Abstract
Cultivated psychropiezophilic (low-temperature- and high-pressure-adapted) bacteria are currently restricted to phylogenetically narrow groupings capable of growth under nutrient-replete conditions, limiting current knowledge of the extant functional attributes and evolutionary constraints of diverse microorganisms inhabiting the cold, deep ocean. This study documents the isolation of a deep-sea bacterium following dilution-to-extinction cultivation using a natural seawater medium at high hydrostatic pressure and low temperature. To our knowledge, this isolate, designated PRT1, is the slowest-growing (minimal doubling time, 36 h) and lowest cell density-producing (maximal densities of 5.0 × 106 cells ml−1) piezophile yet obtained. Optimal growth was at 80 MPa, correlating with the depth of capture (8,350 m), and 10°C, with average cell sizes of 1.46 μm in length and 0.59 μm in width. Through detailed growth studies, we provide further evidence for the temperature-pressure dependence of the growth rate for deep-ocean bacteria. PRT1 was phylogenetically placed within the Roseobacter clade, a bacterial lineage known for widespread geographic distribution and assorted lifestyle strategies in the marine environment. Additionally, the gene transfer agent (GTA) g5 capsid protein gene was amplified from PRT1, indicating a potential mechanism for increased genetic diversification through horizontal gene transfer within the hadopelagic environment. This study provides a phylogenetically novel isolate for future investigations of high-pressure adaptation, expands the known physiological traits of cultivated members of the Roseobacter lineage, and demonstrates the feasibility of cultivating novel microbial members from the deep ocean using natural seawater.
INTRODUCTION
High hydrostatic pressure is one of the most unique physical parameters in deep-ocean environments and plays a significant, albeit underappreciated, role in the distribution of life in the biosphere. The effects of high hydrostatic pressure on microbial physiology are pervasive, with influences ranging from macromolecular structures to diverse cellular processes such as cell division and motility (8).
Studies of microbial assemblages from the abyssal (4,000 m to 6,000 m) and hadopelagic (>6,000 m) environments are riddled with methodological issues, including but not limited to (i) difficulty and expense of sample collection, (ii) bulk seawater collection that mechanically disrupts delicate colloidal and particulate microenvironments, and (iii) specialized high-pressure equipment to maintain samples at in situ pressure and temperature once shipboard (6, 51, 89). Intrinsic biological factors similarly compound the complications of sampling from depth, the most challenging of which is the mixing of allochthonous microbial members from the overlying surface seawater derived from the vertical transport of aggregated material. These “hitchhiking” microorganisms, which are not adapted to the prevailing conditions of the deep, render taxonomic cataloguing and diversity estimates of authentic microbial members difficult. Allochthonous members can readily be recovered in molecular surveys and cultivation efforts, and they influence activity measurements from deep-ocean environments (22, 30, 41, 51, 61). Thus, it is imperative to corroborate the putative high diversity of microorganisms from deep-ocean environments identified using molecular techniques (73) with detailed experiments on phylogenetically diverse psychropiezophilic (low-temperature- and high-pressure-adapted) isolates.
Over 30 years ago, Yayanos and colleagues reported the isolation of the first piezophilic bacterium from the cold, deep ocean (91). Since then, cultivation efforts have yielded psychropiezophilic isolates from phylogenetically narrow groupings within the Gammaproteobacteria and two Gram-positive Carnobacteriaceae strains (16, 18, 30, 34–36, 40, 54–58, 85–88, 90, 91). These isolates have generation times ranging from 6 to 35 h under nutrient-replete conditions at deep-sea temperatures and pressures. Cultivation of meso- and hyperthermophilic isolates has yielded more diverse groupings, including members of the phyla Actinobacteria, Thermotogae, and Euryarchaeota, in addition to the Proteobacteria (77) (Table 1). Biochemical, physiological, genetic, and genomic studies of these isolates have provided valuable information about the mechanisms of high-pressure adaptation (4, 16, 21, 39, 80, 82, 88, 89). However, the high rates of phylogenetic and metabolic diversity identified from metagenomic (17, 37, 47) and small-subunit ribosomal gene surveys (22, 29, 63, 73) beg the question of whether the same adaptive mechanisms identified in the isolated piezophiles are representative of phylogenetically diverse piezophiles.
Table 1.
Growth properties of selected cultivated piezotolerant and piezophilic isolates
| Isolate | Topt (°C) | Popt (MPa) | Maximal growth rate (h−1) | Isolation source (depth [m]) | Reference(s) |
|---|---|---|---|---|---|
| Colwelliaceae | |||||
| Colwellia piezophila Y223GT | 10 | 60 | 0.14 | Japan Trench, sediment (6,278) | 54 |
| Colwellia hadaliensis BNL-1T | 10 | 90 | 0.12 | Puerto Rico Trench (7,410) | 18 |
| Colwellia sp. strain MT41 | 8 | 103 | ∼0.07 | Mariana Trench, decaying amphipod (10,476) | 88, 90 |
| Psychromonadaceae | |||||
| Psychromonas profunda 2825T | 10 | 25 | ∼0.15 | Atlantic Ocean sediment (2,770) | 87 |
| Psychromonas kaikoae JT7304T | 10 | 50 | ∼0.15 | Japan Trench, cold-seep sediment (7,434) | 57 |
| Psychromonas sp. strain CNPT3 | 12 | 52 | ∼0.19 | Central North Pacific, decaying amphipod (5,800) | 88, 91 |
| Psychromonas hadalis K41GT | 6 | 60 | ∼0.14 | Japan Trench, sediment (7,542) | 55 |
| Moritellaceae | |||||
| Moritella profunda 2674T | 6 | 30 | ∼0.17 | Atlantic Ocean, sediment (2,815) | 86 |
| Moritella abyssi 2693T | 10 | 30 | ∼0.20 | Atlantic Ocean, sediment (2,815) | 86 |
| Moritella sp. strain PE36 | 10 | 41 | ∼0.28 | Pacific Ocean, amphipod trap water (3,584) | 88 |
| Moritella japonica DSK1 | 15 | 50 | ∼0.4 | Japan Trench, sediment (6,356) | 36 |
| Moritella yayanosi DB21MT-5 | 10 | 80 | ∼0.2 | Mariana Trench, sediment (10,898) | 34, 56 |
| Vibrionaceae | |||||
| Photobacterium profundum DSJ4 | 10 | 10 | ∼0.45 | Ryukyu Trench, sediment (5,110) | 58 |
| Photobacterium profundum SS9 | 15 | 28 | ∼0.5 | Sulu Trough, amphipod homogenate (2,551) | 16 |
| Shewanellaceae | |||||
| Shewanella violacea DSS12 | 10 | 30 | ∼0.28 | Ryukyu Trench, sediment (5,110) | 36 |
| Shewanella benthica F1A | 8 | 30 | ∼0.15 | Atlantic Ocean, water column (4,900) | 30, 85 |
| Shewanella benthica DB6101 | 10 | 50 | ∼0.35 | Ryukyu Trench sediment (5,110) | 36 |
| Shewanella benthica DB5501 | 15 | 60 | ∼0.35 | Suruga Bay, sediment (2,485) | 36 |
| Shewanella benthica DB6705 | 15 | 60 | ∼0.4 | Japan Trench, sediment (6,356) | 36 |
| Shewanella benthica DB6906 | 15 | 60 | ∼0.35 | Japan Trench, sediment (6,269) | 36 |
| Shewanella benthica DB172R | 10 | 60 | ∼0.45 | Izu-Bonin Trench, sediment (6,499) | 35 |
| Shewanella benthica DB172F | 10 | 70 | ∼0.41 | Izu-Bonin Trench, sediment (6,499) | 35 |
| Shewanella benthica DB21MT-2 | 10 | 70 | ∼0.17 | Mariana Trench sediment (10,898) | 34, 56 |
| Shewanella sp. strain KT99 | ∼2 | ∼98 | Kermadec Trench, amphipod homogenate (9,856) | 40 | |
| Non-Gammaproteobacteria | |||||
| Thioprofundum lithotrophica 106 | 50 | 15 | 0.3 | Mid-Atlantic Ridge, black smoker chimney (3,626) | 77 |
| Desulfovibrio profundus 500-1T | 25 | 15 | —a | Japan Sea, sediment core 518 mbsfb (900) | 7 |
| Carnobacterium sp. strain AT7 | 20 | 20 | Aleutian Trench, water column (2,500) | 40 | |
| Methanopyrus kandleri 116 | 105 | 20 | ∼0.75 | Central Indian Ridge, black smoker fluid (2,415–2,460) | 78 |
| Desulfovibrio hydrothermalis AM13T | 35 | 26 | ∼0.05 | East Pacific Rise, hydrothermal vent chimney (2,600) | 3 |
| Piezobacter thermophilus 108 | 50 | 35 | 0.46 | Mid-Atlantic Ridge, black smoker chimney (3,626) | 77 |
| Marinitoga piezophila KA3T | 65 | 40 | ∼1.9 | East Pacific Rise, hydrothermal vent (2,630) | 2 |
| Dermacoccus abyssi MT1.1T | 28 | 40 | —c | Mariana Trench, sediment (10,898) | 60 |
| Pyrococcus abyssi GE5 | 100 | 40 | 0.98 | Fiji Basin, hydrothermal vent (2,000) | 23 |
| Thermococcus barophilus MPT | 85 | 40 | ∼1.5 | Mid-Atlantic Ridge, hydrothermal vent chimney (3,550) | 46 |
| Methanococcus thermolithotrophicus | 65 | 50 | ∼0.58 | Italy, geothermally heated sediments (0.5) | 9 |
| Pyrococcus sp. strain CH1 | 98 | 52 | —d | Mid-Atlantic Ridge, hydrothermal vent (4,100) | 93 |
| Methanococcus jannaschii | 86 | 75 | 2.36 | East Pacific Rise, hydrothermal vent (2,610) | 31, 48 |
| Rhodobacterales bacterium PRT1 | 10 | 80 | 0.019 | Puerto Rico Trench, seawater (8,350) | This study |
Dilution-to-extinction cultivation methods have resulted in the isolation of numerous members of the bacterioplankton community in the upper ocean, including representatives from the ubiquitous SAR11 clade and phylogenetically novel microorganisms from previously uncultivated phyla (15, 27, 65, 70, 71). While natural seawater as a growth medium, either unamended or supplemented with additional nutrients and vitamins, has been widely successful in obtaining novel microbial isolates from surface seawater assemblages, this methodology has not been extended to cultivation attempts with deep-ocean samples. An exploration of dilution-to-extinction cultivation attempts at high hydrostatic pressure would be a distinct method to isolate microbes from the cold, deep ocean.
In this study, the isolation and characterization of a psychropiezophilic member of the Alphaproteobacteria, designated PRT1, are presented. To our knowledge, this is the first dilution-to-extinction cultivation effort using a natural seawater medium at high hydrostatic pressure and low temperature.
MATERIALS AND METHODS
Sample collection and high-pressure cultivation conditions.
Hadal seawater was collected from 8,350 m (19.763°N, 66.379°W) within the Puerto Rico Trench (PRT) aboard the R/V Pez Mar in November 2006 using the deep-ocean vehicle (DOV) Bobby Ray, an untethered free-fall/free-ascent vehicle designed and constructed by Scripps engineer Kevin Hardy. The temperature of the seawater sample shortly after recovery was approximately 2°C. Upon recovery of DOV Bobby Ray, hadal seawater was returned to in situ temperature and pressure conditions using stainless steel pressure vessels (92). Seawater samples were maintained in 15-ml polyethylene transfer pipette bulbs (Samco) and heat sealed with a handheld heat-sealing clamp (Nalgene) until further processing at Scripps Institution of Oceanography.
Dilution-to-extinction cultivation methods were carried out at high hydrostatic pressure using a natural seawater medium supplemented with 1.0 μM NH4Cl and 0.1 μM KH2PO4 as described by Rappé et al. (65). Natural seawater was collected from the Scripps pier (32.867°N, 117.257°W; La Jolla, CA) and subsequently filtered through a 47-mm-diameter, 0.22-μm pore-size Supor-200 membrane (Pall Corporation), sparged with CO2 (3 min per 1 liter of seawater), and autoclaved for 30 min. The seawater for making the low-nutrient medium was collected when the chlorophyll levels were low and never during or after a rain event. Sterilized seawater was stored in the dark at 4°C to age for at least 3 months. Prior to culture transfers, the medium was again filtered though a sterile 0.22-μm pore-size Supor-200 membrane (Pall Corporation) membrane and supplemented either with phosphate and ammonium or phosphate, ammonium, Thauer vitamin mixture (84) diluted 10−4, and a defined mixture of organic carbon compounds (0.001% [wt/vol] d-glucose, d-ribose, succinic acid, pyruvic acid, glycerol, N-acetylglucosamine, 0.002% [vol/vol] ethanol [65]). For serial dilution transfers, samples were briefly decompressed and immediately inoculated into chilled seawater medium on ice, which was then transferred into 15-ml polyethylene transfer pipette bulbs (Samco), heat sealed, and pressurized to 80 MPa in pressure vessels (89, 92). All transfers and culture manipulations were exposed to atmospheric pressure for less than 30 min and were performed in the dark under a yellow fluorescent lamp (General Electric) to minimize exposure to light. Enrichments were carried out at 80 MPa (corresponding approximately to the in situ pressure condition) and at 8°C. Cell abundances were monitored microscopically using the nucleic acid stain DAPI (4′,6′-diamidino-2-phenylindole) (H-1200; Vector Laboratories, Inc.) for evidence of bacterial growth.
16S rRNA sequencing and phylogenetic analysis.
Genomic DNA was extracted from 150 ml of culture filtered onto a 0.22-μm pore-size Supor-200 membrane (Pall Corporation). The filter was submerged in 3 ml lysis buffer (50 mM Tris-HCl, 40 mM EDTA, 0.75 M sucrose, 1 mg/ml lysozyme) and incubated at 37°C for 1 h with gentle mixing. Proteinase K (final concentration, 0.5 mg/ml) and 1% SDS were added and mixed by inversion, and the mixture was subjected to two freeze-thaw cycles and incubated at 55°C for 2 h. Phenol-chloroform-isoamyl alcohol (25:24:1; Invitrogen) was used to purify DNA, which was subsequently precipitated with 100% ethanol and resuspended in TE (10 mM Tris, 1 mM EDTA) buffer. The 16S ribosomal gene was amplified by PCR using the bacterial 16S-specific primers 27F and 1492R (26). The PCR product was purified with a QIAquick PCR purification kit (Qiagen) and directly sequenced using the general eubacterial primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′), 518R (5′-GTATTACCGCGGCTGCTG-3′), 530F (5′-GTGCCAGCAGCCGCGG-3′), 907R (5′-CCGTCAATTCATTTGAGT-3′), 926F (5′-ACTCAAAGGAATTGACGG-3′), and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The sequences were assembled using the CLC Sequence Viewer (CLC bio), aligned using the SINA Webaligner (64), uploaded into the ARB program (43), and manually checked with the ARB_EDIT4 tool. A filter was applied to aligned sequences to exclude positions with <50% conservation, and Escherichia coli positions 59 to 1406 were exported for bootstrap analysis using the maximum-likelihood methods implemented in RaxML (74) with the JTT model for evolutionary distances through the CIPRES portal (49). Neighbor-joining and parsimony methods implemented in PHYLIP (24) were additionally utilized to compare phylogenies and tree topology. Bootstrap support for nodes is indicated for values above 50%. The outgroup used to calculate phylogeny was Sinorhizobium meliloti (GenBank accession number D14509). Sequence similarity analysis was performed within the ARB program (43).
Identification of GTA using PCR and microscopy.
Degenerate primers developed by Zhao and colleagues (94) were used to screen for the presence of the putative gene transfer agent (GTA) g5 gene (encoding the major capsid protein) from PRT1 genomic DNA extracted as described above. PCR amplification was carried out as described previously (94), and the product was purified with a QIAquick PCR purification kit (Qiagen) and sequenced directly. The PRT1 g5 DNA sequence was translated and aligned with 156 putative GTA g5 proteins (see Table S1 in the supplemental material) using MUSCLE (20). Phylogenetic trees were constructed from the aligned amino acid sequences consisting of 241 unambiguous positions using the maximum-likelihood methods implemented in RaxML (74) with the JTT model for evolutionary distances through the CIPRES portal (49).
GTA particles were collected onto a 0.02-μm Anodisc filter and observed by using epifluorescence microscopy with SYBR green I (Invitrogen) at 1,000× final magnification, following staining with the method described by Patel and colleagues (59).
Characterization of growth under various temperature and hydrostatic-pressure conditions.
For determinations of growth rate as a function of pressure and temperature, mid-log cultures were inoculated 1:100 into 100-ml sterilized Kapak bags (Kapak Corporation) with an attached 0.045-in.-diameter polyethylene tube to allow subsampling from the same culture. All cultures were incubated with sterile glass beads in a temperature-controlled rocking water bath to ensure mixing within the pressure vessels. The same collected batch of natural seawater supplemented with phosphate, ammonium, Thauer vitamins, and the mixture of organic carbon compounds was used for all growth characterization experiments. At 24-h time intervals, pressure vessels were decompressed and 2-ml amounts of culture were removed, fixed with formaldehyde solution (3% final concentration), and frozen at −80°C until analysis.
Growth was monitored with a Becton-Dickson FACsort flow cytometer. Samples were thawed on ice, diluted 10-fold, and stained for 15 min in the dark with SYBR green I (Invitrogen) (45). Quantification of cells was based on green fluorescence and side scatter, and each sample was run with a known concentration of 0.9-μm standard beads. CytoWin (79) was used to process cytometric plots, and the resultant growth curves were fit to a parametric logistic growth model using the program grofit implemented in R (32). The exponential growth rate constants (k) were derived for 17 growth conditions at various temperatures and pressures from a total of 30 discrete growth experiments (experiments resulting in no growth were assigned a k value of 0). The values for k were used to construct the contour plot for the pressure-temperature dependency of the growth rate constant (k) with the DIVA (Data-Interpolating Variational Analysis) gridding software implemented in Ocean Data View (69).
Microscopy. (i) Epifluorescence microscopy for cell sizing.
Formaldehyde-fixed mid-log cultures were filtered onto 0.2-μm Isopore polycarbonate membranes (Millipore) and stored at −80°C until further analysis. Two different staining methods were utilized to obtain cell size measurements as described by Malfatti et al. (44), the nucleic acid stain DAPI (H-1200; Vector Laboratories, Inc.) and the protein stain NanoOrange (N-6666; Invitrogen). The stained samples were viewed at 1,000× on an Olympus BX51 microscope (Olympus) equipped with UV and blue light filter cubes, and images were processed using ImageJ (1, 66). The length and width for 100 cells were measured for both DAPI- and NanoOrange-stained samples.
(ii) Atomic force microscopy.
An aliquot of the formaldehyde-fixed sample was filtered onto 0.2-μm polycarbonate membranes and then rinsed with high-pressure liquid chromatography (HPLC) water in order to remove salt crystals. After drying, the filter was mounted on a glass slide. Atomic force microscopy (AFM) imaging was performed with an MFP-3D BIO (Asylum Research, Santa Barbara, CA) as described by Malfatti et al. (44). Briefly, images were acquired in AC mode in air with a silicon nitride cantilever (AC160TS; Olympus), with a spring constant of 42 N/m and scan rates of 1 Hz. The data from height and phase channels were recorded since the height channel gives quantitative data on the topography of the sample, while the phase channel can suggest qualitative data on the viscoelastic properties of the sample. Topography images were processed with Planfit and Flatten functions.
(iii) Cryo-transmission electron microscopy (cryo-TEM).
Amounts of 3.5 μl of mid-log cultures were applied to holey carbon grids (Quantifoil) that had been glow discharged in an Emitech K350 evaporation unit. The grids were then vitrified using a Vitrobot robot (FEI) and transferred into a precooled, FEI Polara multispecimen holder, which maintained the grids at liquid nitrogen temperature. Micrographs were recorded on a Gatan 4K2 charge-coupled device (CCD) camera at 300 keV in an FEI Polara microscope under low-dose conditions (3 e− per Å2) at nominal magnifications of 12,000× and 23,000× (CCD, 1.88 Å per pixel) and an objective lens defocus setting of −15 μM.
Nucleotide sequence accession numbers.
The 16S rRNA and GTA sequences were deposited in GenBank under accession numbers JF303756 and JF303757, respectively.
RESULTS AND DISCUSSION
Isolation of PRT1 using natural seawater medium at high hydrostatic pressure.
Dilution-to-extinction cultivation methods using a natural seawater medium supplemented with ammonium and phosphate as described by Rappé et al. (65) were carried out at 80 MPa and 8°C. Eleven endpoint serial dilution transfers over the course of approximately 2 years resulted in the isolation of strain PRT1, which was verified using 16S rRNA gene sequencing.
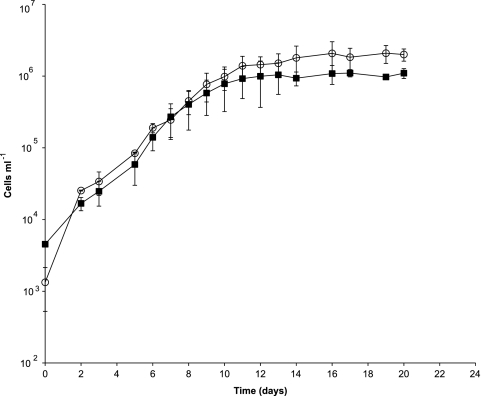
Representative growth plots are presented in Fig. 1. PRT1 was originally isolated and propagated with natural seawater amended only with 1.0 μM ammonium (NH4Cl) and 0.1 μM phosphate (KH2PO4). Under these conditions, the growth rates ranged from 0.3 to 0.4 day−1. Subsequent growth experiments included supplementation with a dilute vitamin solution and a defined mixture of organic carbon compounds, resulting in marginal growth rate increases (0.4 to 0.43 day−1) and higher cell abundances (maximal cell densities of 5.0 × 106 cells ml−1). These growth rate values are comparable to those of surface seawater oligotrophic isolates (0.40 to 0.58 day−1 for Pelagibacter ubique HTCC1062 [65] and 0.40 to 0.73 day−1 for four oligotrophic isolates [71]), yet they are substantially lower than those of all cultured psychropiezophilic isolates (Table 1). However, a compilation of 375 growth rate measurements from microbes in the bathypelagic realm (1,000 to 4,000 m) yielded an average growth rate of 0.061 day−1 (0.019 ± 0.008 [median ± standard error {SE}]) (6). Therefore, the growth rate of PRT1 might be more representative of natural rates of reproduction in the deep ocean than are the growth rates of previously described psychropiezophilic isolates grown under nutrient-replete conditions, in which case it would be considered one of the relatively faster-growing representatives from the deep ocean.
Fig. 1.
Growth of PRT in natural seawater medium amended with ammonium and phosphate (■) and ammonium, phosphate, Thauer vitamins, and a mixed carbon solution (○) measured using flow cytometry. Error bars represent standard deviations of the results of duplicate incubations at 80 MPa and 8°C.
The growth yield of PRT1 was similarly distinct from those of all cultured psychropiezophilic isolates, reaching a cell density (∼106 cells ml−1) that was at least an order of magnitude less than that of even the slowest growing, most piezophilic isolate reported under optimal growth conditions (Colwellia sp. strain MT41, ∼107 cells ml−1) (90). While most psychropiezophilic growth can be tracked using optical density measurements, since cultures reach 108 to 109 cells ml−1, the growth of PRT1 was monitored using flow cytometric techniques in the absence of visible turbidity. The maximal cell densities varied between different batches of seawater collected, as well as the amount of time allowed for the seawater to age. This variation has similarly been observed for the growth properties of SAR11 (65) and suggests that intrinsic factors in the seawater contribute to the growth characteristics of PRT1. Efforts to propagate PRT1 in a variety of synthetic seawater media were unsuccessful. Additionally, increasing the concentration of organic carbon compounds by 1 and 2 orders of magnitude (0.01% and 0.1%, respectively) substantially decreased growth yields (maximal cell densities of 2.6 × 104 cells ml−1).
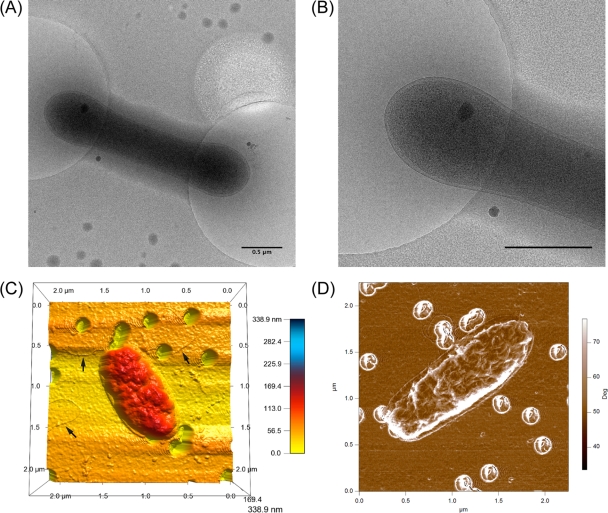
The cell shapes ranged from bilobes to rods, with an average length of 1.46 μm (SE, ±0.039; n = 100) and an average width of 0.59 μm (SE, ±0.0064; n = 100). Size estimates were obtained using NanoOrange-staining cell-sizing methods (the ratios of DAPI to NanoOrange sizing were 0.87 and 0.78 for length and diameter, respectively) (44). The biovolume of PRT1, computed using the formula V = (π/4)W2 × (L − W/3), where L is the length and W is the width (11), was 0.345 μm3. This volume is 36 times greater than that of Pelagibacter ubique HTCC1062 (0.010 μm3, based on size measurements reported by Rappé et al. [65]). Both of these organisms are adapted to low nutrient concentrations; nevertheless, the size is greater for the high-pressure-, low-temperature-adapted strain. PRT1 cells were further visualized using more refined techniques, including cryo-TEM and atomic force microscopy (AFM) to observe morphological features (Fig. 2). Interestingly, AFM imaging yielded the identification of small (∼20 nm in diameter) pilus-like appendages that were consistently found associated with or detached from the cell surfaces and were distinct from flagellar filaments due to their small size (Fig. 2C). These may not have been visible in cryo-TEM images due to the very low electron doses used for imaging.
Fig. 2.
Morphological features of PRT1 visualized using cryo-transmission electron microscopic (cryo-TEM) and atomic force microscopic (AFM) techniques. (A, B) Cryo-TEM images of PRT1 at 12,000× magnification (A) and 23,000× magnification (B). Scale bar, 0.5 μm. (C, D) AFM images of individual PRT1 cells. Color bars indicate the cell height (Z) range in nanometers (C) and degrees (height contrast from the filter base) (D). Black arrows indicate pilus-like appendages (C).
PRT1 is an obligate psychropiezophile.
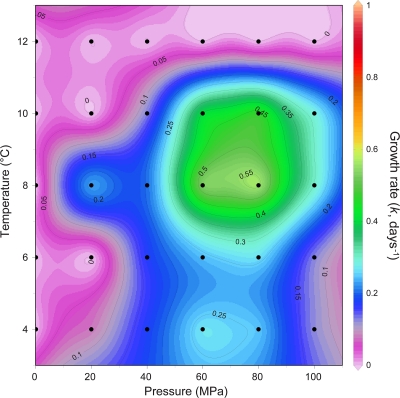
The exponential growth rate constants (k) for PRT1 were derived from a total of 30 discrete growth experiments (using seawater collected from the same batch for purposes of consistency) and used to construct the contour plot for the pressure-temperature dependency of the growth rate constant (Fig. 3). These detailed growth experiments at various temperature and pressure conditions demonstrate the prominence of the “piezophilic trait” and provide further evidence for the temperature-pressure dependence of the growth rate for deep-ocean bacteria as previously described by Yayanos (88). PRT1 was piezophilic under all temperatures tested (4 to 12°C) and found to be an obligate piezophile, or hyperpiezophile (Pkmax > 60 MPa). No growth was observed at atmospheric pressure (Fig. 3). As has been observed previously (88), the growth rate varied with temperature and pressure, indicating a correlation with the habitat pressure (or capture depth) of the isolate. The growth rate of PRT1 near the pressure of the depth of capture (8,350 m) increased with increasing temperature over the interval of 6 to 10°C, with the maximal growth rate among all temperatures being ≈8 to 10°C, consistent with the work of Yayanos and colleagues (88, 89). The results for this isolate further support the assertion that the piezophilic trait is invariant with nutritional state and that, in the absence of a high-nutrient medium (such as 2216 marine broth), bacterial isolates can be recovered that demonstrate adaptations to the prevailing low-temperature and high-hydrostatic-pressure conditions found in the deep ocean.
Fig. 3.
Interpolated contour diagram of the temperature-pressure dependence of the exponential growth rate constant (k) of PRT1. Black dots represent the results of individual growth experiments at the given temperatures and pressures, with color shading and contour lines integrated using DIVA (Data-Interpolating Variational Analysis) gridding software.
PRT1 is a member of the Roseobacter clade within the Alphaproteobacteria.
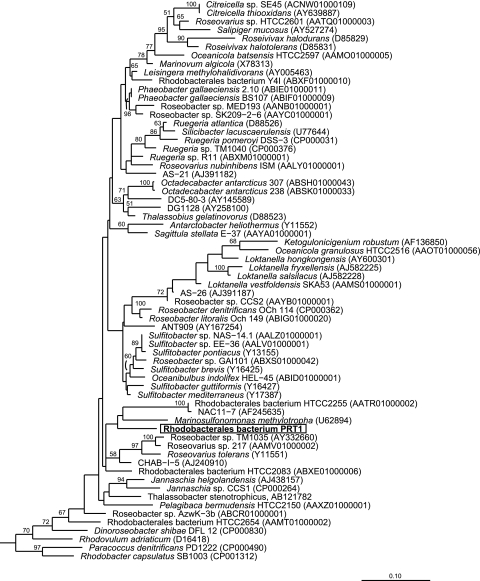
Sequence analysis of the 16S small-subunit ribosomal gene placed PRT1 within the Roseobacter clade, a diverse alphaproteobacterial lineage found ubiquitously in the marine environment (Fig. 4). The within-taxon phylogeny of the Roseobacter lineage is poorly resolved using 16S rRNA gene phylogenies (13, 14, 53), rendering reliable phylogenetic reconstruction for PRT1 difficult. A more robust phylogenetic analysis of over 6,000 Rhodobacterales sequences indicated that PRT1 was affiliated with the abundant marine cluster NAC11-7 (14), a group represented by numerous environmental clone sequences but only two cultivated members (14, 71). The two cultivated members were both obtained using dilution-to-extinction cultivation methods, and a draft genome is available for one of the isolates (Rhodobacterales bacterium HTCC2255; https://moore.jcvi.org/moore/), yet few additional physiological data are available since the initial description of the isolation. The NAC11-7 clade has typically been associated with phytoplankton blooms (83), yet it has also been identified in diverse environments, including oxygen minimum zones (75, 81), in association with deep-sea corals (62) and the hydrothermal vent worm Riftia pachyptila (42), and in hydrothermal chimney biofilms (12), deep-ocean sediments (68), and numerous coastal environments worldwide (67, 76). PRT1 16S rRNA sequence similarity to the 32 Roseobacter isolates with available genome sequences ranged from 90.8 to 94%, which is consistent with the large sequence variation (∼11%) documented for the more than 45 described genera of the Roseobacter lineage (53).
Fig. 4.
Phylogenetic placement of PRT1 within the Roseobacter clade. The PRT1 16S rRNA gene sequence was aligned and compared to reference Roseobacter sequences, and phylogeny was inferred using the maximum-likelihood method implemented in RaxML (74) with the JTT model for evolutionary distances through the CIPRES portal (49). Bootstrap support (100 bootstrap replicates) for nodes is indicated for values greater than 50%. GenBank accession numbers are indicated in parentheses.
The identification of a gene component, the g5 capsid protein gene, for the positive detection of a gene transfer agent (GTA) from PRT1 further supports the phylogenetic placement within the Rhodobacterales order, since almost all members contain this gene (10). GTA particles were additionally visualized from mid-log PRT1 cultures grown at 80 MPa and 8°C (data not shown). GTAs are novel virus-like elements that function in the transfer of random fragments of genomic DNA (4.5 kb for the Rhodobacter capsulatus GTA) while conferring no documented negative effects associated with transfer (i.e., cell lysis) (10, 38). While the g5 phylogeny has previously been found to be congruent with the 16S rRNA phylogeny (94), a more recent study of Rhodobacterales GTAs from the subarctic North Atlantic demonstrated that using a subset of the gene sequence information does not necessarily reflect the expected phylogenetic relationships (25). Analysis of the PRT1 g5 capsid protein with 156 available Rhodobacterales g5 capsid protein sequences (see Fig. S1 in the supplemental material) did not help to resolve the phylogenetic placement of PRT1, although since HTCC2083 and HTCC2255 appear to lack the genetic complements for GTA production, this finding is not surprising. The sequence similarities of the PRT1 g5 capsid protein sequence ranged from 81.1 to 67.4% for the g5 capsid protein sequences from the 32 Roseobacter isolates with available genome sequences. The identification of the g5 capsid protein gene from PRT1 provides insight into a potential mechanism of increased genetic diversification through horizontal gene transfer from GTAs within the hadopelagic environment.
A detailed analysis of available marine Roseobacter genomes indicated that the gene repertoire for a particular trophic strategy (heterotrophy, photoheterotrophy, or autotrophy) was the best predictor of clustering relationships (53). The finding that trophic strategy and not necessarily phylogeny is the best framework for predicting genome content is in line with the hypothesis that horizontal gene transfer (potentially mediated by GTAs) may be the dominant evolutionary force for this generalist lineage. Importantly, the results from the eloquent analysis of available Roseobacter genomes demonstrate the uniqueness of each genome (53), therefore rendering inferences about physiological traits based on phylogenetic relatedness impossible. PRT1 is the third documented psychrophile within the clade, after Octadecabacter arcticus 238 and Octadecabacter antarcticus 307 (28). These psychrophilic Roseobacter genomes contained the greatest number of unique genes, the majority of which were annotated as phage or transposase related, consistent with the proposed hypothesis that transposases play an important role in psychrophilic genome evolution (5). One could hypothesize that the genome of PRT1 is similar to the two psychrophilic Roseobacter genomes, since it must cope with cold temperatures as well as constraints imposed by high hydrostatic pressure. Indeed, molecular analysis of piezophilic gammaproteobacterial isolates suggests that high-pressure adaptation is preceded by preexisting adaptation to low temperature (40).
The majority of published cultivated representatives from the Roseobacter lineage form colonies on nutrient agar media, yet more recent cultivation strategies using dilution-to-extinction methods have resulted in novel isolates that are unable to form colonies, such as Rhodobacterales bacterium HTCC2150 (33). While methods to detect isolated colonies at high hydrostatic pressure are available (19, 52), PRT1 was unable to form colonies under the conditions tested using either gelatin or agar as the solidifying agent. Although PRT1 was not found to form colonies, cells were observed to clump together and form weakly associated aggregates during growth, which might be indicative of the general hypothesis suggested by Moran and colleagues that members of the Roseobacter clade live in nutrient-replete aggregates (e.g., marine snow) found in the oligotrophic ocean (50). The identification of small pilus-like appendages associated with PRT1 mentioned above might contribute to the clumping growth characteristics observed, and additional work is warranted to explore potential colonization and colony formation abilities beyond this initial observation. It is possible that these appendages are pili, which would be consistent with recent genomic analyses indicating that all Roseobacter genomes harbor the genetic complement for assembly of adhesive Flp (fimbrial low-molecular-weight protein) pili (72).
Implications for isolating new piezophiles from novel phylogenetic lineages.
Autochthonous microorganisms of the deep ocean are inherently adapted to the ambient conditions of their environment, i.e., to the high-pressure, low-temperature conditions found throughout the deep ocean. The utility of extinction-to-dilution cultivation techniques has been demonstrated further here as a unique and distinctive strategy to isolate new psychropiezophiles from diverse phylogenetic groups. The isolation of PRT1, a piezophilic alphaproteobacterial representative, demonstrates (i) the ability to cultivate a phylogenetically novel piezophile outside the previously cultivated gammaproteobacterial families Colwelliaceae, Moritellaceae, Psychromonadaceae, Shewanellaceae, and Vibrionaceae from the cold, deep ocean and (ii) the ability to cultivate a low-nutrient-adapted piezophilic isolate using a natural seawater medium, thus providing evidence for a piezophilic phenotype under oligotrophic conditions, and (iii) it enables future biochemical and physiological characterization to further document the unique adaptations of high-pressure-adapted bacteria to the psychropiezosphere. A cautionary note, however, regarding PRT1 is that the slow growth and minimal cell yields preclude many additional biochemical characterizations that require substantial biomass, such as membrane lipid composition or cytochrome analyses.
Supplementary Material
ACKNOWLEDGMENTS
Thanks to the crew of the R/V Pez Mar through the University of Puerto Rico, Mayagüez, for sample collection within the Puerto Rico Trench. Thanks to Brian Palenik and Bianca Brahamsha for use of their FACsort, and special thanks to Anne-Claire Baudoux and Rhona Stuart for help with flow cytometry.
We thank the UCSD Cryo-Electron Microscopy Facility, which is supported by NIH grants to Timothy S. Baker and a gift from the Agouron Institute to UCSD. This work was supported by NSF grants EF-0801793 and EF-0827051 to D.H.B.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 23 September 2011.
REFERENCES
- 1. Abramoff M. D., Magelhaes P. J., Ram S. J. 2004. Image processing with Image J. Biophotonics Int. 11:36–42 [Google Scholar]
- 2. Alain K., et al. 2002. Marinitoga piezophila sp. nov., a rod-shaped, thermo-piezophilic bacterium isolated under high hydrostatic pressure from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 52:1331–1339 [DOI] [PubMed] [Google Scholar]
- 3. Alazard D., et al. 2003. Desulfovibrio hydrothermalis sp. nov., a novel sulfate-reducing bacterium isolated from hydrothermal vents. Int. J. Syst. Evol. Microbiol. 53:173–178 [DOI] [PubMed] [Google Scholar]
- 4. Allen E. E., Facciotti D., Bartlett D. H. 1999. Monounsaturated but not polyunsaturated fatty acids are required for growth at high pressure and low temperature in the deep-sea bacterium Photobacterium profundum strain SS9. Appl. Environ. Microbiol. 65:1710–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allen M. A., et al. 2009. The genome sequence of the psychrophilic archaeon, Methanococcoides burtonii: the role of genome evolution in cold adaptation. ISME J. 3:1012–1035 [DOI] [PubMed] [Google Scholar]
- 6. Arístegui J., Gasol J. M., Duarte C. M., Herndl G. J. 2009. Microbial oceanography of the dark ocean's pelagic realm. Limnol. Oceanogr. 54:1501–1529 [Google Scholar]
- 7. Bale S. J., et al. 1997. Desulfovibrio profundus sp. nov., a novel barophilic sulfate-reducing bacterium from deep sediment layers in the Japan Sea. Int. J. Syst. Bacteriol. 47:515–521 [DOI] [PubMed] [Google Scholar]
- 8. Bartlett D. H., Lauro F. M., Eloe E. A. 2007. Microbial adaptation to high pressure, p. 333–350 In Gerday C., Glandsdorf N. (ed.), Physiology and biochemistry of extremophiles. ASM Press, Washington, DC [Google Scholar]
- 9. Bernhardt G., Jaenicke R., Lüdemann H. D., König H., Stetter K. O. 1988. High pressure enhances the growth rate of the thermophilic archaebacterium Methanococcus thermolithotrophicus without extending its temperature range. Appl. Environ. Microbiol. 54:1258–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biers E. J., et al. 2008. Occurrence and expression of gene transfer agent (GTA) genes in marine bacterioplankton. Appl. Environ. Microbiol. 74:2933–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bratbak G. 1985. Bacterial biovolume and biomass estimations. Appl. Environ. Microbiol. 49:1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brazelton W. J., Baross J. A. 2009. Abundant transposases encoded by the metagenome of a hydrothermal chimney biofilm. ISME J. 3:1420–1424 [DOI] [PubMed] [Google Scholar]
- 13. Brinkhoff T., Giebel H. A., Simon M. 2008. Diversity, ecology, and genomics of the Roseobacter clade: a short overview. Arch. Microbiol. 189:531–539 [DOI] [PubMed] [Google Scholar]
- 14. Buchan A., Gonzalez J. M., Moran M. A. 2005. Overview of the marine Roseobacter lineage. Appl. Environ. Microbiol. 71:5665–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Button D. K., Schut F., Quang P., Martin R., Robertson B. R. 1993. Viability and isolation of marine bacteria by dilution culture: theory, procedures, and initial results. Appl. Environ. Microbiol. 59:881–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeLong E. F., Franks D. G., Yayanos A. A. 1997. Evolutionary relationships of cultivated psychrophilic and barophilic deep-sea bacteria. Appl. Env. Microbiol. 63:2105–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeLong E. F., et al. 2006. Community genomics among stratified microbial assemblages in the ocean's interior. Science 311:496–503 [DOI] [PubMed] [Google Scholar]
- 18. Deming J. W., Somers L. K., Straube W. L., Swartz D. G., MacDonell M. T. 1988. Isolation of an obligately barophilic bacterium and description of a new genus, Colwellia gen. nov. Syst. Appl. Microbiol. 10:152–160 [Google Scholar]
- 19. Dietz A. S., Yayanos A. A. 1978. Silica gel media for isolating and studying bacteria under hydrostatic pressure. Appl. Environ. Microbiol. 36:966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edgar R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eloe E. A., Lauro F. M., Vogel R. F., Bartlett D. H. 2008. The deep-sea bacterium Photobacterium profundum SS9 utilizes separate flagellar systems for swimming and swarming under high-pressure conditions. Appl. Env. Microbiol. 74:6298–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eloe E. A., et al. 2011. Compositional differences in particle-associated and free-living microbial assemblages from an extreme deep-ocean environment. Environ. Microbiol. Rep. 3:449–458 [DOI] [PubMed] [Google Scholar]
- 23. Erauso G., et al. 1993. Pyrococcus abyssi sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Arch. Microbiol. 160:338–349 [Google Scholar]
- 24. Felsenstein J. 2005. PHYLIP (Phylogeny Inference Package) version 3.6. J. Felsenstein, Department of Genome Sciences, University of Washington, Seattle, WA [Google Scholar]
- 25. Fu Y., et al. 2010. High diversity of Rhodobacterales in the subarctic North Atlantic Ocean and gene transfer agent protein expression in isolated strains. Aquat. Microb. Ecol. 59:283–293 [Google Scholar]
- 26. Giovannoni S. J. 1991. The polymerase chain reaction, p. 177–203 In Stackebrandt E., Goodfellow M. (ed.), Modern microbiological methods: nucleic acids techniques in bacterial systematics. John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 27. Giovannoni S. J., Foster R. A., Rappé M. S., Epstein S. 2007. New cultivation strategies bring more microbial plankton species into the laboratory. Oceanography 20:62–69 [Google Scholar]
- 28. Gosink J. J., Herwig R. P., Staley J. T. 1997. Octadecabacter arcticus gen. nov., sp. nov., and O. antarcticus, sp. nov., nonpigmented, psychrophilic gas vacuolate bacteria from polar sea ice and water. Syst. Appl. Microbiol. 20:356–365 [Google Scholar]
- 29. Hewson I., Steele J. A., Capone D. G., Fuhrman J. A. 2006. Remarkable heterogeneity in meso- and bathypelagic bacterioplankton assemblage composition. Limnol. Oceanogr. 51:1274–1283 [Google Scholar]
- 30. Jannasch H. W., Wirsen C. O. 1984. Variability of pressure adaptation in deep sea bacteria. Arch. Microbiol. 139:281–288 [Google Scholar]
- 31. Jones W. J., Leigh J. A., Mayer F., Woese C. R., Wolfe R. S. 1983. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 136:254–261 [Google Scholar]
- 32. Kahm M., Hasenbrink G., Lichtenberg-Frate H., Ludwig J., Kschischo M. 2010. grofit: fitting biological growth curves with R. J. Stat. Softw. 33:1–2120808728 [Google Scholar]
- 33. Kang I., Oh H.-M., Vergin K. L., Giovannoni S. J., Cho J.-C. 2010. Genome sequence of the marine Alphaproteobacterium HTCC2150, assigned to the Roseobacter clade. J. Bacteriol. 192:6315–6316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kato C., et al. 1998. Extremely barophilic bacteria isolated from the Mariana Trench, Challenger Deep, at a depth of 11,000 meters. Appl. Environ. Microbiol. 64:1510–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kato C., Masui N., Horikoshi K. 1996. Properties of obligately barophilic bacteria isolated from a sample of deep-sea sediment from the Izu-Bonin trench. J. Mar. Biotechnol. 4:96–99 [Google Scholar]
- 36. Kato C., Sato T., Horikoshi K. 1995. Isolation and properties of barophilic and barotolerant bacteria from deep-sea mud samples. Biodivers. Conserv. 4:1–9 [Google Scholar]
- 37. Konstantinidis K. T., Braff J., Karl D. M., DeLong E. F. 2009. Comparative metagenomic analysis of a microbial community residing at a depth of 4,000 meters at Station ALOHA in the North Pacific Subtropical Gyre. Appl. Environ. Microbiol. 75:5345–5355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lang A. S., Beatty J. T. 2007. Importance of widespread gene transfer agent genes in alpha-proteobacteria. Trends Microbiol. 15:54–62 [DOI] [PubMed] [Google Scholar]
- 39. Lauro F. M., Bartlett D. H. 2008. Prokaryotic lifestyles in deep sea habitats. Extremophiles 12:15–25 [DOI] [PubMed] [Google Scholar]
- 40. Lauro F. M., Chastain R. A., Blankenship L. E., Yayanos A. A., Bartlett D. H. 2007. The unique 16S rRNA genes of piezophiles reflect both phylogeny and adaptation. Appl. Environ. Microbiol. 73:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lochte K., Turley C. M. 1988. Bacteria and cyanobacteria associated with phytodetritus in the deep sea. Nature 333:67–69 [Google Scholar]
- 42. López-García P., Gaill F., Moreira D. 2002. Wide bacterial diversity associated with tubes of the vent worm Riftia pachyptila. Environ. Microbiol. 4:204–215 [DOI] [PubMed] [Google Scholar]
- 43. Ludwig W., et al. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Malfatti F., Samo T. J., Azam F. 2010. High-resolution imaging of pelagic bacteria by atomic force microscopy and implications for carbon cycling. ISME J. 4:427–439 [DOI] [PubMed] [Google Scholar]
- 45. Marie D., Partensky F., Vaulot D., Brussaard C. 2001. Enumeration of phytoplankton, bacteria, and viruses in marine samples. Curr. Protoc. Cytom. Chapter 11:Unit 11.11 [DOI] [PubMed] [Google Scholar]
- 46. Marteinsson V. T., et al. 1999. Thermococcus barophilus sp. nov., a new barophilic and hyperthermophilic archaeon isolated under high hydrostatic pressure from a deep-sea hydrothermal vent. Int. J. Syst. Bacteriol. 49:351–359 [DOI] [PubMed] [Google Scholar]
- 47. Martín-Cuadrado A. B., et al. 2007. Metagenomics of the Deep Mediterranean, a warm bathypelagic habitat. PLoS One 2:e914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller J. F., Shah N. N., Nelson C. M., Ludlow J. M., Clark D. S. 1988. Pressure and temperature effects on growth and methane production of the extreme thermophile Methanococcus jannaschii. Appl. Environ. Microbiol. 54:3039–3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller M. A., et al. 2009. The CIPRES Portals. Cyberinfrastructure for Phylogenetic Research project. http://www.phylo.org/sub_sections/portal
- 50. Moran M. A., et al. 2007. Ecological genomics of marine roseobacters. Appl. Environ. Microbiol. 73:4559–4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagata T., et al. 2010. Emerging concepts on microbial processes in the bathypelagic ocean—ecology, biogeochemistry, and genomics. Deep Sea Res. Pt. II. 57:1519–1536 [Google Scholar]
- 52. Nakayama A., Yano Y., Yoshida K. 1994. New method for isolating barophiles from intestinal contents of deep-sea fishes retrieved from the abyssal zone. Appl. Environ. Microbiol. 60:4210–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Newton R. J., et al. 2010. Genome characteristics of a generalist marine bacterial lineage. ISME J. 4:784–798 [DOI] [PubMed] [Google Scholar]
- 54. Nogi Y., Hosoya S., Kato C., Horikoshi K. 2004. Colwellia piezophila sp. nov., a novel piezophilic species from deep-sea sediments of the Japan Trench. Int. J. Syst. Evol. Microbiol. 54:1627–1631 [DOI] [PubMed] [Google Scholar]
- 55. Nogi Y., Hosoya S., Kato C., Horikoshi K. 2007. Psychromonas hadalis sp. nov., a novel piezophilic bacterium isolated from the bottom of the Japan Trench. Int. J. Syst. Evol. Microbiol. 57:1360–1364 [DOI] [PubMed] [Google Scholar]
- 56. Nogi Y., Kato C. 1999. Taxonomic studies of extremely barophilic bacteria isolated from the Mariana Trench and description of Moritella yayanosii sp. nov., a new barophilic bacterial isolate. Extremophiles 3:71–77 [DOI] [PubMed] [Google Scholar]
- 57. Nogi Y., Kato C., Horikoshi K. 2002. Psychromonas kaikoae sp. nov., a novel piezophilic bacterium from the deepest cold-seep sediments in the Japan Trench. Int. J. Syst. Evol. Microbiol. 52:1527–1532 [DOI] [PubMed] [Google Scholar]
- 58. Nogi Y., Masui N., Kato C. 1998. Photobacterium profundum sp. nov., a new moderately barophilic bacterial species isolated from a deep-sea sediment. Extremophiles 2:1–7 [DOI] [PubMed] [Google Scholar]
- 59. Patel A., et al. 2007. Virus and prokaryote enumeration from planktonic aquatic environments by epifluorescence microscopy with SYBR Green I. Nat. Protoc. 2:269–276 [DOI] [PubMed] [Google Scholar]
- 60. Pathom-aree W., et al. 2006. Dermacoccus abyssi sp. nov., a piezotolerant actinomycete isolated from the Mariana Trench. Int. J. Syst. Evol. Microbiol. 56:1233–1237 [DOI] [PubMed] [Google Scholar]
- 61. Pathom-aree W., et al. 2006. Dermacoccus barathri sp. nov. and Dermacoccus profundi sp. nov., novel actinomycetes isolated from deep-sea mud of the Mariana Trench. Int. J. Syst. Evol. Microbiol. 56:2303–2307 [DOI] [PubMed] [Google Scholar]
- 62. Penn K., Wu D., Eisen J. A., Ward N. 2006. Characterization of bacterial communities associated with deep-sea corals on Gulf of Alaska seamounts. Appl. Environ. Microbiol. 72:1680–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pham V. D., Konstantinidis K. T., Palden T., DeLong E. F. 2008. Phylogenetic analyses of ribosomal DNA-containing bacterioplankton genome fragments from a 4,000 m vertical profile in the North Pacific Subtropical Gyre. Environ. Microbiol. 10:2313–2330 [DOI] [PubMed] [Google Scholar]
- 64. Pruesse E., et al. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35:7188–7196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rappé M. S., Connon S. A., Vergin K. L., Giovannoni S. J. 2002. Cultivation of the ubiquitous SAR11 marine bacterioplankton clade. Nature 418:630–633 [DOI] [PubMed] [Google Scholar]
- 66. Rasband W. S. 2009. ImageJ. National Institutes of Health, Bethesda, MD: http://rsb.info.nih.gov/ij/ [Google Scholar]
- 67. Rusch D. B., et al. 2007. The Sorcerer II global ocean sampling expedition: Northwest Atlantic through Eastern Tropical Pacific. PLoS Biol. 5:398–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schauer R., Bienhold C., Ramette A., Harder J. 2010. Bacterial diversity and biogeography in deep-sea surface sediments of the South Atlantic Ocean. ISME J. 4:159–170 [DOI] [PubMed] [Google Scholar]
- 69. Schlitzer R. 2010. Ocean Data View. SeaDataNet, Pan-European Infrastructure for Ocean & Marine Data Management, IFREMER/SISMER, Plouzane, France: http://odv.awi.de [Google Scholar]
- 70. Schut F., et al. 1993. Isolation of typical marine bacteria by dilution culture: growth, maintenance, and characteristics of isolates under laboratory conditions. Appl. Environ. Microbiol. 59:2150–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Simu K., Hagström Å. 2004. Oligotrophic bacterioplankton with a novel single-cell life strategy. Appl. Env. Microbiol. 70:2445–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Slightom R. N., Buchan A. 2009. Surface colonization by marine roseobacters: integrating genotype and phenotype. Appl. Environ. Microbiol. 75:6027–6037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sogin M. L., et al. 2006. Microbial diversity in the deep sea and the underexplored “rare biosphere.” Proc. Natl. Acad. Sci. U. S. A. 103:12115–12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stamatakis A., Hoover P., Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 57:758–771 [DOI] [PubMed] [Google Scholar]
- 75. Stevens H., Ulloa O. 2008. Bacterial diversity in the oxygen minimum zone of the eastern tropical South Pacific. Environ. Microbiol. 10:1244–1259 [DOI] [PubMed] [Google Scholar]
- 76. Suzuki M. T., et al. 2004. Phylogenetic screening of ribosomal RNA gene-containing clones in bacterial artificial chromosome (BAC) libraries from different depths in Monterey Bay. Microb. Ecol. 48:473–488 [DOI] [PubMed] [Google Scholar]
- 77. Takai K., et al. 2009. Isolation and physiological characterization of two novel, piezophilic, thermophilic chemolithoautotrophs from a deep-sea hydrothermal vent chimney. Environ. Microbiol. 11:1983–1997 [DOI] [PubMed] [Google Scholar]
- 78. Takai K., et al. 2008. Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc. Natl. Acad. Sci. U. S. A. 105:10949–10954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vaulot D. 1989. CYTOPC: processing software for flow cytometric data. Signal Noise 2:8 [Google Scholar]
- 80. Vezzi A., et al. 2005. Life at depth: Photobacterium profundum genome sequence and expression analysis. Science 307:1459–1461 [DOI] [PubMed] [Google Scholar]
- 81. Walsh D. A., et al. 2009. Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326:578–582 [DOI] [PubMed] [Google Scholar]
- 82. Wang F. P., et al. 2008. Environmental adaptation: genomic analysis of the piezotolerant and psychrotolerant deep-sea iron reducing bacterium Shewanella piezotolerans WP3. PLoS One 3:e1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. West N. J., Obernosterer I., Zemb O., Lebaron P. 2008. Major differences of bacterial diversity and activity inside and outside of a natural iron-fertilized phytoplankton bloom in the Southern Ocean. Environ. Microbiol. 10:738–756 [DOI] [PubMed] [Google Scholar]
- 84. Widdel F., Bak F. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3352–3378 In Ballows A., Trüper H. G., Dworkin M., Harder W., Schleifer K.-H. (ed.), The prokaryotes. A handbook on the biology of Bacteria: ecophysiology, isolation, identification, application, 2nd ed. Springer Verlag, New York, NY [Google Scholar]
- 85. Wirsen C. O., Jannasch H. W., Wakeham S. G., Canuel E. A. 1986. Membrane lipids of a psychrophilic and barophilic deep-sea bacterium. Curr. Microbiol. 14:319–322 [Google Scholar]
- 86. Xu Y., et al. 2003. Moritella profunda sp. nov. and Moritella abyssi sp. nov., two psychropiezophilic organisms isolated from deep Atlantic sediments. Int. J. Syst. Evol. Microbiol. 53:533–538 [DOI] [PubMed] [Google Scholar]
- 87. Xu Y., et al. 2003. Psychromonas profunda sp. nov., a psychropiezophilic bacterium from deep Atlantic sediments. Int. J. Syst. Evol. Microbiol. 53:527–532 [DOI] [PubMed] [Google Scholar]
- 88. Yayanos A. A. 1986. Evolutional and ecological implications of the properties of deep-sea barophilic bacteria. Proc. Natl. Acad. Sci. U. S. A. 83:9542–9546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yayanos A. A. 1995. Microbiology to 10,500 meters in the deep sea. Annu. Rev. Mirobiol. 49:777–805 [DOI] [PubMed] [Google Scholar]
- 90. Yayanos A. A., Dietz A. S., Van Boxtel R. 1981. Obligately barophilic bacterium from the Mariana trench. Proc. Natl. Acad. Sci. U. S. A. 78:5212–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yayanos A. A., Dietz A. S., Vanboxtel R. 1979. Isolation of a deep-sea barophilic bacterium and some of its growth characteristics. Science 205:808–810 [DOI] [PubMed] [Google Scholar]
- 92. Yayanos A. A., Van Boxtel R. 1982. Coupling device for quick high pressure connections to 100 MPa. Rev. Sci. Instrum. 53:704–705 [Google Scholar]
- 93. Zeng X., et al. 2009. Pyrococcus CH1, an obligate piezophilic hyperthermophile: extending the upper pressure-temperature limits for life. ISME J. 3:873–876 [DOI] [PubMed] [Google Scholar]
- 94. Zhao Y., et al. 2009. Gene transfer agent (GTA) genes reveal diverse and dynamic Roseobacter and Rhodobacter populations in the Chesapeake Bay. ISME J. 3:364–373 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.