Abstract
Mutant sorghum (Sorghum bicolor [L.] Moench) deficient in functional phytochrome B exhibits reduced photoperiodic sensitivity and constitutively expresses a shade-avoidance phenotype. Under relatively bright, high red:far-red light, ethylene production by seedlings of wild-type and phytochrome B-mutant cultivars progresses through cycles in a circadian rhythm; however, the phytochrome B mutant produces ethylene peaks with approximately 10 times the amplitude of the wild type. Time-course northern blots show that the mutant's abundance of the 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase mRNA SbACO2 is cyclic and is commensurate with ethylene production, and that ACC oxidase activity follows the same pattern. Both SbACO2 abundance and ACC oxidase activity in the wild-type plant are very low under this regimen. ACC levels in the two cultivars did not demonstrate fluctuations coincident with the ethylene produced. Simulated shading caused the wild-type plant to mimic the phenotype of the mutant and to produce high amplitude rhythms of ethylene evolution. The circadian feature of the ethylene cycle is conditionally present in the mutant and absent in the wild-type plant under simulated shading. SbACO2 abundance in both cultivars demonstrates a high-amplitude diurnal cycle under these conditions; however, ACC oxidase activity, although elevated, does not exhibit a clear rhythm correlated with ethylene production. ACC levels in both cultivars show fluctuations corresponding to the ethylene rhythm previously observed. It appears that at least two separate mechanisms may be involved in generating high-amplitude ethylene rhythms in sorghum, one in response to the loss of phytochrome B function and another in response to shading.
The sorghum (Sorghum bicolor) cv 58M lacks a functional PhyB protein due to a frame-shift mutation (Childs et al., 1997), which causes this cultivar to constitutively exhibit a phenotype typical of shade avoidance. This phenotype includes excessive shoot elongation, low chlorophyll content, and early flowering, even under noninductive photoperiods (Quinby, 1973; Pao and Morgan, 1986; Childs et al., 1991). We have recently reported that although both the wild-type and the mutant cultivars produce ethylene in a circadian rhythm, the phyB-1 mutant produces ethylene with peak values approximately 10 times higher than the wild type (Finlayson et al., 1998). Furthermore, under conditions simulating high shade (low-irradiance, far-red-enriched light), the wild-type plant mimics the phenotype of the phyB-1 mutant and produces diurnal ethylene rhythms with amplitudes similar to the mutant.
Ethylene biosynthesis in plants is likely to be regulated at two possible points. Many studies have demonstrated the regulation of ethylene biosynthesis by controlling the levels of its immediate precursor, ACC, and have shown that this regulation occurs at the level of transcription (Abeles et al., 1992; Kende, 1993) and possibly through phosphorylation-dependent control of ACC synthase degradation (Spanu et al., 1994). Ethylene biosynthesis can also be controlled by regulating the final step of the conversion of ACC to ethylene by ACC oxidase (English et al., 1995). Previous studies examining the phenomenon of ethylene rhythms in plants have implicated both points in the control of ethylene production. Circadian ethylene evolution in Stellaria longipes was demonstrated to correlate with both the abundance of an ACC oxidase mRNA and in vitro ACC oxidase activity (Kathiresan et al., 1996). Emery et al. (1997) and Kathiresan et al. (1998) subsequently found that neither fluctuations in ACC levels nor in ACC synthase mRNA abundances were likely to be contributing to the rhythm. Conversely, Machácková et al. (1997) ascribed diurnal ethylene production in Chenopodium rubrum to rhythms in the activity of ACC synthase, as evidenced by diurnal fluctuations in ACC levels. Circadian ethylene production in cotton was correlated with the conversion of S-adenosyl-l-methionine to ethylene, but not ACC to ethylene, generating the similar conclusion that fluctuations in ACC levels were responsible for the rhythm (Rikin et al., 1984).
The objective of this study was to elucidate the mechanism responsible for rhythmic ethylene biosynthesis by two sorghum cultivars under two different light regimens. As an aid to understanding both how the lesion in PhyB function derepresses cyclic ethylene production, and how simulated high shade generates a similar rhythm in the wild-type plant, we sought to examine the molecular and biochemical basis for the production of these ethylene rhythms.
MATERIALS AND METHODS
Plant Material
Two of the near-isogenic sorghum (Sorghum bicolor [L.] Moench) maturity cultivars, 100M (Ma1Ma1, Ma2Ma2, Ma3Ma3, and Ma4Ma4 for the maturity genes) and 58M (Ma1Ma1, Ma2Ma2, ma3Rma3R, and Ma4Ma4) (Quinby, 1967), were used. Ma3 has been redesignated as PHYB and ma3R as phyB-1, based on the determination that they encode the gene for phytochrome B and a null mutant version of the gene, respectively (Childs et al., 1997). Seeds were germinated vertically in the dark at 27°C on moistened germination paper. After 40 h, four healthy, uniform seedlings were carefully transferred in an upright orientation into plastic 50-mL conical tubes containing 45 mL of fritted clay previously saturated with one-quarter-strength Hoagland solution and drained. The tube walls were covered with aluminum foil and placed in the dark for 24 h until the start of the first photoperiod. The plants received photoperiods and temperature regimens as described, with small fans ensuring constant air circulation. Lighting was provided by a combination of F48T12/CW/HO fluorescent lamps (Phillips, Mahwah, NJ) and 60-W incandescent lamps giving 200 μmol m−2 s−1 PAR with a red-to-far-red ratio of 2.05. For dim, far-red-enriched light treatments, irradiance was provided by four 60-W incandescent lamps producing 29 μmol m−2 s−1 PAR with a red-to-far-red ratio of 0.75. Light measurements were made with a spectroradiometer (model no. 1800, Li-Cor, Lincoln, NE).
Cloning of ACC Oxidase, ACC Synthase, and CAB Genes
A sorghum λgt10 cDNA library prepared from green shoots of young plants was screened with the rice ACC oxidase cDNA clone, X85747. Six positive plaques were purified and cloned into the NOTI site of pBluescript II KS(+). Inserts were sequenced using the ABI dye terminator cycle sequencing ready reaction kit (Perkin-Elmer) and analyzed on an ABI 377 (Perkin-Elmer). Using the primers CTG GAC TGG GAG GAC ATC TT and CAT GTA CTT GGG GTA CGC CT, a PCR product was amplified from sorghum shoot cDNA using hot start, touch down PCR. Sequence analysis indicated that this product was different from the previous clone obtained, and it was used to screen the sorghum cDNA library. One positive plaque was purified and cloned into the NOTI site of pBluescript II KS(+). The insert was sequenced as above.
An insert encoding a fragment of a type-II chlorophyll a/b-binding protein of the PSII gene SbCABII was obtained from the sorghum cDNA library and ligated into the NOTI pBluescript II KS(+) site. The sequence was obtained as described above.
RNA Preparation and Northern Blotting
At each time point, 16 seedlings of each cultivar were harvested and immediately frozen in liquid nitrogen. The samples were stored at −80°C until RNA was extracted. Samples were kept frozen with liquid nitrogen and ground to a fine powder. The powder was then vortexed for 30 s in a test tube with 4 mL of Tris-saturated phenol and 4 mL of 100 mm Tris-HCl, pH 8.0, at 80°C. The tubes were then placed on ice and 4 mL of chloroform:isoamyl alcohol (48:2, v/v) was added and the tubes were vortexed again for 30 s. The mixture was centrifuged for 20 min at 12,000g (4°C), after which the aqueous (upper) phase was transferred to another tube. The RNA was precipitated with 1 volume of 4 m LiCl at −20°C for 3 h, followed by centrifugation for 20 min at 12,000g (4°C). The RNA pellet was washed with 5 mL of cold 70% ethanol and repelleted by brief centrifugation at 12,000g, and the alcohol was removed. The pellet was dried under a vacuum and resuspended in 400 μL of diethyl pyrocarbonate-treated water. The RNA extracts were quantitated in triplicate using a UV spectrometer, and the quantitation was verified by running an ethidium bromide-stained agarose test gel and noting the staining intensities of the rRNA bands. Denaturing agarose gels (0.8% or 1.0%) were then run and the RNA was transferred to charged nylon membranes, which were probed with random primed [32P]dCTP-labeled DNA as previously described.
In Vitro ACC Oxidase Assays
At each time point, four groups of four seedlings of each cultivar were harvested and immediately frozen in liquid nitrogen. The samples were stored at −80°C and then homogenized to a fine powder in a mortar kept frozen with liquid nitrogen. Approximately 0.05 g of homogenized sample was weighed into 1.5-mL microfuge tubes, also kept frozen with liquid nitrogen. These samples were then maintained at −80°C until extracted and assayed as described previously (He et al., 1996) with slight modifications. These modifications included the use of extraction and assay buffers with a pH of 6.9 and the use of 25 mm DTT during extraction and 50 μm FeSO4 during the assay. The ethylene produced was measured using a gas chromatograph (model 10S Plus, Photovac, Markham, Ontario, Canada).
Analysis of ACC Levels
The determination of ACC levels was based on the procedure of McGaw et al. (1985) with modifications for high sample throughput. Replicate samples from the homogenization procedure given above were weighed into 1.5-mL microfuge tubes (approximately 0.05 g). These samples were also kept frozen in liquid nitrogen or at −80°C until analyzed. Twenty nanograms of 2H4-ACC was added to each sample, which was then extracted in 500 μL of 80% ethanol at 70°C for 3 h. The samples were centrifuged at 14,000g for 7 min and the supernatant was transferred to another microfuge tube and dried using a concentrating centrifuge. The residue was redissolved in 500 μL of 0.1 n acetic acid and partitioned with 200 μL of chloroform. The acetic acid fraction was applied to a column of 500 μL of hydrogen-form Dowex AG 50X8 (Bio-Rad) prewashed with 1 mL of 0.1 n acetic acid in a 1-mL plastic pipettor tip. The column was washed with 2.5 mL of 0.1 n acetic acid and then eluted with 400 μL of 2 n NH4OH to waste. A further 350 μL of 2 n NH4OH was applied, collected, and dried. This in turn was redissolved in 500 μL of 0.1 n NH4OH and applied to 500 μL of acetate-form Dowex AG1X8 (Bio-Rad) prewashed with 2 mL of 0.1 n NH4OH in a column as above. The column was washed with 2.5 mL of 0.1 n NH4OH, and then eluted with 950 μL of 0.1 n acetic acid to waste. An additional 450 μL of 0.1 n acetic acid was applied, collected, dried, and redissolved in 500 μL of 30 mm phthalic anhydride in glacial acetic acid.
The samples were heated at 110°C for 1.5 h, 500 μL of water and 1.25 mL of ether were added, and the ether fraction was collected and dried. The sample was dissolved in 250 μL of 30% methanol and 200 μL was injected onto a HPLC system using a 3.9- × 300-mm μBondapak C18 column (Waters) with a linear gradient of 30% to 40% methanol for 5 min, followed by 40% to 48% methanol over 5 min at 1.6 mL min−1. Starting at a retention time of 8.4 min, an 800-μL fraction was collected, dried, and methylated with diazomethane. The sample was analyzed on a HP 5890A GC coupled to a 5970 mass selective detector (Hewlett-Packard) using a 0.32-mm × 15-m DB-5 column (J&W Scientific, Folsom, CA) with on-column injection and a temperature program of 50°C to 250°C at 50°C min−1, followed by 250°C to 270°C at 20°C min−1. The phthalimido-ACC-methyl ester had a retention time of approximately 4.5 min, and ions 244.7 and 248.7 were monitored for quantitation.
RESULTS AND DISCUSSION
The Mechanism of Diurnal/Circadian Ethylene Production under Unshaded Conditions
Six independent clones were obtained from a primary screening of a sorghum cDNA library using the rice ACC oxidase X85747 as a probe. The cloned inserts varied in length, but sequencing analysis revealed that all represented the same gene. The longest insert included the entire coding sequence of 951 bases, as well as 144 untranslated 5′ and 349 untranslated 3′ bases. The cloned product was named SbACO1 and the conceptually translated product showed 49.2% identity to the rice ACC oxidase gene AF049889 over the open reading frame. When used to probe northern blots prepared from young sorghum seedlings, SbACO1 abundance did not show time- or cultivar-dependent differences. The library was again screened using a PCR-generated DNA fragment (described in Methods). The primary screening identified several positive plaques, which contained the same size insert (approximately 900 bases). One insert was cloned into pBluescript II KS(+) and sequenced. Sequencing showed an insert of 872 bases, comprising 581 bases of coding sequence and 291 bases of 3′-untranslated region. The conceptual translation product of this gene fragment was 87.1% identical to the rice ACC oxidase AF049889, and 58% identical to SbACO1.
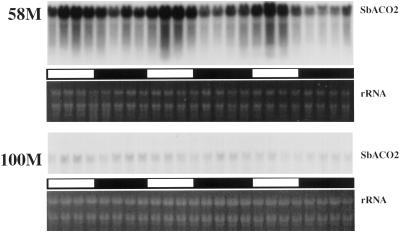
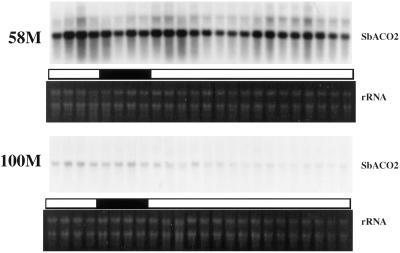
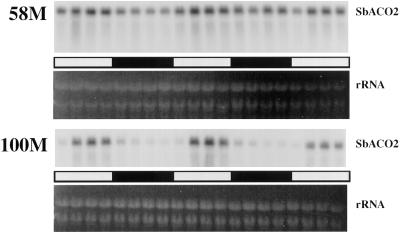
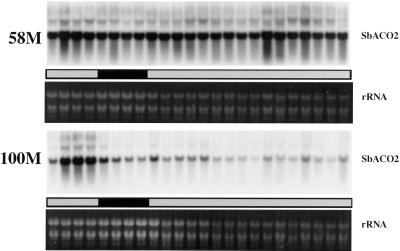
SbACO2 message abundance showed a strong diurnal rhythm coincident with previously reported peak ethylene production in cv 58M (Fig. 1). This mRNA was expressed at a much lower level in the wild-type cv 100M, and rhythmic expression could not be detected. Additional analyses of cv 58M SbACO2 mRNA showed that the variations in abundance were circadian, persisting in constant light/temperature after initial entrainment (Fig. 2).
Figure 1.
Diurnal SbACO2 mRNA abundance from sorghum grown under a 12-h/12-h photoperiod, 31°C/22°C thermoperiod. The first sample was from 5-d-old plants (58M is phyB-1, 100M is PHYB). White and black bars indicate light/warm and dark/cool periods, respectively.
Figure 2.
Diurnal/circadian SbACO2 mRNA abundance from sorghum grown under a 12-h/12-h photoperiod, 31°C/22°C thermoperiod until 8 am of d 6, then in constant light at 27°C. The first sample was from 5-d-old plants (58M is phyB-1, 100M is PHYB). White and black bars indicate light/warm and dark/cool periods, respectively.
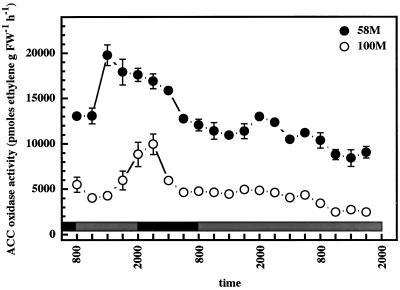
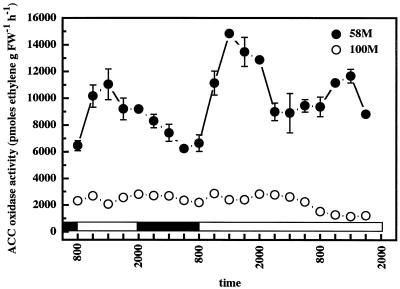
ACC oxidase enzyme activity in cv 58M also fluctuated in a circadian rhythm, paralleling both SbACO2 abundances and previously observed peaks in ethylene production (Fig. 3). The levels of ACC oxidase activity in cv 100M were much lower than those observed in cv 58M, and showed a weak, low amplitude cycle, which is again commensurate with the observed SbACO2 abundances and ethylene production from this cultivar.
Figure 3.
Diurnal/circadian ACC oxidase activity from sorghum grown under a 12-h/12-h photoperiod, 31°C/22°C thermoperiod until 8 am of d 6, then in constant light at 27°C. The first sample was from 5-d-old plants (58M is phyB-1, 100M is PHYB); n = 4, means ± se. White and black bars indicate light/warm and dark/cool periods, respectively. FW, Fresh weight.
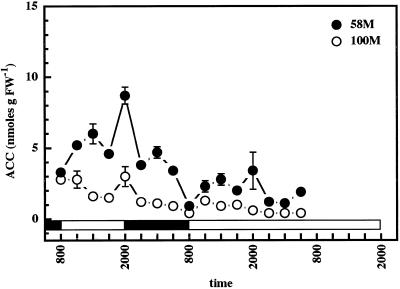
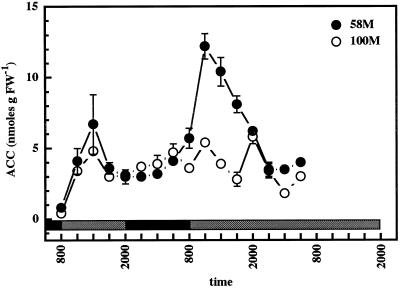
Analyses of ACC levels in the two cultivars demonstrated the absence of a clear-cut single peak rhythm in either cv 58M or cv 100M (Fig. 4). ACC levels were generally higher in cv 58M, and a small peak early in the light period could be observed in this cultivar. However, a second smaller peak was also observed during the dark period, and a larger spike at lights off was also apparent. cv 100M also showed an elevation in ACC content at the transition from light to dark. ACC levels in this cultivar did not demonstrate a clear rhythm but did gradually diminish over the duration of the measurements.
Figure 4.
Diurnal ACC levels from sorghum grown under a 12-h/12-h photoperiod, 31°C/22°C thermoperiod until 8 am of d 6, then in constant light at 27°C. The first sample was from 5-d-old plants (58M is phyB-1, 100M is PHYB); n = 4, means ± se. White and black bars indicate light/warm and dark/cool periods, respectively. FW, Fresh weight.
Our conclusions from the analyses of SbACO2 mRNA abundance, ACC oxidase enzyme activity, ACC substrate levels, and previously reported ethylene measurements (Finlayson et al., 1998) are that circadian ethylene rhythms in sorghum cultivars under nonshaded conditions are the consequence of circadian fluctuations in ACC oxidase activity, themselves a consequence of circadian fluctuations in SbACO2 mRNA abundance. The lesion in phytochrome B, as a result of a frame-shift mutation in the phyB-1 gene of cv 58M, leads to overabundance of SbACO2 message, resulting in enhanced ACC oxidase activity and ethylene production in this cultivar. It is possible that the slightly elevated ACC levels in cv 58M over the wild type also contributed to the increased amplitude of ethylene production; however, the levels of substrate do not correlate well with observed ethylene peaks and are therefore unlikely to be actually driving the rhythm.
The Mechanism of Diurnal Ethylene Production under Dim, Far-Red-Enhanced Simulated High-Shade Conditions
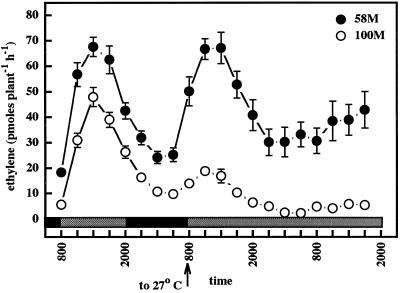
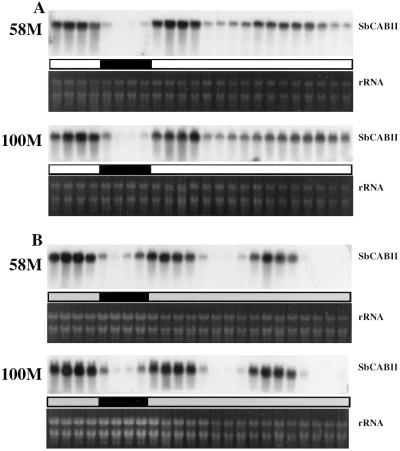
The diurnal abundance of SbACO2 mRNA from cv 58M seedlings grown under conditions simulating extreme shading showed a rhythmic pattern consistent with the pattern of ethylene evolution from the plants (Fig. 5). Under these growth conditions the wild-type cv 100M also produced large peaks of ethylene (Finlayson et al., 1998); and in this case, SbACO2 abundances were elevated over those from “normal light” growth conditions and showed strong diurnal fluctuations in abundance, similar to cv 58M. SbACO2 message abundance in both cultivars showed a very weak circadian pattern when grown in simulated shade (Fig. 6). Seedlings entrained for two cycles and then transferred to constant shaded light/27°C temperature lost the capacity to produce ethylene in rhythmic peaks as soon as the entraining stimuli were removed (Fig. 7). In contrast, SbCABII mRNA abundance cycled with a strong circadian rhythm under this light/temperature regimen, and showed a more robust rhythm than was observed in plants grown under “normal light” (Fig. 8). Apparently, the loss of circadian rhythmicity under this regimen is not a general phenomenon, but is specific to the ethylene rhythm.
Figure 5.
Diurnal SbACO2 mRNA abundance from sorghum grown under a simulated high-shade 12-h/12-h photoperiod, 31°C/22°C thermoperiod. The first sample was from 5-d-old plants (58M is phyB-1, 100M is PHYB). Gray and black bars indicate shaded light/warm and dark/cool periods, respectively.
Figure 6.
Diurnal/circadian SbACO2 mRNA abundance from sorghum grown under a simulated high-shade 12-h/12-h photoperiod, 31°C/22°C thermoperiod until 8 am of d 6, then in constant light at 27°C. The first sample was from 5-d-old plants (58M is phyB-1, 100M is PHYB). Gray and black bars indicate shaded light/warm and dark/cool periods, respectively.
Figure 7.
Ethylene production from sorghum grown under a simulated high-shade 12-h/12-h photoperiod, 31°C/22°C thermoperiod until 8 am of d 6, then given constant simulated high shade at 27°C. The first sample was from 5-d-old plants (58M is phyB-1, 100M is PHYB). n = 5, means ± se. Gray and black bars indicate shaded light/warm and dark/cool periods, respectively.
Figure 8.
Diurnal/circadian SbCABII mRNA abundance from sorghum grown under either “normal light” (A) or simulated high shade (B) with a 12-h/12-h photoperiod, 31°C/22°C thermoperiod until 8 am of d 6, then in constant light at 27°C. The first sample was from 5-d-old plants (58M is phyB-1, 100M is PHYB). White (gray) and black bars indicate light (shaded light)/warm and dark/cool periods, respectively.
Although SbACO2 mRNA abundances in both cultivars showed clear diurnal cycles that correlated well with measured ethylene production, analysis of in vitro ACC oxidase activity did not demonstrate either a circadian or diurnal pattern (Fig. 9). The ACC oxidase activity from cv 58M was generally higher than that observed under “normal light,” showed a rather late peak on the 1st d, and then became chaotic. ACC oxidase activity from cv 100M was also enhanced over “normal light” levels by approximately two to three times, but did not show rhythmicity that would correlate with SbACO2 mRNA abundance or ethylene production under this growth regimen.
Figure 9.
Diurnal/circadian ACC oxidase activity from sorghum grown under a simulated high-shade 12-h/12-h photoperiod, 31°C/22°C thermoperiod until 8 am of d 6, then in constant light at 27°C. The first sample is from 5-d-old plants (58M is phyB-1, 100M is PHYB). n = 4, means ± se. Gray and black bars indicate shaded light/warm and dark/cool periods, respectively. FW, Fresh weight.
When grown in a dim, far-red-enriched environment, the phyB-1 mutant cv 58M exhibited strong peaks in ACC levels coincident with ethylene produced (Fig. 10). Peak ACC levels were greater than those observed with “normal light”; the transitory peak in ACC levels observed at lights off under “normal light” was absent in the simulated shade-grown plants. Wild-type sorghum also produced a peak in ACC coincident with ethylene production on the 1st d measured; however, the following day ACC levels began to fluctuate in an oscillatory pattern of low amplitude. ACC levels in cv 100M grown under the simulated-shade environment were higher than those in “normal-light-grown” plants, especially toward the end of the experiment.
Figure 10.
Diurnal ACC levels from sorghum grown under a simulated high-shade 12-h/12-h photoperiod, 31°C/22°C thermoperiod until 8 am of d 6, then in constant light at 27°C. The first sample was from 5-d-old plants (58M is phyB-1, 100M is PHYB). n = 4, means ± se. Gray and black bars indicate shaded light/warm and dark/cool periods, respectively. FW, Fresh weight.
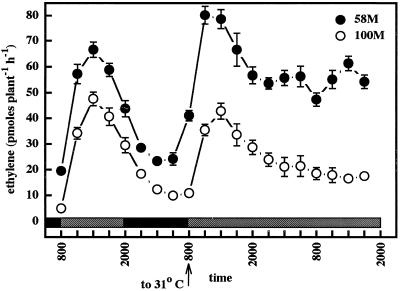
The previous experiments that investigated circadian responses involved a temperature shift to only 27°C on the 1st d of constant light, instead of the 31°C used for diurnal days (22°C at night). The importance of temperature in shade-enhanced ethylene production is demonstrated in Figure 11, in which the temperature shifted from 22°C at night to 31°C on the 1st d of constant light. This regimen caused high-amplitude ethylene peaks in both cultivars, with cv 58M showing weak circadian ethylene production on the 3rd subjective d. Conversely, cv 100M produced a rather low-amplitude ethylene peak in an identical experiment performed with the lower temperature shift (to 27°C on the 1st d of constant light), and under these conditions ethylene production by cv 58M was not circadian (Fig. 7). When these results are compared with the analysis of ACC levels under simulated shade (Fig. 10), it seems likely that the failure of ACC levels in cv 100M to show a clear peak on d 2 (the 1st d of constant light) may be a result of the plants not receiving a sufficient temperature signal.
Figure 11.
Ethylene production from sorghum grown under a simulated high-shade 12-h/12-h photoperiod, 31°C/22°C thermoperiod until 8 am of d 6, then given constant simulated high shade at 31°C. The first sample was from 5-d-old plants (58M is phyB-1, 100M is PHYB). n = 5, means ± se. Gray and black bars indicate shaded light/warm and dark/cool periods, respectively.
Under a simulated high-shade environment, SbACO2 mRNA abundances continued to cycle in cv 58M and were strongly enhanced in cv 100M (Fig. 5), but the fluctuations in mRNA abundance were not translated into rhythmic ACC oxidase activity (Fig. 9). Diurnal ethylene production under these conditions resulted from a pronounced rhythm in ACC levels in both cultivars (Fig. 10).
CONCLUSIONS
From the analyses performed, it becomes apparent that two mechanisms operate to produce rhythmic ethylene evolution in sorghum. Under relatively bright, high red:far-red light, cyclic patterns of ACC oxidase activity in cv 58M drive high-amplitude circadian ethylene rhythms. This ACC oxidase activity was also correlated with the abundance of SbACO2 mRNA. Therefore, it is likely that the actual transcription of SbACO2 was the primary event leading to rhythmic ethylene production. The wild-type cultivar had both low SbACO2 abundance and low ACC oxidase activity, commensurate with the low-amplitude ethylene evolution observed under these conditions.
When seedlings are grown under a dim, far-red-enriched light, both cultivars produced high-amplitude ethylene rhythms; however, this ethylene production was not circadian in cv 100M and was circadian in cv 58M only when entrained with a pronounced temperature shift. Under the simulated shade conditions, SbACO2 mRNA abundance continued to cycle in cv 58M, and also showed high-amplitude, rhythmic abundance in cv 100M; however, ACC oxidase activity did not follow the same pattern in either cultivar. The generally higher levels of ACC oxidase activity in cv 100M under the simulated high-shade conditions may have contributed to the amplitude of ethylene production, but did not appear to be the causative agent for the rhythm itself.
In many cases, high-amplitude cycles of mRNA abundance did not result in similar cycles of protein abundance. For instance, circadianly expressed transcripts cloned from Sinapis alba were observed to cycle strongly, although the corresponding proteins showed little, if any, rhythmicity (Heintzen et al., 1994a, 1994b). Even CAB, which showed very robust cycling at the mRNA level, produced only minor rhythms in protein levels (Piechulla and Gruissem, 1987). It is surprising to observe that cycling SbACO2 transcript abundance does translate into cycling enzyme activity under one light regimen, but not under another. Possibly, there is a light-regulated posttranscriptional regulation of SbACO2 translation or ACC oxidase activity.
Under a simulated high-shade growth environment, the mechanistic basis for diurnal ethylene cycles in both cultivars appeared to shift to rhythmic fluctuations in ACC levels, probably a result of ACC synthase activity. Similar fluctuations in ACC synthesis are thought to control diurnal ethylene rhythms in Chenopodium rubrum (Machackova et al., 1997). Conversely, increased N-malonyl-ACC formation at the expense of ACC levels inhibited ethylene formation in etiolated bean seedlings exposed to red light, an effect reversible by far-red light (Vangronsveld et al., 1988). Although actual enhancement of ACC levels by far-red light was not demonstrated, it is conceivable that the low red:far-red light in the simulated shade environment could increase ACC levels by inhibiting N-malonyl-ACC synthesis.
Both a lesion in the plant's capacity to properly sense the light environment and a simulated-shade regimen elicited a high-amplitude ethylene rhythm. The basic mechanisms generating the rhythm were quite different, however, suggesting that these rhythms may play a fundamentally important role in controlling the phenotype of the plant in environments of varying light qualities and irradiances.
The accession numbers for the sequences described in this article are AF079588 (SbACO1), AF079589 (SbACO2), and AF079590 (SbCABII).
ACKNOWLEDGMENTS
We acknowledge with thanks the seed supplied for this study by Dr. Bill Rooney (Texas A&M University, College Station) and the molecular expertise of Drs. R.A. Creelman, K.L. Childs, and P. Ulanch. We are also grateful to Dr. Jialing Lu for the use of the sorghum cDNA library and to Dr. Hans Kende for his gift of the rice ACC oxidase gene X85747.
Footnotes
This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant no. 97-35304-4820 to P.W.M.), by the Texas Higher Education Board (ATP grant no. 999902-87 to P.W.M.), by a Predoctoral Overseas Korean Government Scholarship to I.-J.L., and by the Texas Agriculture Experiment Station.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology, Ed 2. Academic Press, New York
- Childs KL, Miller FR, Cordonnier-Pratt M-M, Pratt LH, Morgan PW, Mullet JE. The sorghum photoperiod sensitivity gene, Ma3, encodes a phytochrome B. Plant Physiol. 1997;113:611–619. doi: 10.1104/pp.113.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs KL, Pratt LH, Morgan PW. Genetic regulation of development in Sorghum bicolor. VI. The ma3R allele results in abnormal phytochrome physiology. Plant Physiol. 1991;108:345–351. doi: 10.1104/pp.97.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery RJN, Kathiresan A, Reid DM, Chinnappa CC. Ecotypic differences in rhythmicity of ethylene production in Stellaria longipes: the possible roles of ACC, MACC, and ACC oxidase. Can J Bot. 1997;75:1027–1033. [Google Scholar]
- English PJ, Lycett GW, Roberts JA, Jackson MB. Increased 1-aminocyclopropane-1-carboxylic acid oxidase activity in shoots of flooded tomato plants raises ethylene production to physiologically active levels. Plant Physiol. 1995;109:1435–1440. doi: 10.1104/pp.109.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson SA, Lee I-J, Morgan PW. Phytochrome B and the regulation of circadian ethylene production in sorghum. Plant Physiol. 1998;116:17–25. doi: 10.1104/pp.119.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C-J, Finlayson SA, Drew MC, Jordan WR, Morgan PW. Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiol. 1996;112:1679–1685. doi: 10.1104/pp.112.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzen C, Fischer R, Melzer S, Kappeler S, Apel K, Staiger D. Circadian oscillations of a transcript encoding a germin-like protein that is associated with cell walls in young leaves of the long-day plant Sinapis alba L. Plant Physiol. 1994a;106:905–915. doi: 10.1104/pp.106.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzen C, Melzer S, Fischer R, Kappeler S, Apel K, Staiger D. A light- and temperature-entrained circadian clock controls expression of transcripts encoding nuclear proteins with homology to RNA-binding proteins in meristematic tissue. Plant J. 1994b;5:799–813. doi: 10.1046/j.1365-313x.1994.5060799.x. [DOI] [PubMed] [Google Scholar]
- Kathiresan A, Nagarathna KC, Moloney MM, Reid DM, Chinnappa CC. Differential regulation of 1-aminocyclopropane-1-carboxylate synthase gene family and its role in phenotypic plasticity in Stellaria longipes. Plant Mol Biol. 1998;36:265–274. doi: 10.1023/a:1005994118535. [DOI] [PubMed] [Google Scholar]
- Kathiresan A, Reid DM, Chinnappa CC. Light and temperature entrained circadian regulation of activity and mRNA accumulation of 1-aminocyclopropane-1-carboxylic acid oxidase in Stellaria longipes. Planta. 1996;199:329–335. doi: 10.1007/BF00195723. [DOI] [PubMed] [Google Scholar]
- Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- Machácková I, Chauvaux N, Dewitte W, Van Onckelen H. Diurnal fluctuations in ethylene formation in Chenopodium rubrum. Plant Physiol. 1997;113:981–985. doi: 10.1104/pp.113.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaw BA, Horgan R, Heald JK. Selected ion monitoring/isotope dilution mass spectrometric determination of 1-aminocyclopropane-1-carboxylic acid levels in ripening tomato fruit. Anal Biochem. 1985;149:130–135. doi: 10.1016/0003-2697(85)90485-3. [DOI] [PubMed] [Google Scholar]
- Pao CI, Morgan PW. Genetic regulation of development in Sorghum bicolor. I. Role of the maturity genes. Plant Physiol. 1986;82:581–584. doi: 10.1104/pp.82.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechulla B, Gruissem W. Diurnal fluctuations of nuclear and plastid genes in developing tomato fruits. EMBO J. 1987;6:3593–3599. doi: 10.1002/j.1460-2075.1987.tb02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinby JR (1967) The maturity genes of sorghum. In AG Norman, ed, Advances in Agronomy, Vol 19. Academic Press, New York, pp 267–305
- Quinby JR (1973) The genetic control of flowering and growth in sorghum. In AG Norman, ed, Advances in Agronomy, Vol 25. Academic Press, New York, pp 125–162
- Rikin A, Chalutz E, Anderson JD. Rhythmicity in ethylene production in cotton seedlings. Plant Physiol. 1984;75:493–495. doi: 10.1104/pp.75.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanu P, Grosskopf DG, Felix G, Boller T. The apparent turnover of 1-aminocyclopropane-1-carboxylate synthase in tomato cells is regulated by protein phosphorylation and dephosphorylation. Plant Physiol. 1994;106:529–535. doi: 10.1104/pp.106.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangronsveld J, Clijsters H, Van Poucke M. Phytochrome-controlled ethylene biosynthesis of intact etiolated bean seedlings. Planta. 1988;174:19–24. doi: 10.1007/BF00394868. [DOI] [PubMed] [Google Scholar]