows submitted from the same site in Oxfordshire, November 2000.
While the number of salmonellosis cases, and the composition of S. Typhimurium phage types, varied among years, no trends were detected in the PFGE groupings of isolates from the common PFGE groups 5 and 6 (Fig. 1b).
Salmonellosis incidents included within the fully biotyped PFGE study were confirmed at 96 sites in England and Wales, with widespread distribution chiefly across the English Midlands, the English/Welsh border region, and southern England (Fig. 3). Multiple birds were examined from 20 of the 96 sites, with the same phage type and PFGE profile found in all isolates from the same location in 85% (17/20) of these sites. Multiple phage types or PFGE profiles were found from birds examined at three sites: in England in 2000 (house sparrow with S. Typhimurium RDNC, PFGE group 10, and house sparrow with S. Typhimurium DT40, PFGE group 6), in Wales in 2001 (house sparrow with S. Typhimurium DT40, PFGE group 6, and greenfinch with S. Typhimurium DT56v, PFGE group 5), and in Wales in 2005 (greenfinch with S. Typhimurium DT56v, PFGE group 5, and greenfinch with S. Typhimurium DT56v, PFGE group 8).
DT56v and DT40 isolates (Fig. 3a), broadly synonymous with PFGE groupings 5 and 6 (Fig. 3b), respectively, had wide and overlapping geographical distributions within England and Wales, although the former phage type was more restricted to the southern and southwestern counties of England and to the English/Welsh border region.
DISCUSSION
Pulsed-field gel electrophoresis with XbaI digestion found a high level of genetic similarity among the majority of S. Typhimurium isolates from passerines in England and Wales. This is consistent with the generally accepted view that, in Great Britain, passerine species, particularly those using garden bird feeding stations, currently have two host-adapted Salmonella serovars, i.e., S. Typhimurium DT40 and DT56v, and that the reservoir of infection is maintained within wild bird populations (10, 21). For the two most common phage types, DT40 and DT56v, PFGE groupings closely correlated with phage type with remarkably few exceptions. No clustering or variation, therefore, was found in the PFGE groups by bird species, year, or geographical region beyond that revealed by the analyses of phage types conducted previously (14). Since PFGE groupings did not reveal finer-scale variation among isolates than that known for phage type groupings alone (14), no formal species-based or temporo-spatial analyses were conducted in the current study.
For this study, we utilized a convenience sample of Salmonella isolates that was restricted in size by archive and resource availability, and it is possible that undetected bias might exist. While our study focuses on bacterial isolates obtained from dead birds with clinical salmonellosis, available evidence indicates that the S. Typhimurium strains that cause clinical disease are also aclinically or subclinically carried by the same species of birds (7, 26). While the number of isolates each year will be influenced by the overall number of salmonellosis incidents, variation in the intensity of opportunistic surveillance, coupled with the transition between single and dual surveillance schemes, might have altered the efficiency of reporting over the study period. Nevertheless this represents the most comprehensive survey of passerine-derived S. Typhimurium isolates to date in Britain and helps inform our understanding of this important disease.
Alley et al. (2) found a single identical PFGE profile from multiple isolates of S. Typhimurium DT160 from wild birds, humans, and other species during a disease epidemic affecting multiple taxa that occurred following the introduction of this novel phage type to New Zealand in 2000. Similarly, we found minimal variation in the PFGE profiles from the majority of S. Typhimurium isolates. Our longitudinal study, however, spans an 11-year period of endemic infection of common S. Typhimurium phage types in British passerines and therefore represents a contrasting epidemiological situation.
DT40 was first reported in Britain in 1964 (14) and has been isolated from passerine species in Norway (25) and Canada (5). Hughes et al. (10) examined three DT40 isolates and found two distinct PFGE groupings, group 3 and group 6; all of the DT40 isolates in this study clustered with PFGE group 6, and no isolates were identified that clustered with PFGE group 3.
DT56v was not confirmed in Britain until the mid-1990s (14) and, to our knowledge, has not yet been reported in other countries (10). Hughes et al. (10) found no variation in the PFGE profiles for the 23 DT56v isolates examined in their study. Of 85 DT56v isolates in this study, 83 were indistinguishable from those of the Hughes et al. (10) study, but 2 variant DT56v isolates were found from a greenfinch (Wales, 2005) and a house sparrow (England, 2006). This indicates that variant isolate exceptions can occur.
There has been a noticeable shift of phage types isolated from wild passerines in England and Wales between 1999 and 2007. In 1999 to 2003 inclusive, DT40 accounted for 47% (95% confidence interval [CI], 39.1 to 55.3%; 74/157) of isolates (14) making it the more frequently identified phage type in passerines, whereas by 2004 to 2007 inclusive the percentage of DT40 had fallen to 17% (CI, 11.3 to 23.6%; 26/155) of isolates, with DT56v increasing to 77% (CI, 69.3 to 83.2%; 119/155) of isolates.
Phage typing and PFGE are commonly used to subtype Salmonella and are generally used in conjunction with serotyping (16, 24). Since the latter provides valuable information about host-associated subtypes, the emergence of new subtypes, and historical trends in the association of specific subtypes with different host species, when used in combination these methods can provide a high level of discrimination (1).
Multilocus sequence typing (MLST) is a high-resolution genotyping technique for determining the global epidemiology and population structure of bacterial pathogens based on the sequence of selected housekeeping genes (17). While MLST may lack discriminatory power within certain serotypes, it is highly reproducible and infers phylogenetic relationships among Salmonella isolates and subtypes (9). PFGE involves random screening of the entire genome, while MLST analysis is limited to nucleotides within the targeted gene sets. These subtyping methods, therefore, can yield conflicting results and differ considerably in discriminatory power for certain serotypes (6, 31). Therefore, the most appropriate subtyping method, or combination thereof, will depend on both the serotype and the chosen application. MLST does not generally provide high discriminatory power within S. Typhimurium, as the majority of isolates belong to the central sequence type (ST) 19. However, a pilot MLST study of nine passerine S. Typhimurium isolates collected from northern England partitioned this monophyletic serovar and found the six DT56v isolates tested to be ST568 and the two DT40 isolates to be ST19 (11). ST19 is the central sequence type for S. Typhimurium and is shared by a variety of other S. Typhimurium phage types, including DT104 (13). A comprehensive survey of passerine S. Typhimurium isolates from across Great Britain, therefore, should be performed using MLST to further characterize strain diversity.
An understanding of the epidemiology of passerine salmonellosis is important, not only because of its adverse affects on animal welfare and its potential impact on biodiversity but also because it is a zoonotic pathogen. Continued monitoring of passerine Salmonella sp. strains should be conducted to detect any future spillover to domestic animal or human populations.
ACKNOWLEDGMENTS
We thank Malcolm Bennett, Julian Chantrey, James Kirkwood, Howard Leatherbarrow, Kirsi Peck, Rob Robinson, Marcus Rowcliffe, Sara Shopland, Vic Simpson, Mike Toms, Linda Ward, Paul Wigley, Nicola J. Williams, and the members of the public and British Trust for Ornithology's Garden BirdWatch participants who submitted dead birds for postmortem examination.
This work was supported by the Garden Bird Health initiative (British Veterinary Association Animal Welfare Foundation, CJ Wildbird Foods Ltd., Cranswick Pet Products, Defra, Gardman Ltd., RSPB, The Birdcare Standards Association, Tom Chambers Ltd., Universities Federation for Animal Welfare).
Footnotes
Published ahead of print on 23 September 2011.
REFERENCES
- 1. Alcaine S. D., et al. 2006. Multilocus sequence typing supports the hypothesis that cow- and human-associated Salmonella isolates represent distinct and overlapping populations. Appl. Environ. Microbiol. 72:7575–7585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alley M. R., et al. 2002. An epidemic of salmonellosis caused by Salmonella Typhimurium DT160 in wild birds and humans in New Zealand. N. Z. Vet. J. 50:170–176 [DOI] [PubMed] [Google Scholar]
- 3. Anderson E. S., Ward L. R., de Saxe M. J., De Sa J. D. H. 1977. Bacteriophage-typing designations of Salmonella typhimurium. J. Hyg. (Lond.) 78:297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC) 2004. One-day (24–28 h) standardized laboratory protocol for molecular subtyping of Escherichia coli O157:H7, non-typhoidal Salmonella serotypes, and Shigella sonnei by pulsed field gel electrophoresis (PFGE). http://www.cdc.gov/pulsenet/protocols/ecoli_salmonella_shigella_protocols.pdf
- 5. Daoust P. Y., et al. 2000. Salmonellosis in songbirds in the Canadian Atlantic provinces during winter-summer 1997–98. Can. Vet. J. 41:54–59 [PMC free article] [PubMed] [Google Scholar]
- 6. Fakhr M. K., Nolan L. K., Logue C. M. 2005. Multilocus sequence typing lacks the discriminatory ability of pulsed-field gel electrophoresis for typing Salmonella enterica serovar Typhimurium. J. Clin. Microbiol. 43:2215–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grant D., Todd P. A., Pennycott T. 2007. Monitoring wild greenfinch (Carduelis chloris) for Salmonella enterica typhimurium. Ecol. Res. 22:571–574 [Google Scholar]
- 8. Grimont P. A. D., Weill F. X. 2007. Antigenic formulas of the Salmonella serovars. WHO Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur, Paris, France: http://www.pasteur.fr/ip/portal/action/WebdriveActionEvent/oid/01s-000036-089 [Google Scholar]
- 9. Hoelzer K., et al. 2010. The prevalence of multidrug resistance is higher among bovine than human Salmonella enterica serotype Newport, Typhimurium, and 4,5,12:i:− isolates in the United States but differs by serotype and geographic region. Appl. Environ. Microbiol. 76:5947–5959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hughes L. A., et al. 2008. Characterisation of Salmonella enterica serotype Typhimurium isolates from wild birds in northern England from 2005–2006. BMC Vet. Res. 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hughes L. A., Wigley P., Bennett M., Chantrey J., Williams N. 2010. Multi-locus sequence typing of Salmonella enterica serovar Typhimurium isolates from wild birds in northern England suggests host-adapted strain. Lett. Appl. Microbiol. 51:477–479 [DOI] [PubMed] [Google Scholar]
- 12. Kapperud G., Stenwig H., Lassen J. 1998. Epidemiology of Salmonella typhimurium O:4–12 infection in Norway: evidence of transmission from an avian wildlife reservoir. Am. J. Epidemiol. 147:774–782 [DOI] [PubMed] [Google Scholar]
- 13. Kingsley R. A., et al. 2009. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 12:2279–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lawson B., et al. 2010. The epidemiology of salmonellosis in garden birds in England and Wales, 1993 to 2003. Ecohealth doi:10.1007/s10393-010-0349-3 [DOI] [PubMed] [Google Scholar]
- 15. Le Minor L., Popoff M. Y. 1987. Designation of Salmonella enterica sp. nov., nom. rev., as the type and only species of the genus Salmonella. Int. J. Syst. Bacteriol. 37:465–468 [Google Scholar]
- 16. Liebana E., et al. 2002. Multiple genetic typing of Salmonella enterica serotype typhimurium isolates of different phage types (DT104, U302, DT204b, and DT49) from animals and humans in England, Wales, and Northern Ireland. J. Clin. Microbiol. 40:4450–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maiden M. C. J., et al. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nesse L. L., et al. 2005. Molecular epidemiology of Salmonella spp. isolates from gulls, fish-meal factories, feed factories, animals and humans in Norway based on pulsed-field gel electrophoresis. Epidemiol. Infect. 133:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pennycott T. W., et al. 1998. Causes of death of wild birds of the family Fringillidae in Britain. Vet. Rec. 143:155–158 [DOI] [PubMed] [Google Scholar]
- 20. Pennycott T. W., Park A., Mather H. A. 2006. Isolation of different serovars of Salmonella enterica from wild birds in Great Britain between 1995 and 2003. Vet. Rec. 158:817–820 [DOI] [PubMed] [Google Scholar]
- 21. Pennycott T. W., Mather H. A., Bennett G., Foster G. 2010. Salmonellosis in garden birds in Scotland 1995 to 2008: geographic region, Salmonella enterica phage type and bird species. Vet. Rec. 166:419–421 [DOI] [PubMed] [Google Scholar]
- 22. Philbey A. W., Mather H. A., Taylor D. J., Coia J. E. 2008. Isolation of avian strains of Salmonella enterica serovar Typhimurium from cats with enteric disease in the United Kingdom. Vet. Rec. 162:120–122 [DOI] [PubMed] [Google Scholar]
- 23. Philbey A. W., Brown F. M., Mather H. A., Coia J. E., Taylor D. J. 2009. Salmonellosis in cats in the United Kingdom: 1955 to 2007. Vet. Rec. 164:120–122 [DOI] [PubMed] [Google Scholar]
- 24. Rabsch W. 2007. Salmonella typhimurium phage typing for pathogens. Methods Mol. Biol. 394:177–211 [DOI] [PubMed] [Google Scholar]
- 25. Refsum T., Handeland K., Baggesen D. L., Holstad G., Kapperud G. 2002. Salmonellae in avian wildlife in Norway from 1969 to 2000. Appl. Environ. Microbiol. 68:5595–5599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Refsum T., Vikøren T., Handeland K., Kapperud G., Holstad G. 2003. Epidemiologic and pathologic aspects of Salmonella typhimurium infection in passerine birds in Norway. J. Wildl. Dis. 39:64–72 [DOI] [PubMed] [Google Scholar]
- 27. Ribot E. M., et al. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3:59–67 [DOI] [PubMed] [Google Scholar]
- 28. Robinson R. A., et al. 2010. Emerging infectious disease leads to rapid population declines of common British birds. PLoS One 5(8):e12215 doi:10.1371/journal.pone.0012215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thornley C. N., et al. 2003. First incursion of Salmonella enterica serotype Typhimurium DT160 into New Zealand. Emerg. Infect. Dis. 9:493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tizard I. R. 2004. Salmonellosis in wild birds. Semin. Avian Pet Med. 13:50–66 [Google Scholar]
- 31. Torpdahl M., Skov M. N., Sandvang D., Baggesen D. L. 2005. Genotypic characterization of Salmonella by multilocus sequence typing, pulsed-field gel electrophoresis and amplified fragment length polymorphism. J. Microbiol. Methods 63:173–184 [DOI] [PubMed] [Google Scholar]