Abstract
Enteric bacteria deposited into the environment by animal hosts are subject to diverse selective pressures. These pressures may act on phenotypic differences in bacterial populations and select adaptive mutations for survival in stress. As a model to study phenotypic diversity in environmental bacteria, we examined mutations of the stress response sigma factor, RpoS, in environmental Escherichia coli isolates. A total of 2,040 isolates from urban beaches and nearby fecal pollution sources on Lake Ontario (Canada) were screened for RpoS function by examining growth on succinate and catalase activity, two RpoS-dependent phenotypes. The rpoS sequence was determined for 45 isolates, including all candidate RpoS mutants, and of these, six isolates were confirmed as mutants with the complete loss of RpoS function. Similarly to laboratory strains, the RpoS expression of these environmental isolates was stationary phase dependent. However, the expression of RpoS regulon members KatE and AppA had differing levels of expression in several environmental isolates compared to those in laboratory strains. Furthermore, after plating rpoS+ isolates on succinate, RpoS mutants could be readily selected from environmental E. coli. Naturally isolated and succinate-selected RpoS mutants had lower generation times on poor carbon sources and lower stress resistance than their rpoS+ isogenic parental strains. These results show that RpoS mutants are present in the environment (with a frequency of 0.003 among isolates) and that, similarly to laboratory and pathogenic strains, growth on poor carbon sources selects for rpoS mutations in environmental E. coli. RpoS selection may be an important determinant of phenotypic diversification and, hence, the survival of E. coli in the environment.
INTRODUCTION
The fecal bacterial contamination of fresh waters is a recurring issue for public beaches surrounding the Great Lakes of North America (57, 79). Direct fecal deposits (20, 78), as well as input from streams (60) and land runoffs (5), often result in high levels of bacteria in both water and sediment. Populations of Escherichia coli can persist for long periods outside a host organism and possibly multiply in soil (34). Although fecal deposits are implicated as the primary source of E. coli distribution, the environment likely selects for some stress-tolerant E. coli strains (4, 35). These “naturalized” E. coli populations add a newly recognized complexity to the contamination of fresh waters (11, 34, 80). The persistence and possible multiplication of E. coli in the environment may allow for the selection of adaptive mutations comparable to that of long-term laboratory E. coli cultures (82, 84). In particular for environmental E. coli populations, the selection of adaptive mutations may be central for the survival of cells in adverse conditions (24).
E. coli isolated from different hosts can possess high genotypic diversity (63). Spontaneous mutations are estimated to occur at 5.4 × 10−10 mutations per base pair per replication (19), and the selection of some mutations by unique environmental conditions results in niche-specific adaptation (37, 74). The mutation rate itself is dependent on the environment and likely is important in evolution (7, 29). For laboratory E. coli K-12, adaptive mutations are frequently fixed in populations during starvation in long-term batch cultures (28, 84, 85). One such mutation is within the stationary-phase sigma factor gene rpoS (13). The loss of RpoS, however, reduces mutagenesis in aging colonies (7) and the variability of adaptation in an evolving E. coli population (71). As seen with the Lac system, RpoS is required for point mutations and gene amplification in stationary phase (52), likely in part due to the RpoS regulation of error-prone DNA polymerase IV (47). Greater evolutionary potential can result in a more enduring population, despite a possible short-term disadvantage in competitive fitness (81).
RpoS (σs), which is present in E. coli and many proteobacteria (14), regulates stationary-phase and stress-related genes (51, 64). Interestingly, RpoS mutants have shorter doubling times on poor carbon sources and in long-term culture (13, 61). The fixation of rpoS mutations in a population provides enhanced fitness in nutrient-poor conditions but reduced stress resistance (21, 42, 62), as in low pH (70) or osmotic upshift (31). E. coli growth in a glucose-limited chemostat environment selects for multiple rpoS genotype subpopulations (54), which can lead to divergence under environmental selection (24). The rpoS gene can further be regarded as polymorphic (39, 55, 61), and this polymorphism influences the trade-off between self preservation and nutritional competence (SPANC) (22, 26). Phenotypic diversity observed in clinical isolates was attributable, at least in part, to distinct RpoS levels among isolates and its effect on SPANC (49). RpoS mutants have been identified among laboratory (36, 75) and pathogenic (17) E. coli strains, and the ancestral rpoS sequence of laboratory E. coli K-12 likely possessed an amber mutation at codon 33 (2). Nine of 31 tested E. coli strains from the ECOR collection, a standard reference collection of E. coli from hosts (63), carried deleterious rpoS mutations (25). We have previously found that poor carbon sources, such as succinate, readily select for the loss of RpoS function in both laboratory (13) and pathogenic strains (17) at a frequency of 10−8 mutants per cell plated. By extension, stressful environmental conditions, such as poor carbon and nutrient sources, may select for RpoS mutants in environmental E. coli populations.
Adaptive mutations within the rpoS gene are selected in laboratory and pathogenic E. coli strains in nutrient-poor conditions or during growth on nonpreferred carbon sources (13, 17, 61, 84). Because E. coli can be found in a wide range of environments (10, 34, 56), including putative poor carbon environments, we postulated that RpoS mutants are present, and may be selected, in environmental E. coli populations. Recent work using the ECOR strains found RpoS mutants in 29% of natural isolates (25). In this study, the frequency of RpoS mutants among environmental E. coli isolates was determined, as well as the ability to select for RpoS mutants from environmental E. coli on a poor carbon source. Differential RpoS expression is examined as a mechanism for generating phenotypic diversity in environmental E. coli. The RpoS sigma factor is a useful model because it is well-studied, is known to be lost by selection in nutrient-poor conditions, and as a global regulator has phenotypic effects that can be readily assessed in the laboratory.
MATERIALS AND METHODS
Bacterial strains.
Laboratory Escherichia coli K-12 strains used in this study were MG1655 and the precise rpoS deletion of MG1655, which was constructed previously (64). A total of 2,040 environmental E. coli isolates were collected from urban beaches (water and sand samples) and nearby fecal pollution sources (wastewater effluents and animal fecal droppings) in the cities of Hamilton and Toronto on Lake Ontario (Canada). The cities of Hamilton and Toronto, Ontario, are large urban centers along the shoreline at the western end of Lake Ontario. The area has a temperate climate, with summer temperatures reaching more than 30°C at times, and ice cover in the nearshore areas during the winter months. E. coli isolates were obtained from water and sand samples at Bayfront Park and Burlington Beaches around Hamilton (43°15′ N, 79°51′ W) and at Kew and Centre Island Beaches in Toronto (43°40′ N, 79°24′ W). E. coli isolates from wastewater effluent samples were obtained from the final effluents of sewage treatment plants in Hamilton and Toronto. E. coli isolates were obtained from fresh fecal droppings of dogs (Canis lupus familiaris) and cats (Felis catus) at Hamilton and Toronto animal shelters. Isolates also were obtained from fresh fecal droppings of Canada Goose (Branta canadensis), gulls (Larus delawarensis), and Mallard ducks (Anas platyrhynchos) around Hamilton and Toronto beaches. The E. coli isolates were obtained by previously described methods (13), and their environmental sources are summarized in Table 1. A full table of individual isolates is provided in Table S1 in the supplemental material.
Table 1.
Summary of source types for the 2,040 environmental E. coli isolates used in this study
| Type | No. of isolates |
|---|---|
| Beach water | 971 |
| Beach sand | 617 |
| Canada Goose (Branta canadensis) dropping | 131 |
| Sewage plant final effluent | 84 |
| Untreated combined sewer overflow | 68 |
| Dog (Canis lupus familiaris) dropping | 65 |
| Cat (Felis catus) dropping | 48 |
| Gull (Larus delawarensis) dropping | 38 |
| Mallard duck (Anas platyrhynchos) dropping | 18 |
Media and growth conditions.
Environmental isolates were replica plated into sterile 96-well microplates containing 200 μl/well of Luria-Bertani (LB) medium (58). The microplates were incubated overnight without shaking at 37°C. Prior to experiments, isolates were replica plated from the microplates containing LB medium into sterile microplates containing 200 μl/well of 1× M9 salts (58) for the purpose of minimizing nutrient carryover from the LB-rich media. The isolates in 1× M9 salts were used immediately in replica plate experiments on solid medium. For testing growth on tricarboxylic acid (TCA) intermediates and weak acids, isolates were replicated onto 0.5% (wt/vol) glucose, 1% succinate, 1% fumarate, 1% citrate, 1% α-ketoglutarate, 1% acetate, 1% (vol/vol) lactate, 0.5% formate, or 0.5% proprionate M9 minimal media (pH 7) (65). For sequencing and immunoblot analyses, isolates were streaked onto solid LB medium and incubated overnight at 37°C. Single colony isolates were used in all experiments. For calculating generation times, environmental isolates were grown at 37°C with shaking to an optical density at 600 nm (OD600) of ∼1 in 0.5% (wt/vol) glucose M9 minimal medium and subcultured to an OD600 of ∼0.03 in 0.5% glucose, 1% succinate, or 1% fumarate M9 minimal medium (pH 7). Growth was monitored spectrophotometrically (OD600), and the generation time was calculated (17).
RpoS-dependent phenotype tests. (i) Growth on succinate minimal medium.
Environmental isolates were replica plated onto 1% succinate M9 minimal medium (65) and incubated overnight at 37°C. Isolates were observed for growth after 24 and 48 h of incubation. Isolate patches with substantial growth after 24 h at 37°C were considered to grow well on succinate (designated Suc++). As a control for nonselective growth on minimal medium, isolates were replica plated onto 0.5% glucose M9 minimal medium (65).
(ii) Catalase test.
Environmental isolates were replica plated onto solid LB medium without antibiotics. Plates were incubated overnight at 37°C and tested for the presence of catalase by the addition of 5 μl of 30% hydrogen peroxide (H2O2) onto the patches. Isolates were recorded as catalase positive or negative, where catalase-negative strains had a severe lag in bubbling time after the addition of H2O2 (13).
Selection for loss of RpoS activity on succinate minimal media.
Selection for the loss of RpoS activity by growth with succinate as the sole carbon source was performed by growing single colonies of environmental isolates in LB medium at 37°C and 200 rpm overnight. Cultures were washed by centrifugation in 1× M9 salts, and cells (∼109) were plated on succinate minimal plates (13). After 48 to 72 h of incubation at 37°C, fast-growing mutants (Suc++) could be observed. Single Suc++ mutant colonies then were serially streaked onto LB solid medium for purity. Suc++ mutants were tested for catalase activity and succinate growth as described above. The loss of RpoS activity in selected mutants was confirmed by the sequencing of the rpoS gene.
PCR amplification and sequencing of rpoS and fliA genes.
Whole-colony PCR amplicons of rpoS and fliA genes from several isolates were sequenced. Single colonies were picked and boiled in 10 μl of sterile deionized/distilled water (ddH2O) at 95°C for 5 min. A 2-μl aliquot then was transferred to the PCR reagent mix. The primers used for rpoS and fliA open reading frame (ORF) amplification are listed in Table 2 (synthesized by MOBIX Laboratory, McMaster University, Hamilton, Ontario, Canada). PCR was performed with Fermentas Pfu DNA polymerase (Fermentas, Inc., Burlington, Ontario, Canada). PCR conditions consisted of an initial denaturation step at 95°C for 2 min, 30 cycles of 30 s at 95°C, 30 s at 58°C, and 2.5 min at 72°C, and a terminal extension step at 72°C for 5 min. All PCR products were purified using a NucleoSpin Extract II kit (Machery-Nagel GmbH & Co., Inc., Bethlehem, PA) and visualized on a 1% agarose gel for quantification prior to sequencing. Samples were sequenced by MOBIX Laboratory and analyzed with Sequence Scanner, version 1.0 (Applied Biosystems, Inc., Streetsville, Ontario, Canada). Sequencing was performed on both strands of the PCR product.
Table 2.
rpoS and fliA oligonucleotides used in this study for ORF amplification
| Oligonucleotide | ORF | Sequence |
|---|---|---|
| ML-08-145 | rpoS | 5′-CAACAAGAAGTGAAGGCGGG-3′ |
| ML-08-4514 | rpoS | 5′-CTTGCATTTTGAAATTCGTTACA-3′ |
| rpoS754 | rpoS | 5′-GATGACGATATGAAGCAGAG-3′ |
| ML-08-4515 | rpoS | 5′-TTAACGACCATTCTCGGTTTTAC-3′ |
| ML-08-5873 | rpoS | 5′-GGTGCAATCTCCAGCCG-3′ |
| ML-08-5874 | rpoS | 5′-GGAGAATCGTGGCTTAGTCAG-3′ |
| rpoSrv834 | rpoS | 5′-TAACATCAAACGAATCGACC-3′ |
| ML-08-3246 | fliA | 5′-ACCTGTAACCCCCAAATAAC-3′ |
| ML-08-3247 | fliA | 5′-CAATGGGTCTGGCTGTG-3′ |
Analyses of rpoS and fliA sequences in environmental E. coli isolates.
The rpoS and fliA ORF sequences were edited using Sequence Scanner, version 1.0 (Applied Biosystems, Inc., Streetsville, Ontario, Canada) and aligned with Clustal X (IUB DNA weight matrix) (46). The rpoS sequences were compared to the consensus sequence, which is a composite sequence composed of the most frequent (modal) base at each nucleotide position among isolate and laboratory K-12 rpoS sequences. The rpoS sequences were constructed into a dendrogram using the neighbor-joining method (46). The dendrogram was bootstrapped 1,000 times, and it was visualized with MEGA 4.0 (73) as previously described (14). The fliA sequences were compared to the laboratory K-12 fliA sequence.
Motility assay.
Individual colonies of bacteria were stabbed into 200 μl of 0.15% LB agar in a sterile 96-well microplate. The microplate was incubated at 37°C overnight without shaking prior to observation.
Native PAGE analysis of catalase activity.
Overnight E. coli cultures in LB liquid medium were sampled and centrifuged at 4,000 × g for 15 min. Samples were washed with 50 mM potassium phosphate buffer (pH 7.0) and sonicated (17). Five micrograms of protein was separated on 10% nondenaturing polyacrylamide gels and stained for catalase using horseradish peroxidase and diaminobenzidine (30).
Immunoblot analyses of RpoS protein levels.
Overnight E. coli cultures in LB liquid medium were subcultured 1:10,000 into 50 ml of LB and incubated at 37°C with shaking at 200 rpm. Culture samples were taken at exponential phase (OD600 of 0.3), early stationary phase (OD600 of 1.5), and 24 h after subculture. Chloramphenicol was immediately added to the samples to a final concentration of 150 μg/ml to stop protein synthesis, and samples were centrifuged at 12,000 × g for 5 min. The supernatant was removed by pipette, and pellets were resuspended in SDS loading buffer (125 mM Tris-Cl, pH 6.8; 2.5% β-mercaptoethanol; 8.7% glycerol; 1% SDS; 0.01% bromophenol blue) for a final cell concentration equivalent to an OD600 of 1.0. Resuspended pellets were placed in boiling water for 5 min.
Ten microliters of protein samples was resolved on 10% SDS polyacrylamide stacking gels. A second gel was stained for protein with 0.1% Coomassie blue dye to ensure equal protein loading. Resolved proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Inc., Billerica, MA), and the membrane was incubated for 1 h at room temperature in 5% milk made in TBS-T (87 mM NaCl; 10 mM Tris-Cl, pH 8; 0.05% Tween 20). The blocking buffer was replaced with a 1:10,000 dilution of primary antibody (anti-σs antibody; Neoclone, Inc., Madison, WI; or anti-AppA; a gift from C.W. Forsberg, University of Guelph), and the membrane was left overnight with gentle shaking at 4°C. After washing with TBS-T, the membrane then was placed for 1 h at room temperature with shaking in a 1:3,000 dilution of the secondary anti-mouse antibody for the σs membrane or anti-rabbit antibody for the AppA membrane (Bio-Rad Laboratories, Inc., Mississauga, Ontario, Canada). After washing again with TBS-T, the secondary antibody fluorophore was activated in ECL staining solution (1:1 detection reagent mixture; Amersham GE Healthcare, Inc., Baie d'Urfe, Quebec, Canada) for 1 min prior to exposure on Amersham Hyperfilm ECL for 10 s to 1 min.
Survival assays. (i) Acid resistance.
Overnight cultures of environmental isolates in LB were subcultured to ∼105 cells into LB medium adjusted to pH 2.5 with hydrochloric acid. Cultures then were incubated at 37°C with shaking at 200 rpm for 2 h and serially plated onto LB plates to determine the final CFU/ml (76). Percent survival was calculated as (final CFU/ml)/(initial CFU/ml) × 100.
(ii) H2O2 resistance.
Overnight cultures of environmental isolates in LB were washed with 0.9% NaCl and subcultured to ∼107 cells into LB medium containing 15 mM H2O2. Cultures then were incubated at 37°C with shaking at 200 rpm, and CFU/ml was determined over time by serially plating onto LB plates (18, 45). The percent survival was calculated as (final CFU/ml)/(initial CFU/ml) × 100.
Nucleotide sequence accession numbers.
Sequences were deposited at GenBank (3) with accession numbers JN191237 to JN191281 for rpoS, JN191282 to JN191289 for fliA, and JN191290 to JN191292 for succinate-selected RpoS mutants.
RESULTS
Environmental E. coli isolates.
A collection of 2,040 diverse environmental E. coli isolates from urban beaches and nearby fecal pollution sources in the cities of Hamilton and Toronto were used for this study. These isolates were collected between April and September 2004 and between May and June 2005 from beach water, foreshore beach sand, sewage treatment plant final effluent, untreated sewage from combined sewer overflow storage tanks, and fresh fecal droppings from dogs, cats, gulls, ducks, and Canada Goose (Table 1). These isolates were used as a representative collection of E. coli isolated from environmental sources outside a host organism.
Screen for RpoS activity using two RpoS-dependent phenotypes, growth on succinate and catalase activity.
RpoS activity of E. coli isolates were assessed using two RpoS-dependent phenotypes, growth on succinate and catalase activity, to identify the prevalence of environmental RpoS mutants.
RpoS mutants of laboratory E. coli exhibit better growth on poor carbon than wild-type cells (13, 41). After replica plating environmental E. coli isolates onto media with succinate as the sole carbon source, 93 isolates had significant growth after 24 h of incubation (Suc++), which is similar to that of an rpoS mutant control. Seven Suc++ isolates also were catalase negative, as determined by a catalase test. The Suc++ isolates were from beach water (45/971), beach sand (31/617), untreated sewage (6/68), sewage treatment plant final effluent (4/84), and fresh animal fecal droppings (7/300). The largest percentage of Suc++ isolates, therefore, was from untreated sewage (8.8%).
RpoS regulates one of two primary catalase genes, katE, in laboratory E. coli K-12 (67), and consequently an RpoS mutant colony has reduced bubbling compared to that of an RpoS-positive colony with the addition of hydrogen peroxide. Of the 2,040 isolates, 38 isolates were determined to have reduced catalase activity. These isolates were from beach water (14/971), beach sand (11/617), animal fecal droppings (5/300), sewage treatment plant final effluent (5/84), and untreated sewage (3/68). Similarly to the Suc++ phenotype, the largest percentages of catalase-negative isolates were from untreated sewage (4.4%) and final effluent (5.6%).
rpoS alleles confirm presence of RpoS mutants among environmental E. coli isolates.
The rpoS genes of 45 environmental E. coli isolates, including all isolates that were both Suc++ and catalase deficient, as well as representatives of isolates with only one RpoS mutant phenotype, were sequenced to confirm the presence of RpoS mutants. A dendrogram of the 45 rpoS sequences with the isolate sources can be found in Fig. S1 in the supplemental material.
Eight nonsynonymous and 81 distinct synonymous mutations were identified among the isolates (see Table S2 in the supplemental material). A nonsynonymous mutation at codon 33, where glutamine (CAG) is replaced with glutamic acid (GAG), is characteristic of non-K-12 strains (2, 72), and not surprisingly, it was found in all environmental E. coli rpoS sequences determined in this study. Three other nonsynonymous mutations resulted in an amino acid change but yielded a putatively functional RpoS protein, as these mutations had no other notable impact on the RpoS protein. More specifically, isolate ABC01 from beach sand had lysine (AAG) replace glutamic acid (GAG) at codon 122; EKF07 from beach water had tyrosine (TAC) replace asparagine (AAC) at codon 124; and ECH01 from beach water had serine (TCC) replace threonine (ACC) at codon 298. The remaining four nonsynonymous mutations resulted in a nonfunctional RpoS protein. ECE12 isolated from untreated sewage had a 5-bp deletion (nucleotides 255 to 259); BNB03, BNB04, and BNB07 from beach sand had a 1-bp deletion (nucleotide 378); AZB07 from beach sand had a G→A transition (nucleotide 443), resulting in an amber stop codon; and ASC02 from sewage treatment plant final effluent had a 1,329-bp insertion, which has 100% identity to a putative IS10 transposase of E. coli O111:H− strain 11128 (NCBI; February 2011) and a characteristic surrounding 9-bp DNA repeat (43) (Table 3).
Table 3.
Identified mutations in the rpoS gene that predict a nonfunctional RpoS protein in environmental E. coli isolates
| Isolate | Source type | Type of mutation | Effect of mutation on amino acid sequence | Location (nucleotides) | rpoS accession no. |
|---|---|---|---|---|---|
| ECE12 | Untreated combined sewer overflow | 5-bp deletion | Frameshift, short protein | 255–259 | JN191271 |
| BNB03a | Beach sand | 1-bp deletion | Frameshift, short protein | 378 | JN191252 |
| AZB07 | Beach sand | G→A transition | TGG→TAG (amber stop codon) | 443 | JN191250 |
| ASC02 | Sewage treatment plant final effluent | 1,329-bp insertion (IS10 transposase) and 9-bp duplication | Short protein | 776 | JN191249 |
Comparison of mutational frequencies of the rpoS and fliA genes.
Among the environmental E. coli isolates, there were 81 distinct synonymous mutations within the rpoS gene (see Table S2 in the supplemental material), for a frequency of 1.8 × 10−3 synonymous mutations per base per isolate. The rpoS-mutS region of E. coli, as in other enteric bacteria, is an area of high genetic variation and putative high recombination (9, 32, 55). Therefore, we chose a gene of length similar to that of rpoS, the flagellar sigma factor fliA, to determine if the frequency of synonymous mutations in rpoS was unique among other genes in environmental E. coli.
The fliA gene was sequenced for eight isolates of different catalase activity and succinate growth. These isolates were one RpoS mutant (ECE12); two rpoS+ (according to rpoS sequencing), catalase-negative isolates; three rpoS+, Suc++ isolates; and two comparison isolates. All isolates carried synonymous mutations from K-12 fliA, and one nonsynonymous mutation was identified in ABB10 (see Table S3 in the supplemental material). A motility assay in 0.15% LB agar indicated that ABB10 had reduced motility (data not shown). Among the eight isolates, 12 synonymous mutations were identified in 720 nucleotides (see Table S3), for a frequency of 2.1 × 10−3 synonymous mutations per base per isolate. Therefore, the number of synonymous mutations in the fliA gene was comparable to that in the rpoS gene, with a frequency of 10−3 synonymous mutations per base per isolate.
RpoS protein levels in environmental E. coli isolates.
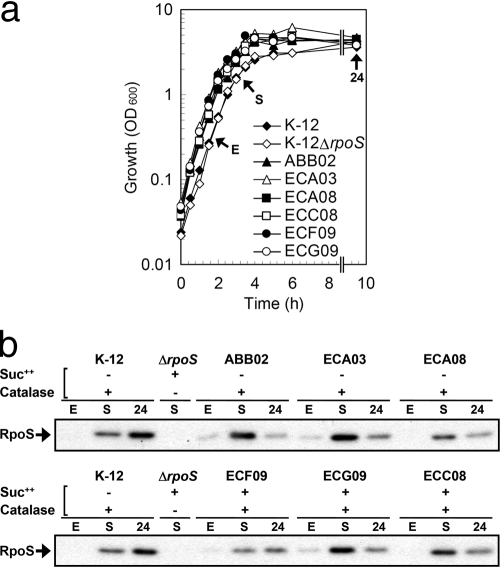
In laboratory E. coli, RpoS protein levels increase during entry into stationary phase (38). For environmental isolates, RpoS expression may be altered through phenotypic diversification and environmental selection. To examine if RpoS in environmental isolates has stationary-phase expression similar to that of laboratory strains, we performed immunoblots on six isolates at exponential phase, early stationary phase, and 24 h after subculture. These isolates were three isolates with a functional RpoS phenotype (ABB02, ECA03, and ECA08) and three isolates with the rpoS+, Suc++ phenotype (ECF09, ECG09, and ECC08). All isolates grew well in rich media (Fig. 1a).
Fig. 1.
(a) Growth of environmental isolates in rich media. ABB02 from beach sand, ECA03 from beach water, and ECA08 from beach water have RpoS-positive catalase and succinate growth phenotypes. ECF09 from untreated combined sewer overflow sewage, ECG09 from beach water, and ECC08 from beach water have RpoS-negative, Suc++ phenotypes but are catalase positive. Samplings are indicated as exponential phase (E), early stationary phase (S), and 24 h postinoculation (24). (b) Immunoblot detection of RpoS protein levels in environmental E. coli isolates.
Like the control E. coli K-12 strain, RpoS expression was stationary phase dependent in all environmental E. coli isolates tested (Fig. 1b). The expression of RpoS from early stationary phase to 24 h postinoculation decreased in environmental isolates, although the overall expression of RpoS was isolate dependent. ABB02, ECA03, and ECG09 had more RpoS protein at early stationary phase than the other three isolates. Importantly, RpoS expression was not decreased in rpoS+, Suc++ isolates.
RpoS-dependent protein expression.
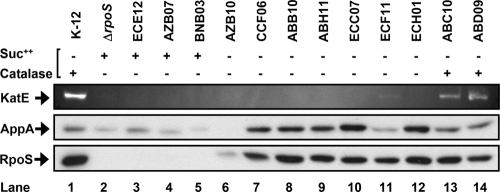
The RpoS sigma factor regulates a large portion of the E. coli genome (44, 64, 77). To examine the effect of RpoS on regulon genes in environmental E. coli, the protein levels from two RpoS-dependent genes, katE (68) and appA (45), were determined (Fig. 2).
Fig. 2.
RpoS-dependent KatE and AppA levels compared to those of RpoS in catalase-deficient environmental E. coli isolates in stationary phase. The upper gel shows 5 μg of protein run on nature PAGE and stained for catalase. The middle and lower panels show 4 μg of protein run on SDS-PAGE, and AppA and RpoS proteins were detected by immunoblotting. Isolates ECE12, AZB07, and BNB03 are confirmed RpoS mutants. Isolates ABC10 and ABD09 are catalase positive.
For confirmed RpoS mutants ECE12, AZB07, and BNB03, RpoS protein could not be detected by immunoblotting (Fig. 2, lanes 3 to 5). KatE expression also was low, comparable to that of the K-12ΔrpoS strain. For all other isolates tested, RpoS protein levels were similar, with the exception of lower RpoS levels in isolate AZB10 from beach sand (Fig. 2, lane 6). Isolate ECF11 from bird fecal droppings had minimal KatE expression (Fig. 2, lane 11), and for the rpoS+, catalase-deficient isolates, KatE expression was too low for detection, even though RpoS was present (Fig. 2, lanes 7 to 10 and 12). As rpoS+ environmental isolate controls, isolates ABC10 and ABD09 from animal feces and untreated sewage, respectively, expressed both RpoS and KatE similarly to K-12 (Fig. 2, lanes 13 and 14).
Despite the presence of RpoS as determined by immunoblotting, KatE expression is low in several isolates, which may be the result of impaired RpoS activity. To determine if RpoS activity is impaired, the levels of a second RpoS-dependent protein, AppA (45), were examined and compared to the levels of KatE and RpoS. As expected, the RpoS mutant isolates had lower AppA expression. The rpoS+, catalase-deficient isolates had greater AppA expression than K-12, with the exceptions of AZB10 and ECF11. AZB10, with lower overall RpoS expression, had undetectable levels of AppA (Fig. 2, lane 6), and ECF11, despite the presence of RpoS, had lower AppA levels (Fig. 2, lane 11).
Selection of RpoS mutants from environmental E. coli on succinate.
In this study, we identified six null RpoS mutants in 2,040 environmental isolates (0.3% among isolates). Using laboratory and pathogenic E. coli, we have previously reported that RpoS mutants are readily selected on poor carbon sources (13, 17). To determine if RpoS in environmental E. coli isolates can be similarly subject to environmental selection, we plated three rpoS+ environmental isolates (ECA01, ECD03, and ECF01) on succinate minimal media.
After 2 to 3 days of incubation at 37°C on succinate minimal media, larger colonies (Suc++) were visible at a frequency of approximately 10−8, as seen with laboratory and pathogenic strains (13, 17). One independent mutant from each isolate was tested and confirmed to be an RpoS mutant by sequencing the rpoS ORF. Single null mutations were identified within the rpoS ORF of each succinate-selected mutant (Table 4).
Table 4.
Mutations within the rpoS gene of succinate-selected mutants of environmental isolates
| Succinate mutant | Selection source | Type of mutation | Effect of mutation on amino acid sequence | Location (amino acids) | rpoS accession no. |
|---|---|---|---|---|---|
| ECA01 Suc++ | ECA01 | 96-bp deletion | 32-amino-acid deletion | 54–85 | JN191290 |
| ECD03 Suc++ | ECD03 | G→A transition | TGG→TAG (amber stop codon) | 148 | JN191291 |
| ECF01 Suc++ | ECF01 | Adenosine insertion | Frameshift, short protein | 32 | JN191292 |
Characterization of RpoS mutants.
For environmental E. coli outside a host, rpoS mutations may be selected under adverse conditions and lead to phenotypic diversity. The loss of RpoS increases the ability of E. coli to grow on nonpreferred carbon sources (13, 17, 23) and concomitantly decreases survival in stress (24, 61, 76). Therefore, to examine the RpoS mutant phenotype of environmental E. coli populations, naturally isolated RpoS mutant AZB07 and succinate-selected RpoS mutant ECA01Suc++ were tested for their abilities to grow on poor carbon sources as well as for their resistance to acidic pH and to oxidative stress.
For growth on the poor carbon sources succinate and fumarate, RpoS mutants had lower generation times than the K-12 control and ECA01Suc++ isogenic parental strain, ECA01 (Table 5). More specifically, RpoS mutants had approximately 18× faster growth on succinate and 6× faster growth on fumarate. As seen in other studies (13, 17), the difference in generation time was not apparent for growth with the preferred carbon source, glucose.
Table 5.
Growth of environmental E. coli RpoS mutants on glucose or on the poor carbon source succinate or fumaratea
| Substrate | Generation time (min) of strain: |
||||
|---|---|---|---|---|---|
| K-12 | K-12ΔrpoS | ECA01 | ECA01Suc++ | AZB07 | |
| Glucose | 93.0 ± 2.1 | 85.1 ± 3.5 | 79.5 ± 3.8 | 78.1 ± 8.8 | 76.3 ± 1.0 |
| Succinate | 1,347.1 ± 62.9 | 141.9 ± 5.1 | 2,403.0 ± 105.2 | 90.8 ± 5.9 | 75.4 ± 2.7 |
| Fumarate | 832.1 ± 100.2 | 129.6 ± 13.3 | 537.4 ± 68.5 | 81.1 ± 7.5 | 118.9 ± 1.2 |
ECA01Suc++ is a succinate-selected RpoS mutant from ECA01, and AZB07 is an RpoS mutant isolated from beach sand. Values are means ± standard errors of the means, where n = 3.
For stress resistance, RpoS mutants were less resistant to both acidic pH (Fig. 3a) and exposure to H2O2 (Fig. 3b). Functional RpoS was essential for cells to withstand low pH, as less than 0.0001% of RpoS mutant cells were able to retain viability. Interestingly, while the K-12 control had just 31% survival after 2 h at pH 2.5, ECA01 showed an increase in numbers of CFU/ml, which suggests a strong resistance in this environmental isolate to acidic conditions. During exposure to H2O2, RpoS mutants lost viability more rapidly than RpoS-positive cells. These results are in agreement with previous research using laboratory E. coli strains (31, 70).
Fig. 3.
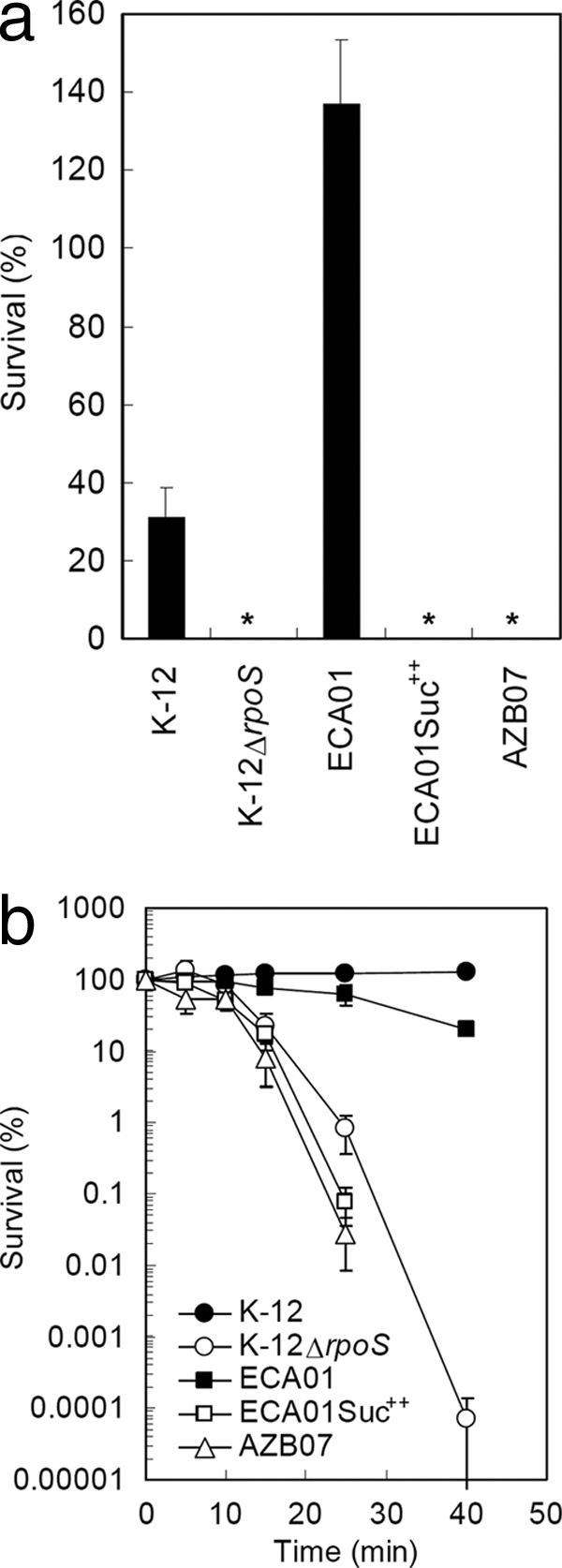
(a) Survival of environmental isolates in acidic conditions. LB medium (pH 2.5) was inoculated with ∼105 cells and incubated at 37°C with shaking for 2 h. Percent survival was calculated as (final CFU/ml)/(initial CFU/ml) × 100, and values of <0.0001% are indicated with an asterisk. ECA01Suc++ is a succinate-selected RpoS mutant from ECA01, and AZB07 is an RpoS mutant isolated from beach sand. The experiment was done in triplicate, and values represent means ± standard errors of the means. (b) Survival of environmental isolates in oxidative stress. LB medium with 15 mM H2O2 was inoculated with ∼107 cells, and the percent survival was calculated over time. At 40 min, ECA01Suc++ and AZB07 had <0.0001% survival. ECA01Suc++ is a succinate-selected RpoS mutant from ECA01, and AZB07 is an RpoS mutant isolated from beach sand. Experiments were done in triplicate, and values represent means ± SEM.
DISCUSSION
Bacterial adaptation depends on complex regulatory systems that may control the differential expression of hundreds of genes. Although such adaptation has been extensively examined in laboratory strains over the last two decades, the role of adaptation in natural E. coli isolates has received little attention. In this study, we examined the RpoS regulon as a model of phenotypic diversity among a large collection of environmental E. coli isolates. This represents the first broad survey of RpoS activity in E. coli isolates collected from the environment. These strains underwent minimal laboratory handling prior to testing and storage, thus they represent a unique collection that can be examined for natural phenotypic diversity. RpoS loss-of-function mutants previously have been identified in several contexts, including laboratory (2, 36, 75) and pathogenic (17) E. coli strains and during long-term culture (25, 61, 83) and growth on poor carbon sources (13), suggesting that such mutants are selected for under some circumstances. Because diverse environmental conditions where E. coli can be found may mimic these conditions, we wished to determine the frequency of RpoS mutants in environmental E. coli isolates.
Two RpoS-dependent phenotype tests, growth on succinate and catalase activity, were used initially in this study to provide an indirect assessment of RpoS activity, since these can be readily adapted to colony screening. The highest percentage of Suc++ isolates and of catalase-negative isolates were found in human fecal sources, which may indicate a favorable selective environment. By screening for these phenotypes among the 2,040 environmental isolates, and the subsequent sequencing of the rpoS gene in candidate mutant strains, six isolates were identified to carry deleterious rpoS mutations. These six isolates, as well as one rpoS+ isolate (EKF07, from beach water), had a consistent RpoS mutant phenotype for both succinate growth and catalase activity. The beach water isolate (EKF07) may have been attenuated by a nonsynonymous mutation at codon 124, where tyrosine (TAC) replaced asparagine (AAC). Considering the spontaneous mutation rate of 5.4 × 10−10 mutations per base pair per replication in E. coli (19), the relatively high abundance of RpoS mutants among environmental E. coli isolates (0.3%) suggests that certain natural environments favor the selective growth of RpoS loss-of-function mutants.
Thirty-one natural isolates from the ECOR collection, a genotypically diverse set of natural E. coli strains from animal and human hosts (63), recently was examined for RpoS expression (25). Interestingly, RpoS mutants were found at a substantially higher frequency (0.29) among the ECOR collection compared to that among the environmental isolates studied here (0.003). Because the ECOR collection was compiled largely based on genotypic diversity (63), the sequence variability of the rpoS gene in these strains may not be surprising. ECOR strains with elevated RpoS expression also accumulate rpoS mutations under nutrient limitation (25). The long-term storage of the ECOR strains, as well as the diverse hosts from which these strains were isolated, may have contributed to a higher observed frequency of RpoS mutants in the ECOR collection than in the environmental isolates examined in this study.
Of the RpoS mutants identified among the environmental isolates, three RpoS mutants (BNB03, BNB04, and BNB07) taken from the same beach sand sample possessed identical rpoS mutations. These beach sand isolates may have resulted from clonal growth in a natural environment, as the multiplication of E. coli in sediment has previously been shown to reach a high cell density of 105 CFU/g nonsterile soil (34). Alternatively, clones from the same fecal deposit may have persisted in the soil. One naturally isolated RpoS mutant (AZB07) from beach sand also was identified to carry the same G→A transition, resulting in an amber stop codon, as a later succinate-selected RpoS mutant (ECD03 Suc++). The selection of this G→A transition in both AZB07 and ECD03 Suc++ is not surprising, given that G/C-to-A/T transitions in bacteria are more common than A/T to G/C (33, 50). The same rpoS mutation in the naturally isolated and the succinate-selected RpoS mutant strains also suggests that growth on succinate can select for mutations that mimic mutations that are naturally selected. Finally, to our knowledge, the presence of a transposon element within the rpoS gene rendering a nonfunctional RpoS protein, as was found in isolate ASC02 from sewage treatment plant final effluent, has not been reported previously for environmental E. coli strains. Unfortunately, using the six RpoS mutants identified, it is not possible to determine a common stimulus that selects for RpoS mutants, as the isolates came from several independent sources.
The rpoS-mutS region of E. coli strains is hypervariable (32) and undergoes frequent recombination (9, 15). The rpoS gene is polymorphic in E. coli (6, 27), as well as in other gammaproteobacteria (39). Despite the reported high sequence variability of the rpoS gene, we found that the frequency of synonymous mutations in the fliA gene, encoding another nonessential sigma factor, was comparable to that of the rpoS gene. At nucleotide position 519 of fliA, all sequenced environmental isolates carried guanosine instead of adenosine (found in the fliA K-12 strain), strongly suggesting that guanosine at this position is ancestral. Isolate ABB10 from untreated sewage, which carried a Ser→Asn mutation at codon 176, also had decreased motility, which suggests that this site is functionally important for motility. The high and comparable number of mutations identified in rpoS and fliA is consistent with the theory that many of these mutations are neutral under natural selection (40). Studies on codon bias, a determinant of synonymous mutation rates in enterobacteria (69), and its effect on selection for rpoS mutations in environmental E. coli would be of future interest. Putatively, synonymous mutations in rpoS accumulate under low environmental selection, while under strong selection, such as succinate minimal media, single rpoS null mutations become fixed in E. coli populations.
As noted above, RpoS regulates the expression of the catalase gene, katE (67), and using the catalase test (see Materials and Methods), environmental isolates were screened for RpoS activity. A total of 38 isolates showed reduced bubbling, indicating lower catalase expression, yet only six of these were RpoS mutants. Previous studies have found that mutations in the rpoS allele result in intermediate bubbling (84). In two rpoS+, catalase-negative isolates, ECH01 and EKF07, threonine is replaced with serine (amino acid 198) and asparagine is replaced with tyrosine (amino acid 124), respectively (see Table S2 in the supplemental material), which indicates the functional importance of these amino acids at these sites. However, the remaining rpoS+, catalase-negative isolates possessed only synonymous rpoS mutations. Environmental E. coli strains may have adaptive modifications in the expression of regulon genes that are independent of those found in RpoS. RpoS regulon composition, compared between species, is diverse and largely species specific (14, 66), but the extent of RpoS regulon plasticity between strains of the same species is not known. By examining the expression of a second RpoS-dependent protein, AppA (45), and the expression of RpoS in several rpoS+, catalase-negative isolates, it was determined that only one isolate (AZB10 from beach sand; Fig. 2, lane 6) had reduced RpoS expression, resulting in lower regulon expression. The remaining rpoS+, catalase-negative isolates exhibited little to no KatE expression, although RpoS and RpoS-dependent AppA were present (Fig. 2, lanes 7 to 12). This indicates that the expression of even prototypical RpoS regulon members is strongly influenced by factors in environmental E. coli isolates. Mutations to RpoS posttranslational factors, such as to Crl (8), also may contribute to differential regulon expression. Interestingly, the AppA expression of rpoS+, catalase-negative isolates, with the exception of isolate AZB10 from beach sand and isolate ECF11 from bird fecal droppings, had higher AppA levels than the laboratory control (Fig. 2, lanes 7 to 10 and 12). A decrease in the expression of one RpoS regulon member may allow for more transcriptional recruitment of RpoS to other dependent promoters. Additional work on RpoS regulon expression is needed to validate this possibility.
Mutations in rpoS can be selected from laboratory and pathogenic E. coli strains grown with succinate as the sole carbon source (13, 17). In this study, we show that rpoS mutants also can be readily selected from environmental E. coli when grown on succinate (Table 4) and have enhanced growth on poor carbon sources (Table 5). Because RpoS mutants were found in 0.3% of isolates, there likely are natural environments that also favor RpoS mutant growth. Of course, it is not clear from this study when rpoS mutations accrue, during passage through the gut or after deposition in the environment, and what natural environments select for RpoS mutants. E. coli can adapt to carbon source and oxygen availability through changes in metabolism (48), and the loss of RpoS previously has been shown to induce the expression of TCA cycle genes (64). RpoS mutants may have an adaptive advantage during nutrient-limited growth in the natural environment as well as in some host environments, such as in the urinary tract, where the TCA cycle is required for infection in vivo (1). On the other hand, fermentation in the gut and the production of short-chain fatty acids, such as acetate, proprionate, and butyrate (16, 53), may select for isolates with functional RpoS protein, as RpoS is important in acid resistance (Fig. 3b). The environmental isolates used in this study exhibit differential abilities to utilize carbon sources, as growth on a range of TCA cycle intermediates or on weak acids present in the gut is strain dependent (data not shown). Metabolic demands on E. coli in the host (1, 12, 59) and in the environment may be important determinants of RpoS selection. Further study is required to identify natural conditions that select for rpoS mutations and diverse metabolic capabilities of environmental E. coli.
In conclusion, RpoS is an alternative sigma factor that is known to be subject to environmental selection (13, 41). As such, mutations in the rpoS gene lead to phenotypic diversity in environmental E. coli isolates. Previous work has focused on rpoS mutations in laboratory and pathogenic E. coli (13, 17), yet the diverse conditions that environmental E. coli experience may have high selective pressures. Indeed, environmental RpoS mutants were determined to exist at a frequency as high as 0.003, and RpoS mutants from environmental E. coli isolates could be selected when grown on a poor carbon source. The natural selection of RpoS mutants outside a host may, therefore, be an important determinant in environmental E. coli adaptation and survival.
Supplementary Material
ACKNOWLEDGMENTS
We thank Stephen Hill for his help with retrieving the 2,040 E. coli isolates and Vithooshan Vijayakumaran for immunoblot analysis of ASC02.
This study was supported by research grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) to H.E.S. S.M.C. was supported by an Ontario Graduate Scholarship.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 23 September 2011.
REFERENCES
- 1. Alteri C. J., Smith S. N., Mobley H. L. 2009. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 5:e1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atlung T., Nielsen H. V., Hansen F. G. 2002. Characterisation of the allelic variation in the rpoS gene in thirteen K12 and six other non-pathogenic Escherichia coli strains. Mol. Genet. Genomics 266:873–881 [DOI] [PubMed] [Google Scholar]
- 3. Benson D. A., Karsch-Mizrachi I., Lipman D. J., Ostell J., Sayers E. W. 2011. GenBank. Nucleic Acids Res. 39:D32–D37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergholz P. W., Noar J. D., Buckley D. H. 2011. Environmental patterns are imposed on the population structure of Escherichia coli after fecal deposition. Appl. Environ. Microbiol. 77:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beversdorf L. J., Bornstein-Forst S. M., McLellan S. L. 2007. The potential for beach sand to serve as a reservoir for Escherichia coli and the physical influences on cell die-off. J. Appl. Microbiol. 102:1372–1381 [DOI] [PubMed] [Google Scholar]
- 6. Bhagwat A. A., et al. 2006. Functional heterogeneity of RpoS in stress tolerance of enterohemorrhagic Escherichia coli strains. Appl. Environ. Microbiol. 72:4978–4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjedov I., et al. 2003. Stress-induced mutagenesis in bacteria. Science 300:1404–1409 [DOI] [PubMed] [Google Scholar]
- 8. Bougdour A., Lelong C., Geiselmann J. 2004. Crl, a low temperature-induced protein in Escherichia coli that binds directly to the stationary phase sigma subunit of RNA polymerase. J. Biol. Chem. 279:19540–19550 [DOI] [PubMed] [Google Scholar]
- 9. Brown E. W., LeClerc J. E., Li B., Payne W. L., Cebula T. A. 2001. Phylogenetic evidence for horizontal transfer of mutS alleles among naturally occurring Escherichia coli strains. J. Bacteriol. 183:1631–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Byappanahalli M., Fowler M., Shively D., Whitman R. 2003. Ubiquity and persistence of Escherichia coli in a Midwestern coastal stream. Appl. Environ. Microbiol. 69:4549–4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byappanahalli M. N., et al. 2006. Seasonal persistence and population characteristics of Escherichia coli and enterococci in deep backshore sand of two freshwater beaches. J. Water Health 4:313–320 [DOI] [PubMed] [Google Scholar]
- 12. Chang D. E., et al. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. U. S. A. 101:7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen G., Patten C. L., Schellhorn H. E. 2004. Positive selection for loss of RpoS function in Escherichia coli. Mutat. Res. 554:193–203 [DOI] [PubMed] [Google Scholar]
- 14. Chiang S. M., Schellhorn H. E. 2010. Evolution of the RpoS regulon: origin of RpoS and the conservation of RpoS-dependent regulation in bacteria. J. Mol. Evol. 70:557–571 [DOI] [PubMed] [Google Scholar]
- 15. Culham D. E., Wood J. M. 2000. An Escherichia coli reference collection group B2- and uropathogen-associated polymorphism in the rpoS-mutS region of the E. coli chromosome. J. Bacteriol. 182:6272–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. 1987. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong T., Chiang S. M., Joyce C., Yu R., Schellhorn H. E. 2009. Polymorphism and selection of rpoS in pathogenic Escherichia coli. BMC Microbiol. 9:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong T., Coombes B. K., Schellhorn H. E. 2009. Role of RpoS in the virulence of Citrobacter rodentium. Infect. Immun. 77:501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drake J. W., Charlesworth B., Charlesworth D., Crow J. F. 1998. Rates of spontaneous mutation. Genetics 148:1667–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edge T. A., Hill S. 2007. Multiple lines of evidence to identify the sources of fecal pollution at a freshwater beach in Hamilton Harbour, Lake Ontario. Water Res. 41:3585–3594 [DOI] [PubMed] [Google Scholar]
- 21. Farrell M. J., Finkel S. E. 2003. The growth advantage in stationary-phase phenotype conferred by rpoS mutations is dependent on the pH and nutrient environment. J. Bacteriol. 185:7044–7052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferenci T. 2003. What is driving the acquisition of mutS and rpoS polymorphisms in Escherichia coli? Trends Microbiol. 11:457–461 [DOI] [PubMed] [Google Scholar]
- 23. Ferenci T. 2005. Maintaining a healthy SPANC balance through regulatory and mutational adaptation. Mol. Microbiol. 57:1–8 [DOI] [PubMed] [Google Scholar]
- 24. Ferenci T. 2008. The spread of a beneficial mutation in experimental bacterial populations: the influence of the environment and genotype on the fixation of rpoS mutations. Heredity 100:446–452 [DOI] [PubMed] [Google Scholar]
- 25. Ferenci T., Galbiati H. F., Betteridge T., Phan K., Spira B. 2011. The constancy of global regulation across a species: the concentrations of ppGpp and RpoS are strain-specific in Escherichia coli. BMC Microbiol. 11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferenci T., Spira B. 2007. Variation in stress responses within a bacterial species and the indirect costs of stress resistance. Ann. N. Y. Acad. Sci. 1113:105–113 [DOI] [PubMed] [Google Scholar]
- 27. Ferreira A., Rendano L., Wiedmann M., Boor K. J. 1999. Characterization of rpoS alleles in Escherichia coli O157:H7 and in other E. coli serotypes. J. Appl. Microbiol. 86:295–301 [DOI] [PubMed] [Google Scholar]
- 28. Finkel S. E., Kolter R. 1999. Evolution of microbial diversity during prolonged starvation. Proc. Natl. Acad. Sci. U. S. A. 96:4023–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galhardo R. S., Hastings P. J., Rosenberg S. M. 2007. Mutation as a stress response and the regulation of evolvability. Crit. Rev. Biochem. Mol. Biol. 42:399–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gregory E. M., Fridovich I. 1974. Visualization of catalase on acrylamide gels. Anal. Biochem. 58:57–62 [DOI] [PubMed] [Google Scholar]
- 31. Hengge-Aronis R., Lange R., Henneberg N., Fischer D. 1993. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J. Bacteriol. 175:259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herbelin C. J., Chirillo S. C., Melnick K. A., Whittam T. S. 2000. Gene conservation and loss in the mutS-rpoS genomic region of pathogenic Escherichia coli. J. Bacteriol. 182:5381–5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hershberg R., Petrov D. A. 2010. Evidence that mutation is universally biased towards AT in bacteria. PLoS Genet. 6:e1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ishii S., Ksoll W. B., Hicks R. E., Sadowsky M. J. 2006. Presence and growth of naturalized Escherichia coli in temperate soils from Lake Superior watersheds. Appl. Environ. Microbiol. 72:612–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ito Y., Toyota H., Kaneko K., Yomo T. 2009. How selection affects phenotypic fluctuation. Mol. Syst. Biol. 5:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ivanova A., Renshaw M., Guntaka R. V., Eisenstark A. 1992. DNA base sequence variability in katF (putative sigma factor) gene of Escherichia coli. Nucleic Acids Res. 20:5479–5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jessup C. M., Bohannan B. J. 2008. The shape of an ecological trade-off varies with environment. Ecol. Lett. 11:947–959 [DOI] [PubMed] [Google Scholar]
- 38. Jishage M., Iwata A., Ueda S., Ishihama A. 1996. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J. Bacteriol. 178:5447–5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jordan S. J., Dodd C. E., Stewart G. S. 1999. Use of single-strand conformation polymorphism analysis to examine the variability of the rpoS sequence in environmental isolates of salmonellae. Appl. Environ. Microbiol. 65:3582–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kimura M. 1968. Evolutionary rate at the molecular level. Nature 217:624–626 [DOI] [PubMed] [Google Scholar]
- 41. King T., Ishihama A., Kori A., Ferenci T. 2004. A regulatory trade-off as a source of strain variation in the species Escherichia coli. J. Bacteriol. 186:5614–5620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. King T., Seeto S., Ferenci T. 2006. Genotype-by-environment interactions influencing the emergence of rpoS mutations in Escherichia coli populations. Genetics 172:2071–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kleckner N. 1981. Transposable elements in prokaryotes. Annu. Rev. Genet. 15:341–404 [DOI] [PubMed] [Google Scholar]
- 44. Lacour S., Landini P. 2004. SigmaS-dependent gene expression at the onset of stationary phase in Escherichia coli: function of sigmaS-dependent genes and identification of their promoter sequences. J. Bacteriol. 186:7186–7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lange R., Hengge-Aronis R. 1991. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 5:49–59 [DOI] [PubMed] [Google Scholar]
- 46. Larkin M. A., et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 47. Layton J. C., Foster P. L. 2003. Error-prone DNA polymerase IV is controlled by the stress-response sigma factor, RpoS, in Escherichia coli. Mol. Microbiol. 50:549–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Le Gac M., et al. 2008. Metabolic changes associated with adaptive diversification in Escherichia coli. Genetics 178:1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Levert M., et al. 2010. Molecular and evolutionary bases of within-patient genotypic and phenotypic diversity in Escherichia coli extraintestinal infections. PLoS Pathog. 6:e1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lind P. A., Andersson D. I. 2008. Whole-genome mutational biases in bacteria. Proc. Natl. Acad. Sci. U. S. A. 105:17878–17883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Loewen P. C., Hu B., Strutinsky J., Sparling R. 1998. Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 44:707–717 [DOI] [PubMed] [Google Scholar]
- 52. Lombardo M. J., Aponyi I., Rosenberg S. M. 2004. General stress response regulator RpoS in adaptive mutation and amplification in Escherichia coli. Genetics 166:669–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Macfarlane S., Macfarlane G. T. 2003. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 62:67–72 [DOI] [PubMed] [Google Scholar]
- 54. Maharjan R., Seeto S., Notley-McRobb L., Ferenci T. 2006. Clonal adaptive radiation in a constant environment. Science 313:514–517 [DOI] [PubMed] [Google Scholar]
- 55. Martinez-Garcia E., Tormo A., Navarro-Llorens J. M. 2003. Polymorphism in the yclC-rpoS region of enterobacteria. Curr. Microbiol. 46:365–370 [DOI] [PubMed] [Google Scholar]
- 56. McLellan S. L. 2004. Genetic diversity of Escherichia coli isolated from urban rivers and beach water. Appl. Environ. Microbiol. 70:4658–4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McLellan S. L., Salmore A. K. 2003. Evidence for localized bacterial loading as the cause of chronic beach closings in a freshwater marina. Water Res. 37:2700–2708 [DOI] [PubMed] [Google Scholar]
- 58. Miller J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, NY [Google Scholar]
- 59. Miranda R. L., et al. 2004. Glycolytic and gluconeogenic growth of Escherichia coli O157:H7 (EDL933) and E. coli K-12 (MG1655) in the mouse intestine. Infect. Immun. 72:1666–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nevers M. B., Whitman R. L., Frick W. E., Ge Z. 2007. Interaction and influence of two creeks on Escherichia coli concentrations of nearby beaches: exploration of predictability and mechanisms. J. Environ. Qual. 36:1338–1345 [DOI] [PubMed] [Google Scholar]
- 61. Notley-McRobb L., King T., Ferenci T. 2002. rpoS mutations and loss of general stress resistance in Escherichia coli populations as a consequence of conflict between competing stress responses. J. Bacteriol. 184:806–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nyström T. 2004. Growth versus maintenance: a trade-off dictated by RNA polymerase availability and sigma factor competition? Mol. Microbiol. 54:855–862 [DOI] [PubMed] [Google Scholar]
- 63. Ochman H., Selander R. K. 1984. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 157:690–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Patten C. L., Kirchhof M. G., Schertzberg M. R., Morton R. A., Schellhorn H. E. 2004. Microarray analysis of RpoS-mediated gene expression in Escherichia coli K-12. Mol. Genet. Genomics 272:580–591 [DOI] [PubMed] [Google Scholar]
- 65. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 66. Santos-Zavaleta A., Gama-Castro S., Perez-Rueda E. 2011. A comparative genome analysis of the RpoS-sigmulon shows a high diversity of responses and origins. Microbiology 157:1393–1401 [DOI] [PubMed] [Google Scholar]
- 67. Schellhorn H. E., Hassan H. M. 1988. Transcriptional regulation of katE in Escherichia coli K-12. J. Bacteriol. 170:4286–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schellhorn H. E., Stones V. L. 1992. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J. Bacteriol. 174:4769–4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sharp P. M., Li W. H. 1987. The rate of synonymous substitution in enterobacterial genes is inversely related to codon usage bias. Mol. Biol. Evol. 4:222–230 [DOI] [PubMed] [Google Scholar]
- 70. Small P., Blankenhorn D., Welty D., Zinser E., Slonczewski J. L. 1994. Acid and base resistance in Escherichia coli and Shigella flexneri: role of rpoS and growth pH. J. Bacteriol. 176:1729–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stoebel D. M., Hokamp K., Last M. S., Dorman C. J. 2009. Compensatory evolution of gene regulation in response to stress by Escherichia coli lacking RpoS. PLoS Genet. 5:e1000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Subbarayan P. R., Sarkar M. 2004. A comparative study of variation in codon 33 of the rpoS gene in Escherichia coli K12 stocks: implications for the synthesis of sigma(s). Mol. Genet. Genomics 270:533–538 [DOI] [PubMed] [Google Scholar]
- 73. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 74. Tyerman J., Havard N., Saxer G., Travisano M., Doebeli M. 2005. Unparallel diversification in bacterial microcosms. Proc. Biol. Sci. 272:1393–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Visick J. E., Clarke S. 1997. RpoS- and OxyR-independent induction of HPI catalase at stationary phase in Escherichia coli and identification of rpoS mutations in common laboratory strains. J. Bacteriol. 179:4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Waterman S. R., Small P. L. 1996. Characterization of the acid resistance phenotype and rpoS alleles of shiga-like toxin-producing Escherichia coli. Infect. Immun. 64:2808–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Weber H., Polen T., Heuveling J., Wendisch V. F., Hengge R. 2005. Genome-wide analysis of the general stress response network in Escherichia coli: sigmaS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 187:1591–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Whitman R. L., Nevers M. B. 2003. Foreshore sand as a source of Escherichia coli in nearshore water of a Lake Michigan beach. Appl. Environ. Microbiol. 69:5555–5562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Whitman R. L., Nevers M. B. 2008. Summer E. coli patterns and responses along 23 Chicago beaches. Environ. Sci. Technol. 42:9217–9224 [DOI] [PubMed] [Google Scholar]
- 80. Whitman R. L., Nevers M. B., Byappanahalli M. N. 2006. Examination of the watershed-wide distribution of Escherichia coli along Southern Lake Michigan: an integrated approach. Appl. Environ. Microbiol. 72:7301–7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Woods R. J., et al. 2011. Second-order selection for evolvability in a large Escherichia coli population. Science 331:1433–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wrande M., Roth J. R., Hughes D. 2008. Accumulation of mutants in “aging” bacterial colonies is due to growth under selection, not stress-induced mutagenesis. Proc. Natl. Acad. Sci. U. S. A. 105:11863–11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zambrano M. M., Kolter R. 1996. GASPing for life in stationary phase. Cell 86:181–184 [DOI] [PubMed] [Google Scholar]
- 84. Zambrano M. M., Siegele D. A., Almiron M., Tormo A., Kolter R. 1993. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science 259:1757–1760 [DOI] [PubMed] [Google Scholar]
- 85. Zinser E. R., Kolter R. 2004. Escherichia coli evolution during stationary phase. Res. Microbiol. 155:328–336 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.