Abstract
The Elecsys hepatitis B surface antigen (HBsAg) II quantitative assay is a new quantitative electrochemiluminescence immunoassay which uses onboard dilution and a simple algorithm to determine HBsAg levels expressed in international units (IU)/ml (standardized against the World Health Organization [WHO] Second International Standard). This study evaluated its performance using routine serum samples from a wide range of HBsAg carriers and patients with chronic hepatitis B (CHB). HBsAg levels were measured in serum samples collected independently by five centers in Europe, Australia, and Asia. Serial dilution analyses were performed to assess the recommended dilution algorithm and determine the assay range free of hook effect. Assay precision was also established. Following assessment of serial dilutions (1:100 to 1:1,000,000) of the 611 samples analyzed, 70.0% and 85.6% of samples tested with analyzers incorporating 1:100 (Elecsys 2010 and cobas e 411) and 1:400 (Modular Analytics E170) onboard dilution, respectively, fell within the linear range of the assay, providing a final result on the first test. No high-dose hook effect was seen up to the maximum HBsAg serum level tested (870,000 IU/ml) using the dilution algorithm. HBsAg levels were reliably determined across all hepatitis B virus (HBV) genotypes, phases of HBV infection, and stages of disease tested. Precision was high across all analyzers (% coefficient of variation [CV], 1.4 to 9.6; HBsAg concentrations, 0.1 to 37,300 IU/ml). The Elecsys HBsAg II quantitative assay accurately and reliably quantifies HBsAg in routine clinical samples. Onboard dilution minimizes retesting and reduces the potential for error.
INTRODUCTION
Hepatitis B surface (HBs) antigen (HBsAg) is the earliest serological indicator of acute hepatitis B virus (HBV) infection and a key diagnostic marker of chronic hepatitis B (CHB) infection when it persists in the serum for more than 6 months (7, 12). HBsAg is found in the serum of patients with CHB, both as a component of the HBV envelope and also as filamentous and spherical noninfectious particles which are present in vast excess over virions. HBsAg levels have been shown to vary during the natural course of chronic infection, with the highest levels being present in the immune tolerance phase, where there are high levels of HBV replication, and with lower levels in the low-replicative phase (4, 10, 18). Although linked, HBsAg production is partially independent of HBV replication, and HBsAg and HBV DNA levels are not directly related (4, 10, 18, 22). Levels of serum HBsAg correlate with the levels of HBV covalently closed circular DNA (cccDNA) in the hepatocytes and therefore can provide an indirect measure of the number of infected cells in the liver, particularly in hepatitis e antigen (HBeAg)-positive patients (22, 23, 24). Data from HBeAg-negative carriers showing that significantly different HBsAg serum levels could be present in spite of comparable cccDNA levels suggest that, at least in this setting, HBsAg serum levels may reflect transcriptionally active cccDNA rather than its absolute amount (2, 4, 24). Clearance of HBsAg during the natural course of CHB is associated with improved long-term clinical outcome, including reduced incidence of cirrhosis and hepatocellular carcinoma and longer survival (7, 12). However, this is an uncommon event, arising in only 1 to 2% of cases per year following several years in which levels of HBV DNA are persistently undetectable (2, 6, 15). Given its association with improved outcome, HBsAg clearance is considered to be the best definition of virological response to treatment and the closest outcome to clinical cure of CHB, making it an important therapeutic goal (7).
A number of studies have shown that the degree and timescale of reductions in HBsAg levels during pegylated interferon (PEG-IFN)-based therapy may predict a sustained response in both HBeAg-positive and HBeAg-negative CHB infection (3, 11, 13, 14, 17, 19) and may also predict the response to treatment with some nucleos(t)ide analogs (NAs) (9, 25). There is also evidence that HBsAg levels, particularly in combination with HBV DNA levels, can be used to identify patients who are in the inactive carrier phase of HBV infection compared with patients with active disease and so distinguish those patients who do not require therapy from those who would benefit from treatment (2, 10, 15). Such data have led to increased interest and research into the potential to individualize CHB therapy based on the measurement of pretreatment and longitudinal on-treatment HBsAg levels. The use of quantitative HBsAg as a clinical tool is therefore increasing, and there is a need for accurate, simple, standardized, and widely available assays to ensure the comparability of findings between laboratories. Although several automated assays have been developed for the qualitative assessment of HBsAg, the Architect HBsAg assay (Abbott Diagnostics, IL) was the only available commercial assay for HBsAg quantification until early 2011. This assay has a linear range of 0.05 to 250 IU/ml. Since most clinical samples have HBsAg levels above this upper limit (4, 10, 18, 22), they require a manual dilution (1:500 recommended; 1:999 should not be exceeded according to the package insert [1]), which is a source of potential error.
The Elecsys HBsAg II quantitative assay is a new in vitro diagnostic electrochemiluminescence immunoassay for the quantitative detection of HBsAg in human serum and plasma, intended for use on Elecsys and cobas e immunoassay analyzers. The assay uses an onboard predilution step and a simple test algorithm to determine HBsAg levels expressed in international units (IU)/ml (standardized against the Second World Health Organization [WHO] International Standard for HBsAg; NIBSC code: 00/588), which allows the majority of samples to be measured without manual predilution, so contributing to the precision of the assay. The aims of the current study were (i) to confirm the proposed dilution algorithm through the assessment of serial dilutions and (ii) to evaluate the performance of the Elecsys HBsAg II quantitative assay in different study centers in Europe, Asia, and Australia using a large number of routine serum samples from HBsAg carriers in different phases of HBV infection and disease and who were infected with a variety of HBV genotypes.
MATERIALS AND METHODS
Samples.
Serum samples from chronic HBsAg carriers and patients with confirmed HBeAg-positive or -negative CHB (HBV DNA and HBsAg positive for >6 months), including follow-up samples from patients with or without antiviral treatment, were included in the analyses. All sera were anonymized, residual, and routine samples collected in a variety of sample tubes according to availability at the various centers. Samples were frozen and stored at −20°C with or without initial storage at 4°C.
Five centers participated in the study: three in Europe (Medizinische Hochschule Hannover, Germany [cobas e 411 analyzer]; University Hospital of Pisa, Italy [cobas e 411 analyzer]; and Roche Diagnostics GmbH, Penzberg, Germany [Elecsys 2010 analyzer]), one in Asia (Siriraj Hospital, Bangkok, Thailand [Modular Analytics E170 analyzer]), and one in Australia (Victorian Infectious Diseases Reference Laboratory, Australia [cobas e 411 analyzer]). All analyses took place between May and August 2010. Where available, data on HBeAg status, genotype, and stage of disease were collected. Phases of disease were classified according to Jaroszewicz et al. (10). Inactive HBsAg carriers were defined as being HBsAg positive, with low or undetectable serum HBV DNA levels and normal aminotransferase levels (7).
Elecsys HBsAg II quantitative assay.
Briefly, the automatic Elecsys HBsAg II quantitative assay procedure is as follows: in the first incubation, two biotinylated monoclonal anti-HBs antibodies and a mixture of monoclonal anti-HBs antibody and polyclonal anti-HBs antibodies labeled with ruthenium form a sandwich complex with HBsAg in the sample. After the addition of streptavidin-coated microparticles, the complex becomes bound to the solid phase via the interaction of biotin and streptavidin. The reaction mixture is aspirated into the measuring cell where the microparticles are magnetically captured onto the surface of the electrode. Application of a voltage to the electrode then induces chemiluminescent emission which is measured by a photomultiplier. Results are determined via a calibration curve which is instrument-specifically generated by 2-point calibration and a master curve provided via the reagent barcode. The calibration method is standardized against the WHO International Standard (NIBSC code 00/588; WHO Second International Standard for HBsAg, subtype adw2, genotype A; IU/ml). The total assay duration is 18 min.
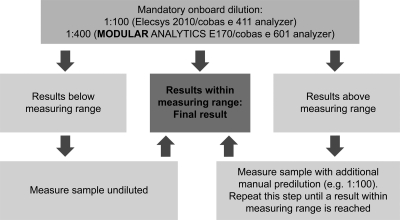
The mandatory dilution algorithm of the assay, as recommended by the manufacturer, is as follows (Fig. 1): an initial measurement of a 1:100 (for the Elecsys 2010 and cobas e 411) or 1:400 (for the Modular Analytics E170, cobas e 601, or cobas e 602) prediluted sample (performed automatically by the analyzer) is carried out. For samples with a result within the range of 5 to 13,000 IU/ml for the 100-fold-diluted sample or 20 to 52,000 IU/ml for the 400-fold-diluted sample, no further dilution is performed and the result is the final level. For samples with results below the ranges described above, samples are reanalyzed undiluted (i.e., without onboard dilution). If results are higher than the above range, a further manual dilution step is performed prior to reanalyzing the sample using onboard dilution. Further dilutions are performed if required until results are found within the measuring range according to the instrument used. The diluent used (Elecsys Diluent HepB, Roche Diagnostics) is based on human serum negative for HBsAg and anti-HBs with added preservatives. The same diluent was used by all centers.
Fig. 1.
Test algorithm for the Elecsys HBsAg II quantitative assay.
To evaluate this dilution procedure, a series of manual dilutions were performed for each sample in this study. As the assay is intended to be used on instruments equipped with an onboard dilution of 1:100 (Elecsys 2010 and cobas e 411) or equipped with an onboard dilution of 1:400 (Modular Analytics E170, cobas e 601, or cobas e 602), these predilutions were used as the first steps of the sample dilution series. The dilution series therefore consisted of eight different manual dilution steps (1:100, 1:400, 1:1,000, 1:4,000, 1:10,000, 1:40,000, 1:100,000, and 1:1,000,000).
Determination of HBsAg levels free of high-dose hook effect.
Serial dilution of serum samples was performed to confirm that the Elecsys HBsAg II quantitative assay showed linear dilution behavior and was unaffected by any prozone phenomenon or high-dose hook effect. Altogether, eight different manual dilution steps (1:100, 1:400, 1:1,000, 1:4,000, 1:10,000, 1:40,000, 1:100,000, and 1:1,000,000) in addition to the undiluted sample were analyzed. The presence or absence of a hook effect was determined by assessment of the mean deviation between the final HBsAg level and the level obtained by subsequent (or previous) dilution steps.
Comparator assay: Abbott Architect HBsAg.
The Abbott Architect HBsAg assay was chosen as the comparator assay, as it was the only commercially available quantitative HBsAg assay. The assay was performed according to the manufacturer's instructions (1).
Data analysis.
The respective dilution factor was used to calculate the final HBsAg level from the obtained measurement values of samples. The lowest dilution step of a sample resulting in a value within the measuring range was used for calculating the final HBsAg level of the sample. For statistical analyses, EXCEL routines were used for the calculation of mean values, the standard deviation, and the coefficient of variation (CV). Correlation data were calculated using WinMC.
RESULTS
A total of 611 serum samples from 345 patients were included in the analysis. The overall genotype distribution of the patients was as follows: genotype A, n = 32; genotype B, n = 26; genotype C, n = 49; genotype D, n = 56; genotype E, n = 6; genotype F, n = 1; genotype G, n = 1; and not determined, n = 174 (Table 1). In all, 148 samples were HBeAg positive and 276 samples were HBeAg negative; the HBeAg status was unknown for 187 samples (including all 184 samples from the Penzberg site).
Table 1.
Number of samples and patient characteristics by site
| Center | No. of samples (no. of patients) | Ethnicity | Genotype (no. of patients) | Patient statusa | Analyses (reference) |
|---|---|---|---|---|---|
| Bangkok | 108 (25) | Asian | B (1); C (24) | Receiving IFN-based treatment | Overall |
| Pisa | 128 (25) | Mediterranean | A (2); D (23) | Receiving IFN-based or NA treatment | Overall, genotype, disease stage (4) |
| Hannover | 100 (20) | Central European | A (5); D (12); not available (3) | Receiving IFN-based treatment | Overall, genotype, disease stage (10) |
| Melbourne | 91 (91) | 53% Asian, 34% Caucasian, 13% Middle Eastern | A (22), B (24), C (24), D (20), G (1) | 88% pretreatment, 4% receiving NAs, 8% status unknown | Overall, genotype |
| Penzberg | 184 (184) | Not available | A (3), B (1), C (1), D (1), E (6), F (1), not available (171) | Untreated | Overall |
IFN, interferon; NA, nucleos(t)ide analog.
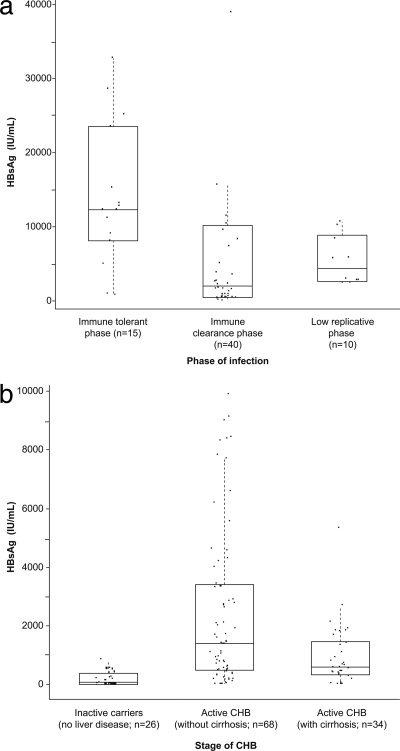
HBV infection phase and disease stage data were available from two sites. The Hannover subcohort included samples from both HBeAg-positive and HBeAg-negative CHB patients, including from the immune tolerant phase (n = 15), immune clearance phase (n = 40), and low-replicative phase (n = 10). The Pisa subcohort included only HBeAg-negative HBsAg carriers: HBeAg-negative CHB patients without (n = 68) or with (n = 34) cirrhosis and inactive carriers (n = 26) without liver disease.
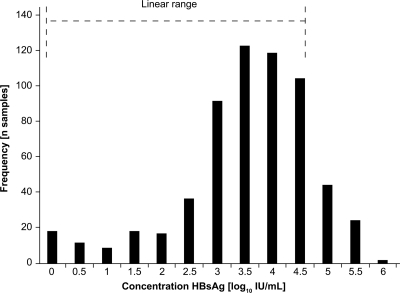
The overall distribution in HBsAg concentrations (log10 IU/ml) is shown in Fig. 2. The median HBsAg concentration in the 611 samples was 2,763 IU/ml (range, 0.11 to 873,300 IU/ml). Median HBsAg levels were highest in the immune tolerant phase (12,290; range, 729 to 32,776 IU/ml) compared with the immune clearance phase (1,973; range, 1.31 to 450,800 IU/ml) and low-replicative phase (4,318; range, 2,412 to 10,650 IU/ml) (Fig. 3 a). Median HBsAg levels were markedly lower in inactive HBsAg carriers without disease (84; range, 0.19 to 822 IU/ml) than in patients with active CHB without (1,392; range, 0.25 to 9,917 IU/ml) or with (587; range, 0.71 to 5,341 IU/ml) cirrhosis (Fig. 3b).
Fig. 2.
Overall HBsAg concentration frequency.
Fig. 3.
HBsAg levels according to disease stage. (a) Data from Hannover cohort. (b) Data from Pisa cohort. CHB patients in the low-replicative phase in the Hannover cohort have higher HBsAg levels than inactive carriers without liver disease in the Pisa cohort; chronic active hepatitis and cirrhotic patients in the Pisa cohort are comparable to those HBeAg-negative patients classified as immune clearance phase in the Hannover cohort.
Assessment of mandatory dilution algorithm. (i) Elecsys 2010 and cobas e 411 analyzers (1:100 onboard dilution).
As a result of serial dilutions from 1:100 to 1:1,000,000, of the 503 samples tested on these platforms, 340 (67.6%) had a final level which was measurable within the initial onboard dilution. In 30 (5.9%) samples, the first measurement was below the detection range and reanalysis of undiluted serum was required. For the remaining 133 (26.5%) samples with results above the range in the first measurement, a manual predilution of the sample before analysis using the respective onboard dilution was performed. Of these, a single predilution step of 1:10 was sufficient to achieve a result within the measurement range in the majority of samples (119/133; 89.5%). The remaining 14 samples required further analysis using a predilution of 1:100. No higher predilutions were necessary to determine the final level in the samples included in this evaluation.
(ii) Modular Analytics E170 (1:400 onboard dilution).
As a result of serial dilutions from 1:100 to 1:1,000,000, of the 108 samples tested on this platform, 101 (93.5%) had a final level which was measurable within the initial onboard dilution. In one sample, the first measurement was below the detection range, and reanalysis of undiluted serum was required. For the remaining six (5.5%) samples with results above the range in the first measurement, a manual predilution of the sample before analysis using the respective onboard dilution was performed. Of these, a single predilution step of 1:10 was sufficient to achieve a result within the measurement range with four samples (66.7%), and the remaining two samples required further analysis using a predilution of 1:100. No higher predilutions were necessary to determine the final level in the samples included in this evaluation.
(iii) Overall results.
Across all platforms, 72.2% of samples had a final level which was measurable within the initial on-board dilution, and therefore, a final result was given by a single analysis. In 5.1% of samples, the first measurement was below the detection range, and reanalysis of undiluted serum was required. For the remaining 139 (22.7%) samples with results above the range in the first measurement, manual predilution of the sample before analysis using the respective onboard dilution was performed. Of these, a single predilution step of 1:10 was sufficient to achieve a result within the measurement range in the majority of samples (123/139; 88.5%). The remaining 16 samples required further analysis using a predilution of 1:100. No higher predilutions were necessary to determine the final level in the samples included in this evaluation.
By extrapolation of these data, regardless of the analyzer used, the final level of the samples included in this study would be determined in the first measurement with the following frequencies: instrument with 1:100 predilution, 428 of 611 samples (70.0%); and instrument with 1:400 predilution, 523 of 611 samples (85.6%).
Determination of HBsAg levels free of high-dose hook effect.
Eight dilutions in addition to the undiluted sample were analyzed where possible. Of the 611 samples, 46 did not give results within the measuring range from two subsequent dilution steps. Of these, 30 samples had a level below the measuring range at a dilution of 1:100, but within the measuring range undiluted (HBsAg < 5 IU/ml). Sixteen further samples had levels within the measuring range at a dilution of 1:100 and undiluted, but below the measuring range at a dilution of 1:400. Serial dilutions could not be performed on these 46 sera, and so, linear dilution and the lack of any hook effect could not be proven with these samples. However, these samples had very low HBsAg levels (maximum level, 22.4 IU/ml), and given that the hook effect is associated with high antigen levels, such an effect would be unlikely or very limited.
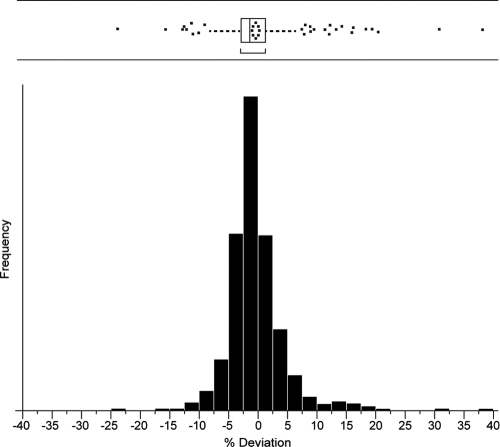
In the remaining 565 samples, the mean deviation between the final level and the level obtained by subsequent (or previous) dilution steps was very low (mean [standard deviation (SD)], −0.464 [4.83%]; 95% percentile, −7.9% to +12.1%). Statistical analysis of the mean deviation is shown in Fig. 4. The assay was therefore regarded to be free of a high-dose hook effect up to the maximum serum level included in the analysis (870,000 IU/ml) if the mandatory dilution algorithm is applied.
Fig. 4.
Deviation in HBsAg measurements between final and subsequent dilution steps (%). Statistics for the percentage of deviation between the final level and the level obtained by subsequent (or previous) dilution steps for the Elecsys HBsAg II quantitative assay.
Precision of the assay.
Precision was determined using the Elecsys HBsAg II quantitative reagents, human sera, and controls (Elecsys PreciControl HBsAg II; Roche Diagnostics) described in protocol EP5-A2 of the Clinical and Laboratory Standards Institute (CLSI; http://www.clsi.org/). Two runs were performed per day in duplicate for 21 days (n = 84). Results for the different analyzers are shown in Table 2.
Table 2.
Precision analyses for the Elecsys HBsAg II quantitative assaya
| Assay | Sample | Mean (IU/ml) | Repeatability |
Intermediate precision |
||
|---|---|---|---|---|---|---|
| SD (IU/ml) | CV (%) | SD (IU/ml) | CV (%) | |||
| Elecsys 2010 and cobas e 411 analyzers (1:100onboard dilution) | HS 1 | 3.07 | 0.056 | 1.8 | 0.17 | 5.6 |
| HS 2 | 54.3 | 0.747 | 1.4 | 3.04 | 5.6 | |
| HS 3 | 6,610 | 237 | 3.6 | 372 | 5.6 | |
| PreciControl HBsAg II 1 | <0.05 | |||||
| PreciControl HBsAg II 2 | 0.109 | 0.003 | 3.1 | 0.010 | 8.9 | |
| Modular Analytics E170, cobas e 601, or cobas e 602 (1:400 onboard dilution) | HS 4 | 3.06 | 0.069 | 2.3 | 0.15 | 4.9 |
| HS 5 | 55.5 | 1.48 | 2.7 | 3.64 | 6.6 | |
| HS 6 | 37,300 | 1,490 | 4.0 | 3,590 | 9.6 | |
| PreciControl HBsAg II 1 | <0.05 | |||||
| PreciControl HBsAg II 2 | 0.130 | 0.007 | 5.6 | 0.010 | 7.6 | |
SD, standard deviation; CV, coefficient of variation; HS, human serum.
Effect of HBV genotype.
Median (range) HBsAg levels according to HBV genotype were as follows: genotype A, 6,080 (0.71 to 873,300) IU/ml (n = 61); genotype B, 1,133 (0.11 to 51,320) IU/ml (n = 25); genotype C, 1,511 (0.47 to 124,600) IU/ml (n = 25); and genotype D, 1,659 (0.15 to 116,200) IU/ml (n = 198). Dilution analyses showed a linear relationship for all genotypes tested, with no evidence of a high-dose hook effect when onboard dilution was used (Table 3).
Table 3.
Representative dilution series for HBV genotypes A to Ea
| Genotype | Undiluted | Dilution |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:100* | 1:400† | 1:1,000 | 1:4,000 | 1:10,000 | 1:40,000 | 1:100,000 | 1:1,000,000 | ||
| A | 123.40 | >130‡ | 33,052 | 32,640 | 32,120 | 31,800 | 31,240 | 30,300 | <0.05# |
| B | >130‡ | 1,193 | 1,148 | 1,220 | 1,196 | 1,340 | <0.05# | <0.05# | <0.05# |
| C | >130‡ | 673 | 728 | 716 | 744 | 740 | <0.05# | <0.05# | <0.05# |
| D | 33.31 | >130‡ | >130‡ | 78,410 | 77,120 | 80,900 | 77,200 | 78,100 | 78,000 |
| E | >130‡ | >130‡ | 24,080 | 23,800 | 24,000 | 23,900 | 24,440 | 23,400 | <0.05# |
Onboard dilution for Elecsys 2010 or cobas e 411 (*) and Modular Analytics E170, cobas e 601, or cobas e 602 (†), respectively. The mandatory dilution algorithm incorporating onboard dilution eliminates any potential high-dose hook effect. >130‡ indicates that the HBsAg level in the diluted sample is above the upper limit of detection for the assay; therefore, the level in the undiluted sample cannot be calculated. <0.05# indicates that the HBsAg level in the diluted sample is below the lower limit of detection for the assay; therefore, the level in the undiluted sample cannot be calculated.
Correlation with the Architect HBsAg assay.
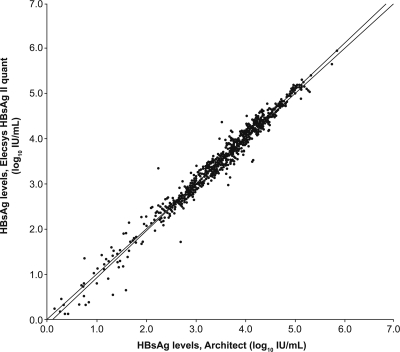
The correlation analysis of the Elecsys HBsAg II quantitative assay to the Architect HBsAg quantitative assay, based on all samples included in the study (n = 611), is shown in Fig. 5. Overall, correlation between the two assays was high (r = 0.988). There was no apparent effect of genotype, and the correlation was high across the entire range.
Fig. 5.
Overall correlation between Elecsys HBsAg II quantitative and Architect HBsAg assays (n = 611). x axis, HBsAg comparison assay (log10 IU/ml); y axis, Elecsys HBsAg II quantitative assay (log10 IU/ml). Passing/Bablok, y = 1.04x − 0.13, τ = 0.900; linear regression, y = 1.03x − 0.10, r = 0.988. The sample concentrations were between approximately 0.11 and 873,300 IU/ml.
DISCUSSION
Our study confirms previous results using a research protocol for the quantification of HBsAg based on the qualitative Elecsys HBsAg II assay that also correlated well with the Architect assay (21, 26). In the current study we have demonstrated that the use of this assay under fully automated conditions with onboard dilution reliably determines serum HBsAg levels in routine samples from HBsAg carriers in different phases of HBV infection, including patients with HBeAg-positive and -negative CHB, and across all genotypes. Our data indicate that the assay performed under the conditions tested is suitable for monitoring clinically relevant changes in HBsAg levels in patients undergoing antiviral therapy. The assay performed reliably and consistently on a range of different analyzers with high precision. For all sera tested, including those with high HBsAg levels up to a maximum of 870,000 IU/ml, the Elecsys HBsAg II quantitative assay was able to determine a level over a broad linear range and free of any high-dose hook effect when the mandatory onboard dilution algorithm was applied. The high-dose hook effect occurs when the capture and detection antibodies in an immunoassay are saturated by high concentrations of antigen in a test sample, which leads to measured levels of antigen being significantly lower than the actual level. Overall, the majority of samples tested using the dilution algorithm fell within the range of the assay and therefore did not require an additional manual predilution step, improving reliability by eliminating the risk of pipetting errors. These features contribute to the accuracy and precision required if absolute HBsAg levels are to be used for clinical decision-making.
The use of onboard dilution is one of the key benefits of the Elecsys HBsAg II quantitative assay. Data for the current study suggest that around 70.0% of samples tested on analyzers incorporating a 1:100 onboard dilution (Elecsys 2010 and cobas e 411) and 85.6% of those tested on analyzers incorporating a 1:400 onboard dilution (Modular Analytics E170 and cobas e 601 or cobas e 602) do not need a manual predilution step and fall within the linear range of the assay using the onboard dilution step. Recent reports on the clinical application of HBsAg-level monitoring during therapy propose clinical decision-making levels of HBsAg in the range of 1,500 to 20,000 IU/ml (19). As such, these clinically relevant levels fall comfortably within the linear range of the Elecsys HBsAg II assay (at least for instruments using a 1:400 predilution), since no further manual dilution is required for samples <52,000 IU/ml, i.e., the majority of samples likely to be encountered in practice. Of the samples which required retesting using a manual predilution step, the vast majority required only an additional predilution of 1:10, with only around 2% of samples overall requiring a manual dilution step of 1:100. This offers advantages over assays requiring manual predilution of all sera, in terms of convenience, laboratory hands-on time, reduced costs, and a lower risk of dilution errors, with reduced potential for intra- and interindividual variation. The use of a defined mandatory onboard dilution algorithm also contributes to standardization of the assay between laboratories. The Elecsys HBsAg II quantitative assay showed high repeatability (intra-assay precision) and intermediate precision for all analyzers tested. Intra-assay and intermediate imprecision (coef-ficient of variation [CV], %) ranged from 1.4 to 5.6 and 4.9 to 9.6 for HBsAg concentrations between 0.1 and 37,300 IU/ml, respectively, across the different instrument platforms, including the onboard dilution. This compares favorably with the ranges of 4.1 to 7.8 and 6.2 to 9.2 for HBsAg concentrations between 0.23 and 182 IU/ml, respectively, reported for the Architect HBsAg quantitative assay, which does not include the additional imprecision due to the necessity of a manual dilution step to achieve levels within this low range (1). A high degree of precision is essential for assays used in clinical practice and particularly if clinical decisions rely on the comparison of serial measurements, for example, as part of patient monitoring during a course of treatment.
Due to its association with improved clinical outcome and survival, sustained HBsAg loss, with or without seroconversion to anti-HBs, is considered to be the ideal endpoint of therapy for CHB (7). A number of recent studies have suggested that monitoring HBsAg levels during treatment with a finite course of PEG-IFN may help identify those patients who are most likely to achieve a sustained response to therapy and go on to clear HBsAg (3, 4, 14, 17, 19) or identify those who may relapse following treatment (17). Recent data have also suggested that HBsAg levels may help predict response to therapy with NAs (9, 25). For example, in analyses of patients treated as part of the pivotal trials of PEG-IFN alfa-2a in HBV, a decline of ≥10% in HBsAg levels from baseline at weeks 12 and 24 of treatment in HBeAg-negative patients (18) or HBsAg levels of <1,500 IU/ml at weeks 12 and 24 of treatment in HBeAg-positive patients (19) were found to be associated with high rates of sustained immune control and HBsAg loss posttreatment. A high rate of sustained response in HBeAg-positive patients with an on-treatment HBsAg level of <1,500 IU/ml at week 12 or 24 was confirmed recently by a second independent study of PEG-IFN (8). In that study, no patient with an HBsAg level of >20,000 IU/ml at week 12 achieved a sustained response, suggesting it may be possible to use this as a potential stopping rule (8) while encouraging those patients with an on-treatment HBsAg level of <20,000 IU/ml to continue therapy. Both the clinically relevant HBsAg levels of 1,500 and 20,000 IU/ml fall within the linear range of the Elecsys HBsAg II quantitative assay. A combined stopping rule using the lack of any decline of HBsAg together with a <2-log10 drop of HBV DNA at week 12 in HBeAg-negative patients treated with PEG-IFN has recently been proposed (20). A rule based on an absolute figure for HBsAg decline may be difficult to justify based on the technical performance of quantitative assays. Although the Elecsys HBsAg II quantitative assay showed excellent intra-assay precision in our study, intra-assay variability of 1.4 to 5.6% was still present, while variability over 10% in a routine testing environment could be expected with the Architect assay, taking into account the need for manual dilution for almost all samples (1).
In agreement with previous studies (10, 18), samples assayed using the Elecsys HBsAg II quantitative assay showed that variations in HBsAg levels varied more according to the phases of HBV infection rather than stages of liver disease. In fact, the lowest levels were found in inactive HBeAg-negative, anti-HBe-positive carriers without liver disease, who showed significantly lower HBsAg serum levels than HBeAg-negative CHB patients in the low-replicative phase. Distinguishing between the low-replicative phase of HBeAg-negative CHB and the inactive HBsAg carrier without liver disease is a major issue for clinical decision-making. Recent data have shown that measuring both HBsAg and HBV DNA levels provides more accurate means of determining inactive carrier status than measuring either marker alone (4, 16). Although these data require verification, they suggest that measurement of HBsAg levels, along with HBV DNA levels, may be useful in monitoring patients during the natural course of HBV infection to distinguish between those patients with active disease who may benefit from therapy and those who are inactive carriers and so do not require treatment (4, 16). Levels of HBsAg that appear to be helpful for differentiating inactive from active disease are in the range of 1,000 to 2,000 IU/ml (4, 16). Again, these clinically relevant levels fall into the linear range covered by the Elecsys HBsAg II quantitative assay.
The differences in HBsAg levels between distinct genotypes, especially the higher median value for HBV genotype A-infected patients compared with those infected with other genotypes seen in this study, were considered to reflect the high proportion of HBeAg-positive patients in this group compared with other genotypes. HBeAg-positive patients have been shown to have significantly and markedly higher HBsAg levels than those with HBeAg-negative disease during the natural course of infection (5).
As the clinical uses of HBsAg quantification continue to be defined and confirmed, it is likely that HBsAg quantification will become an established part of the management of patients. It is therefore anticipated that there will be an associated increase in the numbers of samples to be tested and that the need for rapid, reliable, sensitive, and reproducible assays will intensify. High precision and accuracy are essential to ensuring that changes in HBsAg levels used to guide clinical decision-making are accurate and not a reflection of variability inherent in the assay.
In conclusion, the Elecsys HBsAg II quantitative assay reliably determined HBsAg levels in serum from a wide range of HBsAg carriers and CHB patients across all genotypes, phases of HBV infection, and stages of liver disease, providing results that are standardized against the WHO Second International Standard for HBsAg. Onboard dilution is a key advantage of the assay and has the potential to lower labor costs, decrease turn-around time, and reduce the potential for dilution error, so improving accuracy and precision. These features ensure accurate measurement, which is particularly important if changes in HBsAg levels are to be used for clinical decision-making. Given these findings, the Elecsys HBsAg II quantitative assay is highly suitable for routine clinical use, including those samples with high HBsAg levels.
ACKNOWLEDGMENTS
We thank W. Melchior and R. Hofweber (Roche Diagnostics GmbH) and W. Kantakamala (Department of Microbiology, Siriraj Hospital) for their contributions to this study.
This study was funded by Roche Diagnostics, Rotkreuz, Switzerland.
Editorial support was provided by Elements Communications and funded by Roche Diagnostics, Rotkreuz, Switzerland.
Statement of interests: K.W. has received research funding from Roche Diagnostics and Novartis Pharma. M.R.B. has participated in advisory committees or review panels and has acted as a speaker for Abbott, BMS, Gilead, Merck, Novartis, and Roche. H.W. has received research grants and lecturer and consultant fees from Roche Diagnostics and Abbott Diagnostics. F.B. has participated in advisory committees or review panels and has acted as a speaker for Abbott, Gilead, Novartis, and Roche. All other authors have no potential conflicts of interest to declare.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Abbott Laboratories 2008. Abbott Architect quantitative HBsAg assay package insert. 6C36 84-5655/R8 B6C3B0. Abbott Laboratories, Abbott Park, IL [Google Scholar]
- 2. Brunetto M. R. 2010. A new role for an old marker, HBsAg. J. Hepatol. 52:475–477 [DOI] [PubMed] [Google Scholar]
- 3. Brunetto M. R., et al. 2009. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 49:1141–1150 [DOI] [PubMed] [Google Scholar]
- 4. Brunetto M. R., et al. 2010. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology 139:483–490 [DOI] [PubMed] [Google Scholar]
- 5. Chan H. L., et al. 2010. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology 52:1232–1241 [DOI] [PubMed] [Google Scholar]
- 6. Chu C. M., Liaw Y. F. 2010. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir. Ther. 15:133–143 [DOI] [PubMed] [Google Scholar]
- 7. European Association for the Study of the Liver 2009. EASL clinical practice guidelines: management of chronic hepatitis. J. Hepatol. 50:227–242 [DOI] [PubMed] [Google Scholar]
- 8. Gane E., et al. 2011. Neptune study: on-treatment HBsAg level analysis confirms prediction of response observed in phase 3 study of peginterferon alfa-2a in HBeAg-positive patients. J. Hepatol. 54(Suppl. 1):S31 [Google Scholar]
- 9. Heathcote E. J., et al. 2011. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 140:132–143 [DOI] [PubMed] [Google Scholar]
- 10. Jaroszewicz J., et al. 2010. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J. Hepatol. 52:514–522 [DOI] [PubMed] [Google Scholar]
- 11. Lau G. K., et al. 2008. On-treatment HBsAg decline during peginterferon alpha-2a (40KD)+lamivudine in patients with HBeAg-positive CHB as a potential predictor of durable off-treatment response. Hepatology 48(Suppl.):714A [Google Scholar]
- 12. Lok A. S., McMahon B. J. 2009. AASLD guidelines. Chronic hepatitis B: update 2009. Hepatology 50:1–36 [DOI] [PubMed] [Google Scholar]
- 13. Marcellin P., et al. 2008. In patients with HBeAg-negative chronic hepatitis B HBsAg serum levels early during treatment with peginterferon alfa-2a predict HBsAg clearance 4 years post-treatment. Hepatology 48(Suppl. 1):718A [Google Scholar]
- 14. Marcellin P., et al. 2010. On-treatment decline in serum HBsAg levels predicts sustained immune control 1 year post-treatment and subsequent HBsAg clearance in HBeAg-negative hepatitis B virus-infected patients treated with peginterferon alfa-2a [40KD] (PEGASYS). Hepatol. Int. 4(Suppl.):151 [Google Scholar]
- 15. Martinot-Peignoux M., et al. 2002. Serum hepatitis B virus DNA levels and liver histology in inactive HBsAg carriers. J. Hepatol. 36:543–546 [DOI] [PubMed] [Google Scholar]
- 16. Martinot-Peignoux M., et al. 2010. Quantitative HBsAg: a new specific marker for the diagnosis of HBsAg inactive carriage. J. Hepatol. 52(Suppl.):S282 [Google Scholar]
- 17. Moucari R., et al. 2009. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: a long-term follow-up study. J. Hepatol. 50:1084–1092 [DOI] [PubMed] [Google Scholar]
- 18. Nguyen T., et al. 2010. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J. Hepatol. 52:508–513 [DOI] [PubMed] [Google Scholar]
- 19. Piratvisuth T., et al. 2010. On-treatment decline in serum HBsAg levels predicts sustained immune control 6 months post-treatment and subsequent HBsAg clearance in HBeAg-positive hepatitis B virus-infected patients treated with peginterferon alfa-2a [40KD] (PEGASYS). Hepatol. Int. 4(Suppl.):152 [Google Scholar]
- 20. Rijckborst V., et al. 2010. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology 52:454–461 [DOI] [PubMed] [Google Scholar]
- 21. Sonneveld M. J., et al. 2011. A comparison of two assays for quantification of hepatitis B surface antigen in patients with chronic hepatitis B. J. Clin. Virol. 51:175–178 [DOI] [PubMed] [Google Scholar]
- 22. Thompson A. J., et al. 2010. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology 51:1933–1944 [DOI] [PubMed] [Google Scholar]
- 23. Volz T., et al. 2007. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology 133:843–852 [DOI] [PubMed] [Google Scholar]
- 24. Werle-Lapostolle B., et al. 2004. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 126:1750–1758 [DOI] [PubMed] [Google Scholar]
- 25. Wursthorn K., et al. 2010. Kinetics of hepatitis B surface antigen decline during 3 years of telbivudine treatment in hepatitis B e antigen-positive patients. Hepatology 52:1611–1620 [DOI] [PubMed] [Google Scholar]
- 26. Wursthorn K., et al. 2011. Correlation between the Elecsys HBsAg II assay and the Architect assay for the quantification of hepatitis B surface antigen (HBsAg) in the serum. J. Clin. Virol. 50:292–296 [DOI] [PubMed] [Google Scholar]