Abstract
The production of low-cost biofuels in engineered microorganisms is of great interest due to the continual increase in the world's energy demands. Biodiesel is a renewable fuel that can potentially be produced in microbes cost-effectively. Fatty acid methyl esters (FAMEs) are a common component of biodiesel and can be synthesized from either triacylglycerol or free fatty acids (FFAs). Here we report the identification of a novel bacterial fatty acid methyltransferase (FAMT) that catalyzes the formation of FAMEs and 3-hydroxyl fatty acid methyl esters (3-OH-FAMEs) from the respective free acids and S-adenosylmethionine (AdoMet). FAMT exhibits a higher specificity toward 3-hydroxy free fatty acids (3-OH-FFAs) than FFAs, synthesizing 3-hydroxy fatty acid methyl esters (3-OH-FAMEs) in vivo. We have also identified bacterial members of the fatty acyl-acyl carrier protein (ACP) thioesterase (FAT) enzyme family with distinct acyl chain specificities. These bacterial FATs exhibit increased specificity toward 3-hydroxyacyl-ACP, generating 3-OH-FFAs, which can subsequently be utilized by FAMTs to produce 3-OH-FAMEs. PhaG (3-hydroxyacyl ACP:coenzyme A [CoA] transacylase) constitutes an alternative route to 3-OH-FFA synthesis; the coexpression of PhaG with FAMT led to the highest level of accumulation of 3-OH-FAMEs and FAMEs. The availability of AdoMet, the second substrate for FAMT, is an important factor regulating the amount of methyl esters produced by bacterial cells. Our results indicate that the deletion of the global methionine regulator metJ and the overexpression of methionine adenosyltransferase result in increased methyl ester synthesis.
INTRODUCTION
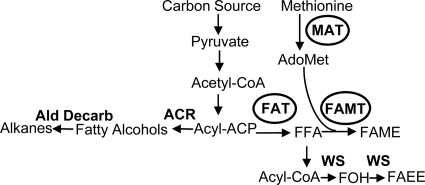
Biofuel research has recently focused on the synthesis of high-energy-density molecules that would serve as gasoline or diesel substitutes. Molecules like butanol, isobutanol, fatty alcohols, fatty acid ethyl esters, and long-chain hydrocarbons have high energy density and limited water solubility and are compatible with existing infrastructures. The cellular pathways that have recently attracted attention are the clostridial pathway for isopropanol and butanol synthesis (12), the amino acid pathway for the synthesis of higher alcohols (3), and the fatty acid pathway for the production of fatty acids (16), fatty alcohols (29), fatty acid ethyl esters (15, 29), and long-chain hydrocarbons (Fig. 1) (5, 24, 31, 32).
Fig. 1.
Overview of the lipid biosynthetic pathways leading to biofuel-related structures. The expression of fatty acyl thioesterases (FATs) leads to the production of free fatty acids (FFAs), which can be converted to (ii) fatty acid methyl esters (FAMEs) by FAMT using S-adenosylmethionine (AdoMet) as the methyl donor or (ii) fatty acid ethyl esters (FAEEs) by the action of a wax synthase (WS). Acyl-ACP can also be converted to fatty alcohols by expressing acyl-ACP reductase (ACR), which is then converted to alkanes by aldehyde decarbonylase. The circled enzymes are overexpressed in our system to produce FAME.
Biodiesel is defined as fatty acid monoalkyl esters, and fatty acid methyl esters (FAMEs) are the most common form. Currently, FAMEs are synthesized predominantly via the transesterification of triacylglycerols, coming mainly from plant oils. However, biodiesel production from plant oils encounters various limitations, particularly the availability of oil-seed supplies in suitable quantities and at competitive prices. Direct intracellular FAME synthesis in bacteria is an attractive alternative to current methods of biodiesel production. It bypasses the transesterification and subsequent purification steps, potentially increasing energy yields and lowering production costs. Direct FAME synthesis could be achieved by using the previously described fatty acid O-methyltransferase (FAMT) (EC 2.1.1.15) enzyme. This enzymatic activity which utilizes S-adenosylmethionine (AdoMet) to methylate the carboxyl group of free fatty acids (FFAs) has been described for mycobacteria; however, no genes have been cloned, and no enzymes have been purified (Fig. 1) (1).
The engineering of bacteria for FAME production via FAMT requires the intracellular production of both FFA and AdoMet. Bacterial fatty acid biosynthesis proceeds via a cytosolic multienzyme system (38). Fatty acid synthesis in Escherichia coli is tightly regulated at multiple points and is coupled to membrane phospholipid biosynthesis by transcriptional and biochemical controls (19). In bacteria, there is no specific mechanism for terminating acyl chain elongation; however, plants have a specific class of enzymes, fatty acyl-acyl carrier protein (ACP) thioesterases (FATs) (EC 3.1.2.14), that terminate acyl chain elongation by hydrolyzing the thioester bond of acyl-ACP, thus releasing FFAs. The expression of plant medium-chain FAT in an E. coli strain deficient in fatty acid oxidation results in the accumulation of FFAs in the bacterial culture (10, 11, 14, 20, 23, 35, 37). In addition to thioesterases, PhaG, a transferase involved in polyhydroxyalkanoate biosynthesis, has been reported to intercept the growing acyl chain during fatty acid biosynthesis (22). PhaG is a 3-hydroxyacyl-ACP:coenzyme A (CoA) transferase (13) and catalyzes the transfer of 3-hydroxyacyl groups from ACP to CoA. PhaG expression in E. coli leads to the accumulation of 3-hydroxydecanoate (40). AdoMet, the second substrate of FAMT, is synthesized by the action of methionine adenosyltransferase (MAT), which catalyzes the reaction between methionine and ATP (17, 18). AdoMet in turn regulates methionine levels by interacting with the global methionine regulator MetJ (26, 36). MetJ is a repressor that controls the expression of the genes involved in methionine biosynthesis (28, 30, 36), and increased levels of AdoMet downregulate the expression of methionine biosynthetic genes.
In this report, we describe the identification of a bacterial FAMT and the engineering of E. coli to produce FAMEs and 3-hydroxy fatty acid methyl esters (3-OH-FAMEs) by expressing FAMT and novel bacterial FATs that exhibit distinct specificities. The production of FAMEs was further enhanced by increasing intracellular AdoMet levels, by deleting metJ, and by overexpressing MAT.
MATERIALS AND METHODS
Reagents.
3-Hydroxy free fatty acids (3-OH-FFAs) and 3-OH-FAMEs were purchased from Matreya. FFAs and FAMEs were purchased from Sigma. E. coli BL21(DE3) and BL21(DE3)(pLysS) cells were purchased from Novagen. Restriction enzymes were purchased from New England BioLabs. S-Adenosylmethionine was purchased from Sigma-Aldrich, and S-adenosyl-l-methionine (methyl-3H) was purchased from MP Biomedicals. Gene synthesis was performed by Genscript Inc. The full-length rat MAT (rMAT) clone was purchased from Invitrogen. Genomic DNA for Clostridium acetobutylicum ATCC 824, Geobacter metallireducens, Mycobacterium marinum, and Mycobacterium smegmatis was purchased from the ATCC. Double-expression pETDuet and pCDFDuet vectors were purchased from Novagen.
Bacterial strains.
The Keio collection ΔmetJ mutant (4) was purchased from the Yale E. coli genetic stock center, and the metJ mutation was transduced into E. coli strain BL21(DE3) using P1 vir phage transduction. Kanamycin-resistant colonies for ΔmetJ mutants were selected and verified. The integration of the kanamycin cassette was determined by PCR using primers metJfor (5′-CGGTAACGCCTGTACGGTAAACTATG) and metJrev (5′-GTCCATGTATAAAAAGCGGTGGGTCGC), which are external to the site of integration. A PCR fragment of 1.6 kb, which was sequenced, confirmed the integration of the kanamycin cassette into the metJ site. All DNA sequencing was performed at the University of California, Berkeley, sequencing facility.
Cloning.
Rat MAT was PCR amplified from the full-length clone (clone 7368255; Invitrogen) using primers ratMAT-Nde1F (5′-GCACCATATGAATGGACCTGTGGATGGCTTGTGTGAC) and ratMAT-Kpn1R (5′-GCACGGTACCAAACACAAGCTTCTTGGGGACCTC) and cloned into the NdeI-KpnI sites (underlined) of the pETDuet vector, generating an in frame S-tagged protein.
The CAC_3591 gene was PCR amplified from C. acetobutylicum genomic DNA and inserted into the pCDFDuet vector within BamHI and PstI sites (underlined) to generate pCDF-CaFAT; the primers used were cacFATfor (5′-CGGGATCCGTCAAAGGTTGTTACTAAAAGAA) and cacFATrev (5′-GGCTGCAGTTATGATTTAATAAAATCAGTCTTTATTA).
Mmar_3356 and Msmeg_4347 were amplified from M. marinum and M. smegmatis genomic DNA, respectively, and inserted into the pETDuet vector within the EcoRI and HindIII sites (underlined) to generate pETDuet-MmFAMT and pETDuet-MsmegMT; the primers used were MmFAMTfor (5′-CCGGAATTCGCCACGGGAGATCAGGCTG), MmFAMTrev (5′-CCGAAGCTTTCAGGCGCGCTTGGCAAG), MsmegMTfor (5′-CCGGAATTCGCCCAAATTCCGAGTGGC), and MsmegMTrev (5′-CCGAAGCTTTCAGCCCGAGCGGCG).
FAT genes from Clostridium phytofermentans, C. sporogenes, C. tetani, and M. marinum as well as Pseudomonas putida phaG and tesB from E. coli were synthesized by Genscript and cloned into the BamHI-NotI sites of pCDFDuet to generate the respective expression vectors. Synthesized sequences were codon optimized for E. coli expression.
Bacterial growth conditions.
The strains used for the thioesterase expression studies were grown in flasks with Luria broth (LB) with shaking at 37°C. For the FAMT expression studies, the cells were grown in flasks with M9 minimal medium supplemented with 2% glucose with shaking at 37°C. The appropriate antibiotics were added to the cultures at the following concentrations: 50 mg/liter ampicillin, 50 mg/liter kanamycin, 50 mg/liter spectinomycin, and 34 mg/liter chloramphenicol. The transcription of heterologous genes was induced by the addition of 0.25 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG) when the cells reached an optical density at 600 nm (OD600) of 0.4 to 0.6. To determine the preferred substrate for FAMT, a fatty acid mixture composed of 400 μg of each of the 3-OH FFAs C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, and C18:0 was dried in a glass vial and resuspended in ethanol. Two hundred fifty microliters of the ethanol-fatty acid mixture was added to 50 ml of culture of FAMT-expressing strains at the time of induction. The same experiment was also conducted with the following FFAs: C10:0, C12:0, C14:0, C16:0, C16:1, and C18:1. The shaking cultures were collected 20 h after induction, and lipids were extracted from cell pellets and supernatants. The extracted FFAs, FAMEs, and 3-OH-FAMEs were separated and quantified as described below.
Lipid analysis.
Bacterial cultures were harvested by centrifuging cultures for 20 min at 3,220 × g to separate the pellet from the supernatant. The pellet was resuspended in phosphate-buffered saline (PBS), followed by the extraction of both the pellet and supernatant lipids with chloroform-methanol (2:1). Prior to lipid extraction, 50 μg of the internal standards FFA C17:0, methyl ester (ME) C17:0, 3-OH C17:0, and 3-OH-ME C17:0 was added to both the pellet and supernatant. The lower organic phase was extracted, evaporated, and loaded onto thin-layer chromatography (TLC) Silica Gel 60 plates (250 μm thick). FFAs and FAMEs were separated by using the solvent petroleum ether-diethyl ether-acetic acid (95:5:1, vol/vol/vol) (FFA Rf = 0.15; FAME Rf = 0.41). FFAs, 3-OH FFAs, and 3-OH FAMEs were separated by using petroleum ether-diethyl ether-acetic acid (50:50:1, vol/vol/vol) (3-OH FFA Rf = 0.15; 3-OH FAME Rf = 0.35; FFA Rf = 0.55). The lipids on the TLC plate were visualized by amido black staining (21), and spots corresponding to FFAs and 3-OH FFAs were scraped into glass vials and derivatized with methanol-sulfuric acid (96:4) at 62°C. The derivatized esters were extracted with hexane and analyzed by gas chromatography (GC). Methyl esters were scraped from the TLC plate and directly extracted with hexane for analysis by gas chromatography. Gas chromatography was performed with a Clarus600 gas chromatogram (Perkin-Elmer) equipped with an Elite-5 column (Perkin-Elmer) and a flame ionization detector for effluent analysis. Hydrogen was used as the carrier gas at an initial flow rate of 2 ml/min for 6 min, dropping to 1.5 ml/min for 8 min with a rate of 0.5 ml/min. The GC program was as follows: an initial temperature of 125°C ramped sequentially to 185°C at 12°C/min, 215°C at 4°C/min, and 275°C at 25°C/min.
Enzyme purification and FAMT assays.
The plasmid carrying the FAMT gene was transformed into E. coli BL21(DE3)(pLysS) cells, and expression was initiated by the addition of 1 mM IPTG. The cells were grown overnight at 20°C and harvested by centrifugation, and protein purification was performed by using the Ni-nitrilotriacetic acid (NTA) Spin kit (Qiagen) according to the manufacturer's recommendations. The in vitro FAMT assay was modeled similarly to methyl jasmonate synthase assays (25). FFAs or 3-OH-FFAs were dried in glass vials, resuspended in 100 mM Tris-HCl (pH 7.8), and solubilized by sonication. The second substrate, AdoMet, was added to the reaction mixture in both cold and radiolabeled methyl-3H (0.55 μCi per reaction) forms. The substrates were combined in an in vitro reaction mixture also containing 300 mM KCl and purified FAMT in a final volume of 100 μl. The reaction mixture was incubated for 30 min at 37°C, and the reaction was stopped by the addition of 100 μl hexane to the mixture. After the addition of hexane, the reaction mixture was vortexed and centrifuged; methyl-3H levels in the organic hexane phase were determined by liquid scintillation.
Mass spectrometry.
Samples representing culture pellets or supernatants were derivatized with N,O-bistrimethylsilyl-trifluoracetamide–1% trimethylsilyl chloride (70°C for 30 min). The injection consisted of 1 μl, 1:10 split; the injector temperature was 280°C; the oven program was 3 min isocratic at 120°C and then 20°C/min to 320°C and 3 min isocratic; the carrier gas was helium at 1 ml/min; and the mass spectrum was recorded from m/z 50 to 600 at a scan rate of 2.66 scans/s. Samples were derivatized with 0.2 ml dimethyldisulfide and 10 μl iodine (60 mg/ml in diethyl ether) at 70°C for 30 min. After cooling to room temperature, 0.2 ml iso-octane–isopropanol (9 + 1, vol/vol) and 0.4 ml sodium thiosulfate (0.5 g/10 ml) were added. After vigorous mixing, the mixture was centrifuged (3,000 × g for 5 min), and the upper phase was derivatized with 100 μl N,O-bistrimethylsilyl-trifluoracetamide–1% trimethylsilyl chloride at 70°C for 30 min. The injection consisted 1 μl, 1:10 split; the injector temperature was 280°C; the oven program was 3 min isocratic at 120°C and then 20°C/min to 325°C and 5 min isocratic; the carrier gas was helium at 1 ml/min; and the mass spectrum was recorded from m/z 50 to 600 at a scan rate of 2.66 scans/s. GC mass spectrometry (GCMS) was performed with an Agilent 7890A gas chromatograph equipped with a Varian FactorFour VF5-ms (30 by 0.25 mm by 0.25 μm) capillary column coupled to an Agilent 5975C MSD model.
RESULTS
Identification of bacterial FAMTs.
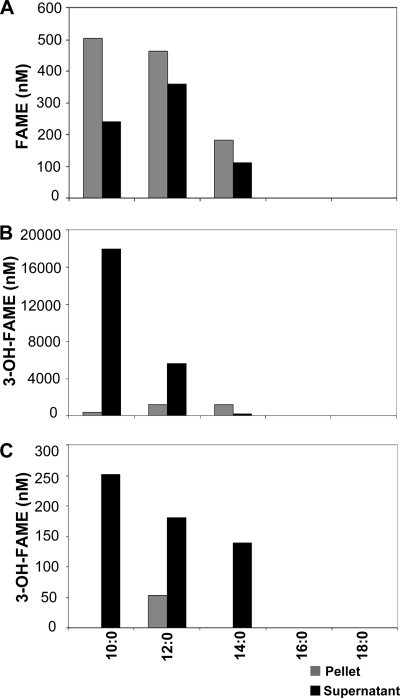
The existence of FAMT enzyme activity was reported previously for mycobacteria (1). Such a reaction would presumably involve the transfer of a methyl group from AdoMet to the carboxyl group of an FFA, generating FAMEs. Similar reactions have been described previously for plants, such as jasmonate methyltransferase (25), benzoate and salicylate methyltransferase (6), and gibberellin methyltransferase (34). These Arabidopsis enzymes form a well-defined protein family, the SABATH methyltransferase family (Pfam03492), and Arabidopsis thaliana contains 24 such genes. BLAST searches of the mycobacterial genomes using Pfam03492 identified genes belonging to the carboxyl methyltransferase enzyme family. We cloned and expressed two genes, one from M. marinum (Mmar_3356) and another from M. smegmatis (Msmeg_4347), in E. coli. These genes share 52% identity in their amino acid sequences, and their closest homolog in the A. thaliana genome is 25% identical (see Fig. S1 in the supplemental material). To test whether these genes exhibit FAMT activity, we expressed Mmar_3356 and Msmeg_4347 in E. coli and provided either an extracellular mixture of fatty acids or 3-OH fatty acids (Fig. 2). To ensure adequate AdoMet availability, we performed this set of experiments with BL21 metJ::kan strains. The deletion of metJ prevents the feedback inhibition of methionine synthesis, thus allowing increased levels of methionine for the production of AdoMet (36). We also utilized a double-expression vector to coexpress rat MAT, an enzyme previously shown to significantly increase AdoMet levels in E. coli (2), with the mycobacterial methyltransferases. We provided free fatty acids or 3-OH free fatty acids with acyl chain lengths ranging from 10 to 18 carbons. In the cultures expressing the M. marinum gene, we detected the formation of FAMEs and 3-OH-FAMEs; no FAMEs were detected in the absence of a methyltransferase (data not shown). The total concentration of FAMEs in the culture was 1.87 μM (Fig. 2A). Approximately 40% of the FAMEs were detected in the supernatant, while the rest remained in the cell pellet. When cells expressing Mmar_3356 were provided with 3-OH-FFAs, we also detected the formation of 3-OH-FAMEs (Fig. 2B). However, we detected a much higher concentration of 3-OH-FAMEs in the culture, 25.5 μM, than FAMEs. Approximately 94% of the 3-OH-FAMEs were detected in the supernatant. The two predominant methyl esters formed were 3-OH decanoic acid and 3-OH dodecanoic acid methyl esters. These data strongly suggest that the M. marinum gene encodes a functional FAMT with a preference for C10:0 and C12:0 3-hydroxy fatty acids.
Fig. 2.
Expression of MmFAMT leads to formation of FAMEs and 3-OH-FAMEs. E. coli BL21 ΔmetJ::kan cells were transformed with double-expression vectors expressing Mmar_3356 (A and B) or Msmeg_4347 (C) and rat MAT. An FFA (A) or 3-OH-FFA (B and C) cocktail mixture was added exogenously to each culture during induction. Lipids from cell pellets and supernatants were extracted 20 h after induction, and FAMEs and 3-OH-FAMEs were quantified as described in Materials and Methods. Data are representative of experiments done three independent times.
We performed similar experiments with E. coli cells expressing the M. smegmatis gene. The cells were treated with the same mixture of either FFAs or 3-OH-FFAs as that described above. In these cultures, we were unable to detect FAMEs, only 3-OH-FAMEs, albeit in significantly lower quantities than those with the M. marinum gene (Fig. 2C). These observations suggest that although Msmeg_4347 has some residual activity against 3-OH-FFAs, its primary substrate may be a different molecule.
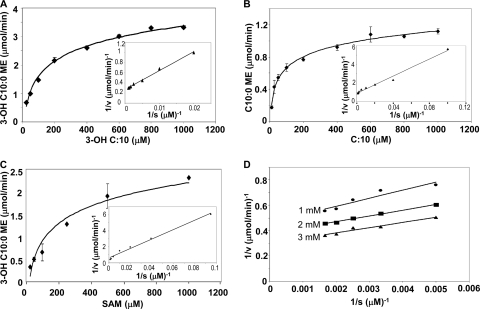
In order to determine if Mmar_3356 is sufficient for methyl ester production, biochemical assays were developed to determine if the purified protein exhibits in vitro FAMT activity and to determine its kinetic constants (Table 1). The catalytic efficiency of Mmar_3356 was highest toward C10 and C12 3-OH-FFAs as substrates. The apparent Km for C10 3-OH-FFA was 99 μM, and that for AdoMet was 80 μM (Fig. 3). The Km was slightly higher for 3-OH C12 (129 μM) (Table 1). Kinetic studies of 3-OH-FAME production as a function of the 3-OH C10 concentration at different fixed concentrations of AdoMet yielded parallel lines indicative of a ping-pong reaction mechanism (Fig. 3D) (7–9). These data correlate well with the in vivo observations and support the conclusion that Mmar_3356 has a preference for the soluble C10:0 3-OH-FFA.
Table 1.
FAMT kinetic parameters for various fatty acid substrates
| FFA | Mean Km (μmol) ± SD | Mean kcat (s−1) ± SD | kcat/Km (mol/liter−1 s−1) |
|---|---|---|---|
| 3-OH C8:0 | 196 ± 15 | 358 ± 17 | 1.82 × 106 |
| 3-OH C10:0 | 99 ± 6 | 1,600 ± 78 | 16.1 × 106 |
| 3-OH C12:0 | 129 ± 11 | 1,346 ± 102 | 10.4 × 106 |
| C8:0 | 77 ± 4 | 510 ± 32 | 6.6 × 106 |
| C10:0 | 46 ± 2 | 489 ± 26 | 10.6 × 106 |
| C12:0 | 103 ± 5 | 1,165 ± 63 | 11.3 × 106 |
Fig. 3.
Kinetic characterization of MmFAMT. (A to C) Kinetic parameters for 3-OH C10 FFA (A), C10 FFA (B), and AdoMet (C) were determined for MmFAMT. Experiments for panels A and B were performed with 2 mM AdoMet. Experiments with variable AdoMet concentrations (C) were performed with 800 mM 3-OH C10 FFA. (D) Experiments performed with 3-OH C10 FFA at three different AdoMet concentrations, 1, 2, and 3 mM. Enzyme assays were performed as described in Materials and Methods. Error bars represent the standard deviations of experiments done in triplicate.
Identification of bacterial thioesterases with distinct acyl group specificities.
The above-described experiments utilized exogenous fatty acids to generate methyl esters. The in situ generation of fatty acids in E. coli is accomplished by the expression of FATs. Based on the above-described results, we sought to identify FAT enzymes that would generate intracellular 3-OH-FFAs. Many experiments have been performed with plant FATs (11, 14, 20, 23, 35, 37), but none have reported the generation of 3-OH-FFAs. Therefore, we sought to identify novel FATs with the potential to synthesize the desired 3-OH-FFAs. A computational survey of available genomic data identified a plethora of bacterial genes belonging to the acyl-ACP thioesterase protein family (Pfam01643). These genes are widely distributed, and their sequences are quite divergent, even in organisms of the same phylogenetic group (see Table S1 in the supplemental material). The pairwise identity level of these genes was between 20 and 45%, indicating their significant sequence divergence. To determine whether these genes possess thioesterase activity with distinct specificity compared to that of their plant counterparts, we cloned and expressed 6 bacterial genes in E. coli (Table S1). We chose four clostridial genes, CAC_3591 from C. acetobutylicum ATCC 824, CTC00119 from Clostridium tetani E88, CPHY_0251 from Clostridium phytofermentans ISDg, and CLOSPO00958 from Clostridium sporogenes ATCC 15579, and two genes from M. marinum, Mmar_0791 and Mmar_2977. For comparative purposes, we also expressed the A. thaliana fatA gene. Figure 4 shows the specificities of the selected bacterial thioesterases. The overexpression of A. thaliana FATa (AtFATa) in E. coli was previously shown to lead to the formation of C16:1 and C16:0 FFAs (11); we observed the same result (data not shown). The expression of bacterial FATs leads to the overproduction of FFAs, although differences in FAT specificities are apparent. Contrary to A. thaliana FATa, the bacterial thioesterases have broader substrate specificities, leading to the accumulation of saturated and unsaturated FFAs with chain lengths ranging between 10 and 18 carbons. The FFAs with shorter acyl chain lengths (C8 to C12) were found mainly in the supernatant, whereas FFAs with longer acyl chains were distributed among the cell pellet and the supernatant. Furthermore, the expression of C. acetobutylicum and C. phytofermentans thioesterases leads to a significant production of 3-OH-FFAs with acyl chain lengths ranging mainly from C10 to C14. In addition to saturated 3-OH-FFAs, the expression of CAC_3591 and CPHY_0251 resulted in the synthesis of monounsaturated C12 and C14 3-OH-FFAs (Fig. 4A and B and see Fig. S2 and S3 in the supplemental material). The identity of these compounds and the position of the double bond (omega-7) were verified by mass spectrometry (Fig. S2 and S3). Unlike the bacterial thioesterases, the expression of the plant thioesterase (AtFATa) does not lead to the formation of 3-OH-FFA. 3-OH-FFAs with acyl chain lengths of up to 14 carbons were found mainly in the supernatant, whereas 3-OH-FFAs with longer acyl chains were partitioned among the cell pellet and the supernatant (Fig. 4).
Fig. 4.
FFA and 3-OH-FFA production by E. coli strains expressing bacterial thioesterases. (A) C. acetobutylicum CAC_3591; (B) C. phytofermentans CPHY_0251; (C) C. sporogenes Clospo00958; (D) C. tetani CTC_0119; (E) M. marinum Mmar_2977; (F) M. marinum Mmar_0791. Bacterial FATs were expressed in E. coli cells, and FFAs and 3-OH-FFAs were analyzed as described in Materials and Methods. Data are representative of experiments done three independent times.
In situ generation of FAMEs and 3-OH-FAMEs.
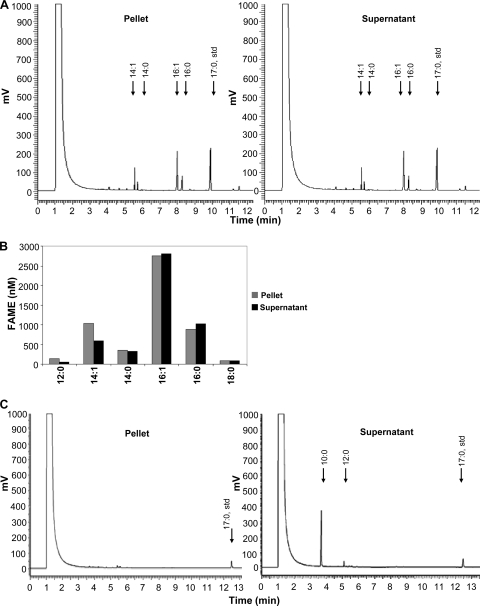
To generate strains that will synthesize FAMEs from endogenously produced FFAs, we coexpressed M. marinum FAMT (MmFAMT) with AtFATa or C. acetobutylicum FATa (CaFATa) (CAC_3591) in shake flask experiments under aerobic conditions. The coexpression of AtFATa and MmFAMT in the presence of rMAT resulted in the formation of FAMEs (Fig. 5A). The total concentration of FAMEs in the culture was 10.2 μM (Fig. 5B). Approximately 48% of the FAMEs were detected in the supernatant, while the rest remained in the cell pellet (Fig. 5A). The resulting FAME mixture consisted mainly of methyl palmitate and methyl palmitoleate, with smaller quantities of methyl myristate and methyl myristoleate, matching the profile of the FFAs produced by the Arabidopsis thioesterase (Fig. 5A and B). These data indicate that MmFAMT is able to generate FAMEs utilizing FFAs released endogenously by a plant thioesterase. Our results shown in Fig. 2, utilizing exogenous fatty acids, indicated that MmFAMT exhibits a higher specificity toward 3-OH fatty acids. Thus, we coexpressed CaFAT with MmFAMT in the presence of rMAT, resulting in the synthesis of 3-OH-FAMEs at a final concentration of 33.25 μM (Fig. 5C). The predominant methyl ester was 3-hydroxydecanoic acid methyl ester (30.3 μM), with a small percentage of 3-hydroxydodecanoic acid methyl ester (2.95 μM) (Fig. 5C). The majority of the methyl esters (95%) were found in the supernatant, indicating an efficient mechanism for the secretion of 3-OH-FAMEs.
Fig. 5.
Coexpression of thioesterases with MmFAMT leads to methyl ester formation. BL21 ΔmetJ::kan cells expressed either AtFATa (A and B) or CaFAT (C) with MmFAMT and rMAT. Cultures were collected at 24 h postinduction, lipids were extracted, and FAMEs and 3-OH-FAMEs were separated by TLC and quantified by GC as described in Materials and Methods. (A) GC chromatograms from extracts of the cell pellet and supernatant of AtFATa-expressing cells, illustrating the generation of FAMEs. (B) Relative concentrations and distributions of the different FAME types in the pellet and supernatant from AtFATa-expressing cells. (C) GC chromatograms from extracts of the cell pellet and supernatant of CaFAT-expressing cells, illustrating the production of 3-OH-FAMEs. Data are representative of three independent experiments with similar results.
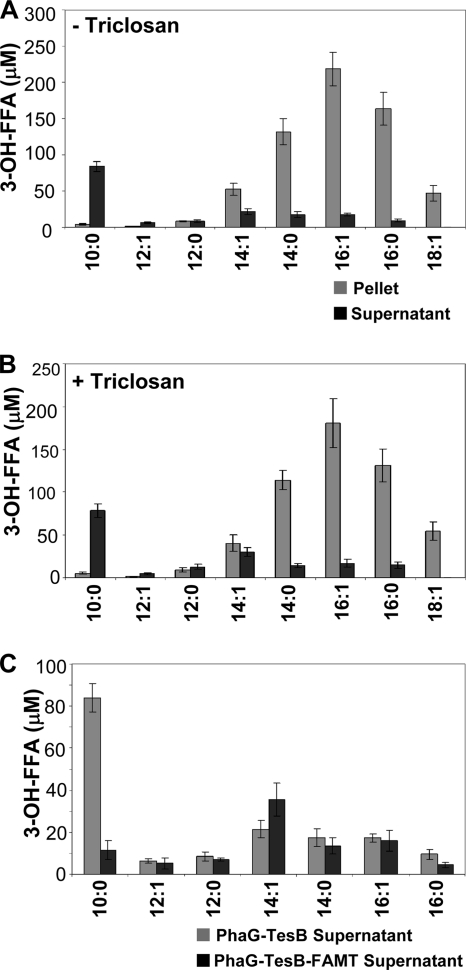
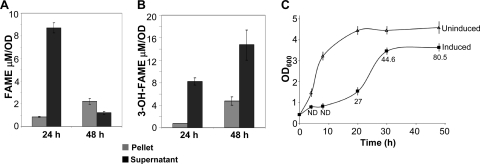
The total quantity of 3-hydroxydecanoic acid produced by CaFAT makes up less than 33% of the total FFAs and 3-OH-FFAs (Fig. 4A). Thus, in order to maximize 3-OH-FAME production, a more efficient enzyme is desirable for 3-OH-FFA production. An alternative pathway for synthesizing in situ 3-OH-FFAs involves the coexpression of the (R)-3-hydroxydecanoyl-ACP:CoA transacylase gene (phaG) from Pseudomonas putida and thioesterase II (tesB) from E. coli (39, 40). Previous work has shown that the coexpression of phaG and tesB results in significantly higher levels of 3-hydroxydecanoic acid production than the thioesterases that we tested (39, 40). When we coexpressed phaG and tesB, in addition to 3-OH C10:0, the predominant 3-OH-FFAs detected were 3-OH C14:1, 3-OH C14:0, 3-OH C16:1, and 3-OH C16:0 FFAs (Fig. 6A). The coexpression of phaG and tesB with FAMT resulted in the accumulation of 3-OH-FAMEs, at 24 and 48 h postinduction, reaching a concentration of 70.5 μM at 48 h (Fig. 7). The majority of these 3-OH-methyl esters (∼91%) consisted of 3-OH C10:0. In addition to 3-OH-FAMEs, we also detected the production of FAMEs at 24 h (∼15 μM) and at 48 h (∼10 μM) (Fig. 7). It is not clear why there is a decrease in FAME accumulation from 24 to 48 h; there may be some loss due to the volatile nature of FAMEs or the turnover of FAMEs. All of the FAME detected outside the cell corresponded to C12:0. A separate set of experiments that included triclosan, a FabI inhibitor implicated in increasing 3-OH C10:0 production (39, 40) in cells expressing phaG and tesB, led to a slight decrease in the concentrations of all of the 3-OH-FFAs; no increase in the 3-OH C10:0 concentration was observed (Fig. 6B). The addition of triclosan in phaG-, tesB-, and FAMT-expressing cells did not result in an increase in methyl ester production, suggesting that triclosan does not significantly affect fatty acid production in our experimental system.
Fig. 6.
3-OH-FFA profiles of phaG- and tesB-expressing cells with and without triclosan. E. coli BL21 ΔmetJ::kan cells were transformed with a Duet expression vector expressing PhaG and TesB. (A) Cells without triclosan. (B) Cells with triclosan. Cells were collected 48 h after induction, and concentrations of 3-OH-FFAs in the cell pellet and supernatant were determined. (C) Distribution of 3-OH-FFAs in the supernatant of PhaG-TesB-expressing cells without FAMT or with FAMT. Data are representative of three independent experiments with similar results.
Fig. 7.
Expression of phaG and tesB with FAMT leads to production of FAME and 3-OH-FAME. E. coli BL21 ΔmetJ::kan cells were transformed with Duet coexpression vectors expressing PhaG, TesB, FAMT, and rMAT. (A and B) Cells were collected 24 h and 48 h after induction, and FAMEs (A) and 3-OH-FAMEs (B) in the cell pellet and supernatant were quantified as described in Materials and Methods. (C) Growth curves of uninduced (triangles) and induced (filled squares) E. coli BL21 ΔmetJ::kan transformed plasmids encoding PhaG, TesB, FAMT, and rMAT. Aliquots from each culture were collected at 4, 8, 20, 30, and 48 h postinduction and analyzed for growth and methyl ester production. For the induced culture, the numbers below each time point represent methyl ester production in μM. ND, not detectable. Error bars represent the standard deviations from triplicate experiments.
In order to determine how FAME production affects cell growth, we analyzed the growth rates of cultures transformed with plasmids carrying the phaG, tesB, FAMT, and rMAT genes. Compared to uninduced cultures, the induced cells grew at a significantly low rate; however, after about 30 h postinduction, their growth had reached within 75% of the levels of uninduced cells (Fig. 6C). Analysis of methyl esters revealed undetectable levels until 20 h postinduction, and maximal accumulation was detected at 48 h postinduction (Fig. 7C).
Regulation of FAME and 3-OH-FAME synthesis by methionine metabolism.
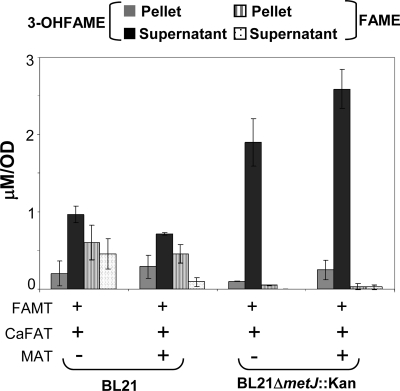
An important factor in FAME and 3-OH-FAME biosynthesis via FAMT is the concentration of AdoMet. Previous work reported that the expression of rMAT in E. coli cells leads to increased intracellular levels of AdoMet (2). To test whether the expression of rMAT leads to increased FAME synthesis, we cloned and expressed rMAT. The coexpression of rMAT with CaFATa and MmFAMT in wild-type E. coli BL21 cells did not result in any significant increase in 3-OH-FAME synthesis (Fig. 8).
Fig. 8.
MAT overexpression in ΔmetJ cells increases 3-OH-FAME production. E. coli BL21 or BL21 ΔmetJ::kan cells were transformed with Duet coexpression vectors expressing FAMT, CaFAT, and rMAT. Cells were collected 24 h after induction, and FAMEs and 3-OH-FAMEs in the cell pellet and supernatant were quantified. Error bars represent the standard deviations from triplicate experiments.
MetJ has been described to be a major regulator of methionine biosynthesis, acting by sensing AdoMet levels and repressing the transcription of the genes operating in the methionine biosynthetic pathway. Experiments utilizing a metJ deletion mutant resulted in an increase of 3-OH-FAME synthesis when CaFAT and MmFAMT were coexpressed (Fig. 8). In addition, the expression of rMAT in metJ mutants resulted in a further increase in concentrations of 3-OH-FAMEs. These results indicate that intracellular AdoMet concentrations are important determinants of methyl ester formation.
DISCUSSION
The fatty acid biosynthetic pathway has taken center stage in efforts to generate advanced biofuels (Fig. 1). We report the identification and characterization of a novel methyltransferase that methylates fatty acids and 3-OH fatty acids utilizing AdoMet as the methyl donor. Our bioinformatic analysis identified several mycobacterial genes as potential FAMTs; we cloned and tested two genes from M. marinum and M. smegmatis. We have demonstrated both in vivo and in vitro that the M. marinum gene is an FAMT and that its expression in E. coli leads to FAME and 3-OH-FAME accumulation. In contrast, we were unable to determine any activity for the M. smegmatis gene, although its overall level of identity with Mmar_3356 is relatively high (52%), indicating that the two genes have distinct enzymatic activities. The M. smegmatis gene may have an activity against a fatty acid that is absent from E. coli, or some other variation of the fatty acid core structure. Mycobacteria synthesize a plethora of fatty acid structures, including saturated and unsaturated meroacids with variable degrees of substitutions (33), and it is possible that the M. smegmatis gene product methylates one of these structures. Alternatively, the M. smegmatis gene may methylate a molecule unrelated to fatty acids.
The in vitro assays indicated that MmFAMT is active on the soluble substrates C10:0, C12:0, and C14:0. The Kms for 3-OH C10:0 and 3-OH C12:0 fatty acids are essentially identical, whereas the Km for 3-OH C14:0 is approximately three times lower. Based on these Km values, we would still expect to produce significantly higher levels of 3-OH C12:0 and 3-OH C14:0 methyl esters in cells generating in situ fatty acids via thioesterases, particularly CAC_3591, which generates significant amounts of saturated and unsaturated 3-OH C14 (Fig. 4A). We attribute these observations to substrate solubility. Most probably, 3-OH tetradecanoic acid is located on the membrane, exhibiting limited solubility, and therefore is inaccessible to MmFAMT, which is clearly a soluble enzyme.
The higher specificity of MmFAMT toward 3-OH fatty acids prompted us to investigate the existence of FATs that would be able to generate 3-OH fatty acids. Plant FATs have received broad attention, since they are able to deregulate E. coli fatty acid biosynthesis. However, no publication has reported the generation of 3-OH-FFAs from plant thioesterases. Therefore, we decided to investigate whether the bacterial homologs of plant FATs had similar activities. All six genes that we tested increased FFA levels, indicating that they are active thioesterases. However, only two genes (CAC_3591 and CPHY_0251) generated significant quantities of 3-OH-FFAs, indicating that this activity is not shared by all thioesterases. Although we did not perform direct in vitro assays, we postulate that these FATs act on acyl-ACP rather acyl-CoA, because we obtained similar results when we used fadD mutants that lacked acyl-CoA synthetase activity (data not shown). 3-OH-FFAs have been described in the literature as being effective antifungal compounds secreted by Lactobacillus plantarum (27). This is a probable physiological function of the two bacterial FATs that generate 3-OH-FFAs. However, the physiological function of the bacterial thioesterases that do not release 3-OH-FFAs remains unclear.
A combination of FATs and FAMTs leads to FAME and 3-OH-FAME production (Fig. 5). Besides FATs, the expression of PhaG constitutes an alternative route to 3-OH-FFA production. The combined expression of PhaG-TesB and FAMT leads to the highest concentrations of FAMEs and 3-OH-FAMEs (Fig. 7), indicating that this pathway may be more efficient in intercepting the growing acyl chain than the FATs described above.
In addition, methyl ester production also depends on the intracellular AdoMet concentrations. Our data suggest that the expression of the MAT enzyme alone is not enough to increase methyl ester production. A deregulation of the transcriptional network that controls methionine and AdoMet biosynthesis by deleting the global regulator MetJ is necessary to increase methyl ester synthesis. These results are consistent with the current model of methionine and AdoMet biosynthesis, which places MetJ as a central regulator that responds to elevated AdoMet concentrations and downregulates the methionine biosynthesis pathway.
The work described in this report provides an alternative route for the synthesis of biofuel molecules. The direct intracellular synthesis of FAMEs and 3-OH-FAMEs in E. coli bypasses the extraction and transmethylation steps currently necessary for biodiesel production. The coexpression of PhaG, TesB, FAMT, and rMAT achieved the highest yield of biodiesel, 80.5 μM (16 mg/liter). The FAME yields that we achieved are roughly 40 times lower than the maximal fatty acid ethyl ester yields reported previously and ∼19 times lower than those of alkanes (24, 29). However, our results suggest that FAME production can be improved. In PhaG-TesB-expressing cells, FAMT is able to convert virtually all of the 3-OH C10:0 FFA (90%) into 3-OH C10:0 FAMEs (Fig. 6C). This strongly suggests that the bottleneck in methyl ester production in E. coli is due to a lack of an efficient thioesterase that hydrolyzes 3-OH C10:0. The main concern arising from the current work is the mismatch between the specificities of the FAMT and FATs that we used. MmFAMT prefers soluble 3-OH C10:0 and C12:0 fatty acids; however, these fatty acids represent only a small percentage of the fatty acid pool released by the thioesterases that we identified. Further work will focus on identifying or engineering FAMT and FAT enzymes with similar specificities to explore the limits of direct intracellular FAME synthesis. Furthermore, our in vitro assays with purified FAMT show that C12:0 fatty acid is an efficient FAMT substrate; however, FAMT-expressing cultures did not produce significant titers of FAME. We hypothesize that this may be due to FAMT being a soluble enzyme distributed within the cytoplasm, while free fatty acids are predominately on the membrane; thus, fatty acids are unlikely to have a significant interaction with FAMT. Localizing FAMT to the cytoplasmic side of the inner membrane may lead to significantly more interactions with fatty acids and, therefore, significantly higher levels of FAME production. The pathway described here is a first step in the generation of FAMEs and, with further optimization, may lead to the production of a cost-efficient next-generation biofuel.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maria Billini for her excellent technical assistance.
This work was funded by a grant from the Energy Bioscience Institute to A.L. and by the Office of Science of the U.S. Department of Energy under contract no. DE-AC02-05CH112.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 16 September 2011.
REFERENCES
- 1. Akamatsu Y., Law J. H. 1970. The enzymatic synthesis of fatty acid methyl esters by carboxyl group alkylation. J. Biol. Chem. 245:709–713 [PubMed] [Google Scholar]
- 2. Alvarez L., Mingorance J., Pajares M. A., Mato J. M. 1994. Expression of rat liver S-adenosylmethionine synthetase in Escherichia coli results in two active oligomeric forms. Biochem. J. 301(Pt. 2):557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atsumi S., Hanai T., Liao J. C. 2008. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89 [DOI] [PubMed] [Google Scholar]
- 4. Baba T., et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beller H. R., Goh E.-B., Keasling J. D. 2010. Genes involved in long-chain alkene biosynthesis in Micrococcus luteus. Appl. Environ. Microbiol. 76:1212–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen F., et al. 2003. An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. Plant J. 36:577–588 [DOI] [PubMed] [Google Scholar]
- 7. Cleland W. W. 1963. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim. Biophys. Acta 67:104–137 [DOI] [PubMed] [Google Scholar]
- 8. Cleland W. W. 1963. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim. Biophys. Acta 67:173–187 [DOI] [PubMed] [Google Scholar]
- 9. Cleland W. W. 1963. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim. Biophys. Acta 67:188–196 [DOI] [PubMed] [Google Scholar]
- 10. Davis M. S., Solbiati J., Cronan J. E., Jr 2000. Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J. Biol. Chem. 275:28593–28598 [DOI] [PubMed] [Google Scholar]
- 11. Dormann P., Voelker T. A., Ohlrogge J. B. 1995. Cloning and expression in Escherichia coli of a novel thioesterase from Arabidopsis thaliana specific for long-chain acyl-acyl carrier proteins. Arch. Biochem. Biophys. 316:612–618 [DOI] [PubMed] [Google Scholar]
- 12. Hanai T., Atsumi S., Liao J. C. 2007. Engineered synthetic pathway for isopropanol production in Escherichia coli. Appl. Environ. Microbiol. 73:7814–7818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffmann N., et al. 2002. Biochemical characterization of the Pseudomonas putida 3-hydroxyacyl ACP:CoA transacylase, which diverts intermediates of fatty acid de novo biosynthesis. J. Biol. Chem. 277:42926–42936 [DOI] [PubMed] [Google Scholar]
- 14. Jiang P., Cronan J. E., Jr 1994. Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J. Bacteriol. 176:2814–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalscheuer R., Stolting T., Steinbuchel A. 2006. Microdiesel: Escherichia coli engineered for fuel production. Microbiology 152:2529–2536 [DOI] [PubMed] [Google Scholar]
- 16. Lu X., Vora H., Khosla C. 2008. Overproduction of free fatty acids in E. coli: implications for biodiesel production. Metab. Eng. 10:333–339 [DOI] [PubMed] [Google Scholar]
- 17. Markham G. D., DeParasis J., Gatmaitan J. 1984. The sequence of metK, the structural gene for S-adenosylmethionine synthetase in Escherichia coli. J. Biol. Chem. 259:14505–14507 [PubMed] [Google Scholar]
- 18. Markham G. D., Hafner E. W., Tabor C. W., Tabor H. 1980. S-Adenosylmethionine synthetase from Escherichia coli. J. Biol. Chem. 255:9082–9092 [PubMed] [Google Scholar]
- 19. Marrakchi H., Zhang Y. M., Rock C. O. 2002. Mechanistic diversity and regulation of type II fatty acid synthesis. Biochem. Soc. Trans. 30:1050–1055 [DOI] [PubMed] [Google Scholar]
- 20. Ohlrogge J., Savage L., Jaworski J., Voelker T., Post-Beittenmiller D. 1995. Alteration of acyl-acyl carrier protein pools and acetyl-CoA carboxylase expression in Escherichia coli by a plant medium chain acyl-acyl carrier protein thioesterase. Arch. Biochem. Biophys. 317:185–190 [DOI] [PubMed] [Google Scholar]
- 21. Plekhanov A. Y. 1999. Rapid staining of lipids on thin-layer chromatograms with amido black 10B and other water-soluble stains. Anal. Biochem. 271:186–187 [DOI] [PubMed] [Google Scholar]
- 22. Rehm B. H., Kruger N., Steinbuchel A. 1998. A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis. The PHAG gene from Pseudomonas putida KT2440 encodes a 3-hydroxyacyl-acyl carrier protein-coenzyme A transferase. J. Biol. Chem. 273:24044–24051 [DOI] [PubMed] [Google Scholar]
- 23. Salas J. J., Ohlrogge J. B. 2002. Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch. Biochem. Biophys. 403:25–34 [DOI] [PubMed] [Google Scholar]
- 24. Schirmer A., Rude M. A., Li X., Popova E., del Cardayre S. B. 2010. Microbial biosynthesis of alkanes. Science 329:559–562 [DOI] [PubMed] [Google Scholar]
- 25. Seo H. S., et al. 2001. Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate-regulated plant responses. Proc. Natl. Acad. Sci. U. S. A. 98:4788–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shoeman R., et al. 1985. Regulation of methionine synthesis in Escherichia coli: effect of metJ gene product and S-adenosylmethionine on the expression of the metF gene. Proc. Natl. Acad. Sci. U. S. A. 82:3601–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sjogren J., Magnusson J., Broberg A., Schnurer J., Kenne L. 2003. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 69:7554–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith A. A., Greene R. C. 1984. Cloning of the methionine regulatory gene, metJ, of Escherichia coli K12 and identification of its product. J. Biol. Chem. 259:14279–14281 [PubMed] [Google Scholar]
- 29. Steen E. J., et al. 2010. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463:559–562 [DOI] [PubMed] [Google Scholar]
- 30. Su C. H., Greene R. C. 1971. Regulation of methionine biosynthesis in Escherichia coli: mapping of the metJ locus and properties of a metJ plus-metJ minus diploid. Proc. Natl. Acad. Sci. U. S. A. 68:367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sukovich D. J., Seffernick J. L., Richman J. E., Gralnick J. A., Wackett L. P. 2010. Widespread head-to-head hydrocarbon biosynthesis in bacteria and role of OleA. Appl. Environ. Microbiol. 76:3850–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sukovich D. J., et al. 2010. Structure, function, and insights into the biosynthesis of a head-to-head hydrocarbon in Shewanella oneidensis strain MR-1. Appl. Environ. Microbiol. 76:3842–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takayama K., Wang C., Besra G. S. 2005. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 18:81–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varbanova M., et al. 2007. Methylation of gibberellins by Arabidopsis GAMT1 and GAMT2. Plant Cell 19:32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Voelker T. A., Davies H. M. 1994. Alteration of the specificity and regulation of fatty acid synthesis of Escherichia coli by expression of a plant medium-chain acyl-acyl carrier protein thioesterase. J. Bacteriol. 176:7320–7327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weissbach H., Brot N. 1991. Regulation of methionine synthesis in Escherichia coli. Mol. Microbiol. 5:1593–1597 [DOI] [PubMed] [Google Scholar]
- 37. Yuan L., Voelker T. A., Hawkins D. J. 1995. Modification of the substrate specificity of an acyl-acyl carrier protein thioesterase by protein engineering. Proc. Natl. Acad. Sci. U. S. A. 92:10639–10643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y. M., Rock C. O. 2008. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 6:222–233 [DOI] [PubMed] [Google Scholar]
- 39. Zheng Z., et al. 2004. Thioesterase II of Escherichia coli plays an important role in 3-hydroxydecanoic acid production. Appl. Environ. Microbiol. 70:3807–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zheng Z., Zhang M. J., Zhang G., Chen G. Q. 2004. Production of 3-hydroxydecanoic acid by recombinant Escherichia coli HB101 harboring phaG gene. Antonie Van Leeuwenhoek 85:93–101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.