Abstract
Neisseria gonorrhoeae surface protein A (NspA) is a highly conserved gonococcal antigen. To explore the potential of NspA in vaccine development against gonorrhea, BALB/c mice were immunized with pcNspA containing the NspA gene from N. gonorrhoeae strain WHO-A via intramuscular (i.m.) injection, intranasal (i.n.) immunization, or intravaginal (i.vag.) immunization. Following the last DNA immunization, mice were boosted with recombinant NspA (rNspA). Enzyme-linked immunosorbent assays (ELISAs) indicated that all immunized mice generated measurable NspA-specific IgG and IgA in serum and secretory IgA (sIgA) in vaginal wash fluids. The antisera had bactericidal and opsonic activities. These data demonstrated that NspA induced antibodies with antigonococcal activity.
INTRODUCTION
Gonorrhea is one of the most prevalent sexually transmitted diseases. The World Health Organization estimated that 62 million new gonococcal infections occur annually (46). Clinical manifestations of gonococcal diseases mainly include acute or chronic purulent infection of the mucous membrane of the urogenital system, such as urethritis, prostatitis, and epididymitis in men (10). Asymptomatic infection is more often seen in females (10) and results in delayed medical treatment. Thus, asymptomatic infection not only contributes to the persistence of the gonococcus among general population but also leads to more serious conditions such as pelvic inflammatory disease (PID), infertility, ectopic pregnancy, and disseminated gonococcal infection (DGI) (10, 35). Moreover, gonococcal infections also facilitate HIV transmission (23). Since a high frequency of multiple-antibiotic resistance makes therapy for gonococcal diseases problematic (42), the development of effective antigonococcal vaccines is the key to prevent and control gonococcal infections.
For vaccine development, the identification of conserved gonococcal components capable of inducing protective immune responses is required. Martin and colleagues have recently identified a widely distributed and highly conserved surface protein, NspA, in the outer membrane (OM) of Neisseria meningitidis (28). Two monoclonal antibodies (MAbs) against NspA exhibit bactericidal activities against serogroup B, A, and C of the meningococcal isolates. These MAbs passively protect mice against experimental infections (6, 28, 32). Immunization with purified recombinant NspA protein (rNspA) efficiently protects the mice against a meningococcal deadly challenge (28). The cross-reactive antibodies (Abs) are present in the sera of the immunized mice and efficiently attach to and kill the four strains in serogroup B (27). This result emphasizes that the NspA protein has characteristics of a vaccine candidate. Phase 1 first-in-human studies show that the unfolded rNspA meningococcal vaccine is well tolerated and immunogenic in healthy adult volunteers but did not elicit bactericidal antibodies, indicating that the development of the rNspA vaccine needs to proceed with some modifications (14). Mouse anti-rNspA antiserum and MAbs produced by the laboratory of Moe et al. led to less antibody-dependent and complement-mediated bacteriolysis (31, 32); the capsule expressed by the six meningococcal strains that they used may limit the accessibility of NspA surface epitopes to the antisera (32). Given the difference in the NspA surface accessibility of different strains, it is proposed that an rNspA-based meningococcal B vaccine may have to be supplemented with additional antigens and that optimal immunogenic vaccines using rNspA may require formulations that permit proper folding of the protein (17, 31, 32).
Orthologs of the nspA gene are also present in the genomes of N. gonorrhoeae strains (37). Plante and colleagues have cloned and sequenced the nspA gene from N. gonorrhoeae B2. This gene contains an open reading frame of 525 nucleotides coding for a polypeptide of 174 amino acid residues with a calculated molecular weight of 18,316 and a pI of 10.21 (37). The predicted amino acid sequences of the NspA polypeptides of the gonococcal strains B2 and FA1090 are 98% similar (37). Seven NspA-specific MAbs have been generated, and four of them recognize the corresponding epitope in all 51 N. gonorrhoeae strains tested, revealing a high level of antigenic conservation of the gonococcal NspA proteins (37). Radioimmunobinding assays indicate that the gonococcal NspA protein is exposed at the surface of the intact cells (37). Similar to the meningococcal NspA, the wide distribution and high conservation confer vaccine potential to gonococcal NspA.
DNA vaccine consists of a DNA plasmid containing a gene of interest from a pathogen that is able to express the corresponding target antigen in eukaryotic cells under the control of a suitable promoter (19). DNA vaccines are versatile, safe, and simple. They can be developed rapidly and inexpensively for the prevention of infections by a wider range of pathogens. Therefore, DNA vaccines are potentially powerful in the prevention of infectious diseases (1, 19). To enhance the immune responses, several new strategies have been developed (1). For example, boosting with protein antigens significantly increases the antibody responses (48). In the present study, we analyze the antibody responses of mice immunized with a recombinant plasmid encoding the gonococcal NspA and boosted with rNspA via three inoculation routes.
MATERIALS AND METHODS
Immunization of mice.
The expression plasmid containing the gonococcal nspA gene (accession number AY157539.1) from N. gonorrhoeae strain WHO-A, pcNspA, was described previously (20). The open reading frame of the gene was amplified and inserted into prokaryotic expression vector pQE-31 according to standard molecular techniques as previously described (40). The expression and refolding of recombinant NspA were performed as described previously (29). To confirm that the renatured rNspA was folded properly and expressed conformational epitopes, an enzyme-linked immunosorbent assay (ELISA) with whole gonococcal cells was carried out using the mouse antiserum induced by it as the primary antibody (37). Six- to 7-week-old female BALB/c mice were divided into four groups and immunized five times at 2-week intervals. Three different routes were adopted to immunize the mice. For intramuscular (i.m.) immunization, 100 μg of purified pcNspA in 50 μl of Tris-EDTA buffer (5 mM Tris, pH 7.4, 0.1 mM EDTA) was injected into the musculus quadriceps femoris of the mouse. For intranasal (i.n.) immunization, 100 μg of pcNspA was dropped into the noses of methoxyflurane-anesthetized mice. For intravaginal (i.vag.) immunization, the mice were massaged gently on the end of the back to be stimulated to excrete feces and urine, and then 100 μg of pcNspA was injected slowly into the vaginas. After the fourth DNA immunization, the mice were boosted with 100 μg of renatured rNspA emulsified with Freund's incomplete adjuvant through the same immunization routes as DNA vaccinations. The control mice were immunized with only Tris-EDTA buffer.
Sample collection.
Sera and vaginal secretions were collected from the mice before immunization and 2 weeks after each immunization; i.e., samples were collected on days 0, 14, 28, 42, 56, and 70. To obtain serum, tail vein blood samples were incubated at 4°C for 12 h and centrifuged at 12,000 × g for 5 min. The supernatants were collected and stored at −20°C. To collect vaginal secretions, 100 μl of phosphate-buffered saline (PBS) was pipetted in and out of the vaginal vault three times. The procedure was repeated twice, and the vaginal washes were pooled (39) and centrifuged at 12,000 × g for 5 min. The collected supernatants were stored at −80°C.
ELISA.
Microtiter wells (96-well Nunc microtiter plates) were coated with 200 ng of purified gonococcal rNspA protein in carbonate coating buffer (0.15 M sodium carbonate, 0.35 M sodium bicarbonate, 0.03 M sodium azide, pH 9.6) at 4°C overnight. After three washes in PBS buffer containing 0.05% Tween 20 (PBST), the wells were blocked with 3% bovine serum albumin (BSA) in PBST at 37°C for 1 h. Following the washes, sera or vaginal secretions serially diluted in PBST were added into the wells, and the plates were incubated at 4°C overnight. The wells were then washed and incubated with secondary antibodies at room temperature for 1 h. Secondary antibodies included horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG diluted in PBST at 1:5,000 and HRP-conjugated goat anti-mouse IgA at 1:2,500. After addition of the Ultra-TMB (where TMB is 3,3′,5,5′-tetramethylbenzidine) substrate (Pierce) to the washed wells and incubation at room temperature for 30 min, the absorbance was measured at 490 nm (UltraMicroplate Reader ELx800; Bio-Tek Instruments, Inc.). The quantity of NspA-specific antibody bound to the plates was determined by comparing the optical density of the rNspA-coated wells to that obtained by coating the wells with goat anti-mouse IgG Fc-affinity-purified coating antibody at 1:100 (or IgA-affinity-purified coating antibody at 1:100 for IgA ELISA), comparing them to a standard curve of mouse Ig reference serum with known quantities of immunoglobulins (Bethyl Laboratories), and developing them in parallel with the rNspA-specific wells (48).
Serum antibacterial assay.
Gonococcal strain WHO-A was a gift of Yin Yueping, Institute of Academy of Dermatology, Beijing, China. The bacteria were resuscitated on GC chocolate agar from freezer stocks directly. The plates were allowed to incubate at 37°C and 5% CO2 for 16 to 18 h, and the isolated colonies were picked from the plate and suspended in 37°C prewarmed fastidious broth (FB) (8, 41). The cell suspension was adjusted to about 2 × 103 CFU/ml. Mouse sera were pooled by group and heat inactivated at 56°C for 30 min. A total of 50 μl of serially diluted serum samples and 40 μl of bacterial suspension were mixed and incubated at 37°C and 5% CO2 for 15 min. Then, 10 μl of normal human fresh serum was added into the mixtures to supply the complement sources, and the incubation was continued for an additional 45 min. The human serum was collected from a healthy adult volunteer with no reactivity against gonococcal strain WHO-A. Samples were plated onto GC chocolate agar (three to five plates for the pooled sera from each group) and incubated for approximately 24 h, and then viable gonococcus colonies were enumerated. Greater than 50% killing compared to negative controls was defined as significant killing (33, 39, 48).
Opsonic activity analysis.
Luminol-enhanced chemiluminescence (CL) assays were performed to investigate the opsonic activity of the NspA-specific antisera as described previously (48). Briefly, 2 × 106 log-phase gonococcus cells were added to pooled sera from each group in 96-well white Nunc microtiter plates and incubated at 37°C for 15 min. Ten microliters of normal human serum was added as complement and incubated for 30 min at 37°C, followed by the addition of 2 × 105 Percoll-isolated human polymorphonuclear neutrophils (PMNs) and 0.1 μM luminol. The final volume of each reaction mixture was 100 μl, and pooled sera from each immunized group of mice or nonimmunized control groups were diluted at the final concentration of 1:100. Light emission from the reaction was measured in a 1420 Victor D Multilabel Counter (Wallac) at approximately 2-min intervals. Zymosan A was used as a positive control, and gonococcus cells incubated in normal mouse serum were used as negative controls.
Statistics.
Analysis of variance was performed using the software SPSS, version 10.0. A P value of less than 0.05 was considered significant.
RESULTS
IgG response against gonococcal NspA.
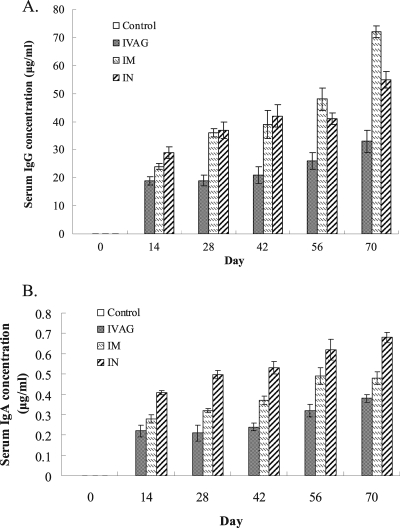
Quantitative ELISAs indicated that the levels of serum IgG to gonococcal NspA varied with different immunization routes (Fig. 1A). The highest level of IgG response was seen in the i.m. immunization group, in which gonococcal NspA-specific IgG responding to the NspA DNA vaccination kept increasing until the fourth immunization (day 56), and boosting with rNspA after the last DNA vaccination resulted in a significant increase in serum IgG (day 70). Immunization with NspA DNA and boosting with rNspA via the intranasal route also induced a high level of IgG response similar to that of the i.m. route (P > 0.05). Intravaginal immunization stimulated less specific IgG titers than the other two routes, and only a slight increase in IgG response was generated by boosting with rNspA.
Fig. 1.
The levels of serum IgG (A) and IgA (B) antibodies against N. gonorrhoeae NspA in mice immunized via different routes (n = 6). Mouse sera obtained from tail vein blood at days 0, 14, 28, 42, 56, and 70 postimmunization were analyzed by quantitative ELISA.
Gonococcal NspA-specific IgA responses.
Specific serum IgA responses to rNspA were also detected. All three immunization groups were stimulated to produce more NspA-specific serum IgA than the control groups although the levels of IgA were lower than the level of IgG (Fig. 1B). In contrast to IgG production, the highest level of IgA was induced in the i.n. immunization group. Mice immunized by i.m. and i.vag. routes generated similar levels of IgA (P > 0.05), which were significantly lower than the level produced in the i.n. immunization group (P < 0.05). Boosting with rNspA also caused increases in the serum IgA levels in the immunized mice; however, the increases were insignificant.
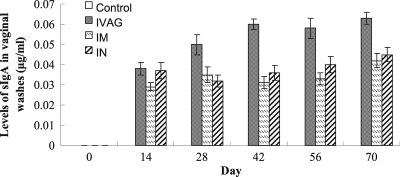
Mucosal sIgA responses against gonococcal NspA.
All immunization groups generated secretory IgA (sIgA) in vaginal washes. The highest level of sIgA was found in the i.vag. immunization group. The i.m. and i.n. immunization groups produced similar amounts of sIgA (P > 0.05) (Fig. 2). Boosting with rNspA did not result in significant increases in the amount of sIgA in all groups. Antibody titers of serum IgG and IgA and of mucosal sIgA against whole bacteria were also determined, and the results were similar to the antibody titers against purified rNspA.
Fig. 2.
Levels of sIgA antibodies against N. gonorrhoeae NspA in vaginal washes from mice immunized via different routes (n = 6). Vaginal secretions from immunized mice were collected at 0, 14, 28, 42, 56 and 70 days postimmunization and analyzed by quantitative ELISA.
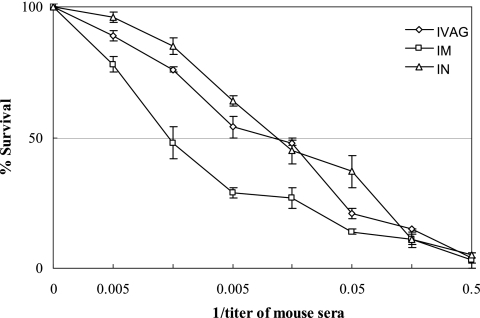
Bactericidal activity of the immune sera.
The results of ELISA with whole gonococcal cells revealed that both serum IgG and IgA bound the native bacterial cells. Then in vitro serum bactericidal assays were performed using pooled mouse and human sera as a complement source. Sera from the i.m. immunization group had the strongest bactericidal activity against N. gonorrhoeae, followed by sera from the i.n. and i.vag. immunization groups (Fig. 3). The lower bactericidal activity of the sera from the i.n. and i.vag. immunization groups might be related to the difference in immunization routes and/or the lower levels of gonococcal NspA-specific IgG in the sera. The exact reasons for this remain to be explored.
Fig. 3.
N. gonorrhoeae-specific bactericidal activity of sera from immunized mice detected using an antibody complement-mediated bactericidal activity assay. A series of diluted serum samples were mixed with gonococcal cells and incubated. Normal human fresh serum was added into the mixtures as complement sources. Then, the mixtures were plated onto GC chocolate agar and cultured. The recovered gonococcal colonies from the mixtures containing the immunized serum samples were enumerated and compared to those from negative controls (gonococci incubated with normal mouse serum). For all groups, n = 3.
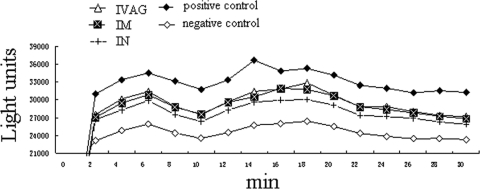
Opsonic activity of the immune sera.
Antiserum-opsonized gonococcus cells can react with human PMNs and result in the production of reactive oxygen radicals, which is associated with the generation of light energy known as chemiluminescence (CL) (48). The results of luminol-enhanced CL assays are shown in Fig. 4. Gonococci induced low levels of CL in the absence of antibody, possibly due to the presence of bacterial adhesins that bind to PMNs (48). Coincubation of gonococci with immune sera resulted in enhanced CL, probably due to the increased gonococcal interaction with PMNs through antibodies (48). CL enhanced by i.m. or i.n. immunization was significantly higher than that induced by controls (P < 0.05). Sera from the i.vag. immunization group showed only slightly enhanced CL, possibly because the levels of specific antibodies produced in this group were lower than those produced in the other two immunization groups. The CL value showed two peaks. The first (in the first 10 min) peak might be the result of a harmful effect to PMNs caused by the stimulation of gonococci. After this adaptive period, the respiratory burst of PMNs increased gradually. Subsequently, probably with the engulfing and elimination of gonococci in PMNs and/or with the decline in the activity of the PMNs, respiratory activity declined.
Fig. 4.
Respiratory burst effect enhanced by immune sera. Luminol-enhanced chemiluminescence was measured using a 1420 Victor D Multilabel Counter (Wallac) at approximately 2-min intervals in 96-well white Nunc microtiter plates containing a mixture of pooled serum samples, 2 × 106 gonococcus cells, 10% normal human serum, 2 × 105 Percoll-isolated human PMNs, and 0.1 μM luminol. The positive control contained zymosan A but not mouse serum or bacterial cells; the negative control was gonococcus cells incubated in normal mouse serum. Representative data from one of the three experiments are shown.
DISCUSSION
Various components of N. gonorrhoeae have been investigated for the development of effective vaccines. Gonococcal pilus vaccines have been developed but may not be protective (21). The lipooligosaccharide (LOS) epitope or its mimicker also represents an excellent target for a potentially protective gonococcal vaccine (13). Antiserum induced by a conserved epitope of gonococcal outer membrane protein IB is bactericidal to both homologous and heterologous strains (15). DNA vaccination of mice with the PorB gene from N. gonorrhoeae strain FA1090 by i.m. injection or epidermal gene gun bombardment generates a measurable amount of specific antibodies that recognize the surface of the homologous gonococcal strain and are opsonic to human neutrophils (48). Different delivery systems and administration routes result in different immune responses against PorB (47, 48). Natural infection of gonococci induces a paucity of systemic and local antibody responses to recombinant transferrin-binding proteins (38), and complexes of transferrin-binding proteins TbpA and TbpB (or their epitopes) and inactivated cholera toxin are able to stimulate the generation of protective antibodies by the host. Thus, these complexes can be used in the development of vaccines against gonococcal diseases (39). Immunization with the TbpB virus replicon particle (VRP) system results in specific IgG and IgA in mucosal secretions of mice (44). Competence-associated lipoproteins have been identified as candidate vaccine targets (3). Nasal administration of gonococcal outer membrane (OM) preparations can induce OM-specific systemic and vaginal immune responses and significantly accelerate gonococcal clearance in estradiol-treated mice (36). Opacity (Opa) protein loops can induce antibodies that recognize a broad spectrum of antigenically distinct Opa variants and have agglutination and adherence blocking abilities (9). All of this research indicates that the generation of an effective gonococcal vaccine is feasible if the gonococcal antigens are carefully selected and formulated to elicit only host-protective antibodies (5).
N. gonorrhoeae NspA is a highly conserved and widely distributed outer membrane protein (31). We evaluated its potential as a gonorrhea vaccine candidate and compared the different antibody responses of NspA vaccination via three routes of antigen delivery. Intramuscular injection has been considered one of the preferred routes of DNA vaccination (43). Some gonorrhea vaccine studies have been performed using the i.m. or subcutaneous injection route (47, 48). However, some investigators believe that the i.m. injection of DNA vaccine cannot induce significant mucosal immune responses (45). Immunization with plasmid DNA containing the gene of interest, followed by boosting with the protein antigen, can induce effective antibody responses (48). N. gonorrhea infection occurs through mucous membranes of the urogenital system, eye conjunctiva, pharynx, or rectum; thus, mucosal immune responses may play important roles in the defense against gonococcal infections. In the present study, we found that the intramuscular injection of mice with gonococcal NspA DNA, followed by boosting with rNspA, induced the generation of high levels of specific antibodies in serum and some sIgA responses in vagina. Boosting with rNspA protein enhanced the antibody responses in serum but failed to promote sIgA production in vaginal secretions.
Administration of an i.n. immunization is safe, simple, and noninvasive. More importantly, it induces strong systemic and mucosal immune responses (18, 47, 48). Moreover, i.n. immunization is effective in inducing specific immune responses in the genital tract (18). The bronchus-associated lymphoid tissue (BALT) and the nasal mucosa-associated lymphoid tissue (NALT) constitute organized lymphoid aggregates and are believed to be sites of induced T- and B-cell responses to inhaled antigens (7). Membranous (M) cells are portals of entry for antigens in these nasopharyngeal lymphoid tissues (34). Our data indicate that i.n. immunization of mice induces not only high titers of specific and protective serum IgG and IgA but also a significant amount of sIgA in vaginal secretions, which provide important immune protection by inducing the mucosal immune responses.
Intravaginal inoculation is another commonly used mucosal vaccination route in the development of vaccines against sexually transmitted diseases (24). Although nasal administration of mucosal vaccines induces immunity at multiple sites, including the female reproductive tract (2, 11, 24, 36, 39), it is evident that local immunization induces stronger immune responses at or adjacent to the region of antigen exposure than at distant sites (24, 30). The genital tract lacks organized lymphoid structures (30); however, it can initiate immune responses against several sexually transmitted infections, including the infection by N. gonorrhoeae (16), and genital tract immunization via the vaginal route can result in the generation of specific immunity (22). In this study, mice inoculated with NspA DNA and boosted with rNspA protein by i.vag. immunization generated stronger specific sIgA responses in vaginal secretions than mice that received i.m. or i.n. immunization.
Antigen delivery route is an important consideration in vaccine development (47). Our present study deals with the characterization of the antibody responses induced in mice immunized with gonococcal NspA DNA and boosted with rNspA administered via the i.m., i.n., or i.vag. route. Effective antibody responses can be induced by all three immunization routes. The levels and protective activities of antibodies in sera are lower in the i.vag. immunization group; however, stronger mucosal immune responses are generated.
Microbial complement inhibitor-binding molecules can be promising vaccine targets by eliciting Abs that neutralize this microbial defense mechanism (26). However, human factor H transgenic mice immunized with meningococcal fH-binding protein (fHbp) vaccine have 4- to 8-fold lower serum bactericidal Ab responses than those of control mice, indicating that Ab responses are impaired by the potential effect of fH binding on vaccine immunogenicity (4). The mutant fHbp vaccine that does not bind fH but that retains immunogenicity is predicted to be superior in humans to the fHbp vaccine that binds fH (4). Meningococcal NspA also binds fH (25). Since the NspA protein is highly conserved among pathogenic Neisseria strains (37), gonococcal NspA is probably a receptor for fH. Thus, the potential fH binding ability of gonococcal NspA should be considered in further studies of the vaccine candidate (4, 12, 25, 26).
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (31100652), Department of Science and Technology of Jiangsu Province (BK2009193 and BK2009615), Department of Education of Jiangsu Province (07KJD310244), and Yangzhou University (2008CXJ049 and 2007CXJ031).
Footnotes
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Abdulhaqq S. A., Weiner D. B. 2008. DNA vaccines: developing new strategies to enhance immune responses. Immunol. Res. 42:219–232 [DOI] [PubMed] [Google Scholar]
- 2. Balmelli C., et al. 1998. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J. Virol. 72:8220–8229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barh D., Kumar A. 2009. In silico identification of candidate drug and vaccine targets from various pathways in Neisseria gonorrhoeae. In Silico Biol. 9:225–231 [PubMed] [Google Scholar]
- 4. Beernink P. T., et al. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J. Immunol. 186:3606–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blake M. S., Wetzler L. M. 1995. Vaccines for gonorrhea: where are we on the curve? Trends Microbiol. 3:469–474 [DOI] [PubMed] [Google Scholar]
- 6. Cadieux N., et al. 1999. Bactericidal and cross-protective activities of a monoclonal antibody directed against Neisseria meningitidis NspA outer membrane protein. Infect. Immun. 67:4955–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canessa C., Vierucci S., Azzari C., Vierucci A. 2010. The immunity of upper airways. Int. J. Immunopathol. Pharmacol. 23:8–12 [PubMed] [Google Scholar]
- 8. Cartwright C. P., Stock F., Gill V. J. 1994. Improved enrichment broth for cultivation of fastidious organisms. J. Clin. Microbiol. 32:1825–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cole J. G., Jerse A. E. 2009. Functional characterization of antibodies against Neisseria gonorrhoeae opacity protein loops. PLoS One 4:e8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furuya R., Tanaka M. 2009. Neisseria gonorrhoeae infections. Nihon Rinsho 67:129–135 [In Japanese] [PubMed] [Google Scholar]
- 11. Gallichan W. S., et al. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451–3457 [DOI] [PubMed] [Google Scholar]
- 12. Giuntini S., Reason D. C., Granoff D. M. 2011. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect. Immun. 79:3751–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gulati S., Ngampasutadol J., Yamasaki R., McQuillen D. P., Rice P. A. 2001. Strategies for mimicking neisserial saccharide epitopes as vaccines. Int. Rev. Immunol. 20:229–250 [DOI] [PubMed] [Google Scholar]
- 14. Halperin S. A., et al. 2007. Phase 1 first-in-human studies of the reactogenicity and immunogenicity of a recombinant meningococcal NspA vaccine in healthy adults. Vaccine 25:450–457 [DOI] [PubMed] [Google Scholar]
- 15. Heckels J. E., Virji M., Tinsley C. R. 1990. Vaccination against gonorrhoea: the potential protective effect of immunization with a synthetic peptide containing a conserved epitope of gonococcal outer membrane protein IB. Vaccine 8:225–230 [DOI] [PubMed] [Google Scholar]
- 16. Hedges S. R., Sibley D. A., Mayo M. S., Hook E. R., Russell M. W. 1998. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J. Infect. Dis. 178:742–751 [DOI] [PubMed] [Google Scholar]
- 17. Hou V. C., Moe G. R., Raad Z., Wuorimaa T., Granoff D. M. 2003. Conformational epitopes recognized by protective anti-neisserial surface protein A antibodies. Infect. Immun. 71:6844–6849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ichinohe T., et al. 2010. Induction of cross-protective immunity against influenza A virus H5N1 by an intranasal vaccine with extracts of mushroom mycelia. J. Med. Virol. 82:128–137 [DOI] [PubMed] [Google Scholar]
- 19. Ingolotti M., Kawalekar O., Shedlock D. J., Muthumani K., Weiner D. B. 2010. DNA vaccines for targeting bacterial infections. Expert Rev. Vaccines 9:747–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ji M., Chen H. 2004. Cloning the NspA gene of Neisseria gonorrhoeae and its expression in eukaryotic cells. Acta Microbiol. Sin. 44:104–106 [Google Scholar]
- 21. Johnson S. C., et al. 1991. Human immunization with Pgh 3-2 gonococcal pilus results in cross-reactive antibody to the cyanogen bromide fragment-2 of pilin. J. Infect. Dis. 163:128–134 [DOI] [PubMed] [Google Scholar]
- 22. Kanazawa T., et al. 2010. Local gene expression and immune responses of vaginal DNA vaccination using a needle-free injector. Int. J. Pharm. 396:11–16 [DOI] [PubMed] [Google Scholar]
- 23. Klotman M. E., et al. 2008. Neisseria gonorrhoeae-induced human defensins 5 and 6 increase HIV infectivity: role in enhanced transmission. J. Immunol. 180:6176–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwant A., Rosenthal K. L. 2004. Intravaginal immunization with viral subunit protein plus CpG oligodeoxynucleotides induces protective immunity against HSV-2. Vaccine 22:3098–3104 [DOI] [PubMed] [Google Scholar]
- 25. Lewis L. A., et al. 2010. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 6:e1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madico G., et al. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin D., et al. 2000. Candidate Neisseria meningitidis NspA vaccine. J. Biotechnol. 83:27–31 [DOI] [PubMed] [Google Scholar]
- 28. Martin D., Cadieux N., Hamel J., Brodeur B. R. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J. Exp. Med. 185:1173–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsuka Y. V., Dilts D. A., Hoiseth S., Arumugham R. 1998. Characterization of a subunit structure and stability of the recombinant porin from Neisseria gonorrhoeae. J. Protein Chem. 17:719–728 [DOI] [PubMed] [Google Scholar]
- 30. Mestecky J., Fultz P. N. 1999. Mucosal immune system of the human genital tract. J. Infect. Dis. 179(Suppl. 3):S470–S474 [DOI] [PubMed] [Google Scholar]
- 31. Moe G. R., Zuno-Mitchell P., Lee S. S., Lucas A. H., Granoff D. M. 2001. Functional activity of anti-neisserial surface protein A monoclonal antibodies against strains of Neisseria meningitidis serogroup B. Infect. Immun. 69:3762–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moe G. R., Tan S., Granoff D. M. 1999. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect. Immun. 67:5664–5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ngampasutadol J., Rice P. A., Walsh M. T., Gulati S. 2006. Characterization of a peptide vaccine candidate mimicking an oligosaccharide epitope of Neisseria gonorrhoeae and resultant immune responses and function. Vaccine 24:157–170 [DOI] [PubMed] [Google Scholar]
- 34. Park H. S., Francis K. P., Yu J., Cleary P. P. 2003. Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group A streptococcus. J. Immunol. 171:2532–2537 [DOI] [PubMed] [Google Scholar]
- 35. Pellati D., et al. 2008. Genital tract infections and infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 140:3–11 [DOI] [PubMed] [Google Scholar]
- 36. Plante M., et al. 2000. Intranasal immunization with gonococcal outer membrane preparations reduces the duration of vaginal colonization of mice by Neisseria gonorrhoeae. J. Infect. Dis. 182:848–855 [DOI] [PubMed] [Google Scholar]
- 37. Plante M., et al. 1999. Antigenic and molecular conservation of the gonococcal NspA protein. Infect. Immun. 67:2855–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Price G. A., Hobbs M. M., Cornelissen C. N. 2004. Immunogenicity of gonococcal transferrin binding proteins during natural infections. Infect. Immun. 72:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Price G. A., Russell M. W., Cornelissen C. N. 2005. Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice. Infect. Immun. 73:3945–3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 41. Takei M., Yamaguchi Y., Fukuda H., Yasuda M., Deguchi T. 2005. Cultivation of Neisseria gonorrhoeae in liquid media and determination of its in vitro susceptibilities to quinolones. J. Clin. Microbiol. 43:4321–4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tapsall J. W. 2009. Neisseria gonorrhoeae and emerging resistance to extended spectrum cephalosporins. Curr. Opin. Infect. Dis. 22:87–91 [DOI] [PubMed] [Google Scholar]
- 43. Thacker E. L., Thacker B. J., Kuhn M., Hawkins P. A., Waters W. R. 2000. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am. J. Vet. Res. 61:1384–1389 [DOI] [PubMed] [Google Scholar]
- 44. Thomas C. E., et al. 2006. Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan equine encephalitis viral replicon particles. Infect. Immun. 74:1612–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wetzler L. M., Blake M. S., Barry K., Gotschlich E. C. 1992. Gonococcal porin vaccine evaluation: comparison of Por proteosomes, liposomes, and blebs isolated from rmp deletion mutants. J. Infect. Dis. 166:551–555 [DOI] [PubMed] [Google Scholar]
- 46. World Health Organization. 2001. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/pub/sti/who_hiv_aids_2001.02.pdf [Google Scholar]
- 47. Zhu W., et al. 2005. Comparison of immune responses to gonococcal PorB delivered as outer membrane vesicles, recombinant protein, or Venezuelan equine encephalitis virus replicon particles. Infect. Immun. 73:7558–7568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu W., Thomas C. E., Sparling P. F. 2004. DNA immunization of mice with a plasmid encoding Neisseria gonorrhea PorB protein by intramuscular injection and epidermal particle bombardment. Vaccine 22:660–669 [DOI] [PubMed] [Google Scholar]