Abstract
Dengue virus (DV) IgM/IgG ratio and IgG avidity value (AV) can reliably distinguish between primary and secondary DV infections using sera collected within 30 days of disease onset, but little is known about their efficacies using sera collected >30 days after onset. To investigate this issue, we analyzed specimens submitted to our reference laboratory for DV antibody testing. We first classified patients as having primary (n = 55) or secondary (n = 58) infections based on seroconversion patterns in a comparison of two sera collected <30 days apart. We then evaluated IgM/IgG ratios and IgG AVs of the second specimens by using receiver operating characteristic curve analysis. The IgM/IgG ratio that best discriminated primary from secondary infection was 1.32; 95% of 55 primary infections exhibited ratios of >1.32, whereas 93% of 58 secondary infections exhibited ratios of ≤1.32. The discriminatory AV was 0.39; 95% of 41 primary infections exhibited AVs of ≤0.39, whereas 95% of 38 secondary infections exhibited AVs of >0.39. We then evaluated the IgM/IgG ratios and AV for primary-infection patients whose second serum samples were collected ≥30 days after the first serum samples; only 56% of 27 sera exhibited ratios of >1.32, whereas 81% of 21 sera exhibited AVs of ≤0.39. Assuming that the first specimens were collected within a week after symptoms appeared, these findings indicate that IgG AV is superior to the IgM/IgG ratio for distinguishing primary from secondary DV infections when using samples collected more than 5 weeks after disease onset.
INTRODUCTION
Infection with dengue virus (DV) poses a major public health burden in tropical and subtropical areas worldwide; many cases are associated with significant morbidity, ranging from a nonspecific febrile illness to severe hemorrhagic fever (8, 16, 23). Primary infection with any of the four DV serotypes induces an immune response that protects against later infection by that serotype; however, later infection by another serotype, referred to as secondary DV infection, is a risk factor for dengue hemorrhagic fever (17, 19, 25). Discrimination of primary from secondary DV infections is also a valuable epidemiological tool, providing information useful in determining if specific DV serotypes have been recently introduced or reintroduced within a given geographic area (22).
DV IgM and IgG seroconversion patterns accurately distinguish primary from secondary DV infections (2, 5); for many patients, however, only one sample is available for testing, and it exhibits a DV IgM+ IgG+ reactivity pattern indicating that seroconversion has already occurred. In this setting, a reliable approach for determining if this IgM+ IgG+ result represents primary or secondary DV infection would be advantageous. Several groups have shown that the DV IgM/IgG ratio for sera collected within 30 days of symptom onset can be used to accurately classify patients as having primary or secondary infection (5, 6, 11, 13, 22). The discriminatory ratios range from 1.2 to 2.0, depending on the laboratory's assays and interpretation protocols. Within a given laboratory, however, the IgM/IgG ratio shows approximately 95% accuracy for discriminating primary from secondary DV infection. The discriminatory power of the IgM/IgG ratio reflects differences in DV-specific IgM and IgG production in primary versus secondary infections. In primary infection, high levels of DV IgM develop within a few days of disease onset, followed a few days later by production of DV IgG at moderate levels (1, 2, 8, 10, 12, 20, 21, 26). In secondary infection, IgM is detected a few days later and at lower levels than in primary infection, and IgG rapidly increases to very high levels (8, 10, 11, 20, 24–26). Thus, sera from patients with recent primary DV infection typically exhibit ratios greater than the discriminator ratio, whereas sera from patients with recent secondary infection typically exhibit ratios less than the discriminator ratio. Over time, primary-infection patients are expected to show a decrease in IgM levels and an increase in IgG levels and thus at some point should exhibit IgM/IgG ratios characteristic of recent secondary DV infection (8, 10, 11, 13, 24, 25); however, the nature of this temporal shift from high ratio to low ratio in primary DV infection has not been systematically evaluated in either cross-sectional or cohort follow-up studies.
DV IgG avidity, a measure of the strength with which IgG attaches to antigen, is also an effective discriminator of primary from secondary DV infections. Studies have shown that during the first month after disease onset, DV IgG avidity value (AV) is typically low in primary infections but high in secondary infections (4–6, 14, 15). The accuracy of DV IgG AV for distinguishing primary from secondary infections is approximately 98% (4–6, 14, 15). As is the case for IgG levels, the IgG AV is expected to increase over time following primary infection (9), eventually reaching a value characteristic of recent secondary infection; however, the timeline for DV IgG avidity maturation is not well-characterized.
Most studies of the efficacy of DV IgM/IgG ratio and IgG AV for distinguishing between primary and secondary DV infection utilized sera collected within 30 days of symptom onset. Little is known about the reliability of the IgM/IgG ratio and IgG AV for discriminating primary and secondary infections using samples collected at later time points. We thus sought to establish the discriminatory IgM/IgG ratio and IgG AV for distinguishing primary from secondary infection in our laboratory using second-draw sera collected <30 days after first-draw sera. We then assessed the reliability of these discriminatory cutoffs when applied to second sera collected ≥30 days after first sera from primary-infection patients.
MATERIALS AND METHODS
Specimens.
Serum specimens submitted to Focus Diagnostics for DV antibody testing were evaluated using validated laboratory-developed assays for DV IgG and IgM. Clinical information (e.g., time since onset of symptoms) was not supplied for any of the specimens.
DV IgG measurement.
The DV IgG assay was an indirect enzyme-linked immunosorbent assay (ELISA) (7) that utilized polystyrene microtiter wells coated with inactivated purified DV types 1 to 4 (internally developed). Each assay run included internally developed negative control and positive control sera as well as a calibrator serum sample. Control, calibrator, and patient sera were diluted 1:101 in sample buffer, and 0.1 ml of diluted specimen was added to assigned microtiter wells. Following incubation for 1 h at room temperature (RT), the wells were washed with phosphate-buffered saline containing Tween (PBST) and then received peroxidase-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). After 30 min at RT and washing, wells received tetramethylbenzidine (Neogen Corp., Lexington, KY); after 10 min, wells received dilute sulfuric acid (Ricca Chemicals, Arlington, TX) to stop the reaction. Absorbance was measured at 450 nm using an ELISA reader (BioTek, Winooski, VT). Results were expressed as an index, calculated by dividing the specimen absorbance value by the calibrator serum absorbance value; index values of >1.10 were considered positive.
DV IgM measurement.
The DV IgM assay was a mu-capture ELISA performed as previously described (3, 7, 18). Briefly, diluted control, calibrator, and patient sera were added to microtiter wells coated with rabbit anti-human IgM (Jackson ImmunoResearch); after 1 h at RT, the wells were washed and then received inactivated DV antigen (containing all 4 DV serotypes, internally developed). After 2 h at RT and washing, the wells received peroxidase-conjugated 6B6C anti-flavivirus monoclonal antibody (Scantibodies Laboratory, Santee, CA). After incubation for 30 min at RT and washing, the wells received tetramethylbenzidine and 10 min later received dilute sulfuric acid. Absorbance at 450 nm was measured, and results were expressed as an index, calculated and interpreted as described for the IgG ELISA. Sera with positive results were further tested using a background subtraction assay to identify samples giving false-positive results due to heterophilic activity (18).
DV IgM/IgG ratio.
The DV IgM/IgG ratio was calculated for sera positive for DV IgG and IgM by dividing the IgM index by the IgG index.
Definition of acute primary and secondary DV infections.
Criteria consistent with those described by other investigators (2, 5) were used to define primary and secondary DV infections; these criteria were based on seroconversion patterns in a comparison of two specimens from a given patient. Primary infection was defined as an IgM-negative/IgG-negative (pattern 1) or IgM-positive/IgG-negative (pattern 2) first specimen and an IgM-positive/IgG-positive second specimen. Secondary infection was defined as an IgM-negative/IgG-positive first specimen and an IgM-positive/IgG-positive second specimen (pattern 3).
DV IgG avidity measurement.
The routine DV IgG ELISA procedure was modified as previously described (4, 5) to measure DV IgG avidity. IgG-positive sera were diluted as described for the IgG ELISA and added to duplicate microtiter wells. After an hour at RT, the well contents were discarded. PBST was then added to one of each pair of duplicate wells, whereas dissociating buffer (PBST containing 7 M urea [MP Biomedicals, Santa Ana, CA]) was added to the other well. After 10 min at RT, the well contents were discarded and the wash procedure was repeated (including the 10-min incubation step). All wells were washed once more with PBST; the assay was then completed as described for the DV IgG ELISA, and absorbance at 450 nm was measured. For a given specimen, the avidity value (AV) was calculated by dividing the absorbance value obtained for the well washed with urea buffer by the absorbance value obtained for the well washed with PBST. Specimens giving absorbance values of >3.5 for the PBST-washed well were retested at 1:1,010 and 1:10,100 dilutions; the dilution giving a PBST-washed well absorbance closer to, but not greater than, 3.5 was used to calculate the AV.
Statistical analyses.
Receiver operating characteristic (ROC) curve analyses were performed using MedCalc software (Mariakerke, Belgium). Differences among proportions were evaluated by chi-square analysis (MedCalc software), and group means were compared using Student's t test (MedCalc software). Significance was defined as P < 0.05.
RESULTS
Table 1 presents information on the seroconversion patterns used to define primary and secondary infections and the number of patients per group segregated by the number of days between the first and second serum samples. Overall, 82 patients with primary infection and 63 patients with secondary infection were identified. Patients with primary infection were divided roughly evenly between seroconversion pattern 1 and pattern 2. Median index values for sera exhibiting seroconversion pattern 1 were as follows: first-sample IgM 0.44/IgG 0.31, second-sample IgM 10.07/IgG 3.58. Median index values for sera exhibiting pattern 2 were as follows: first-sample IgM 5.04/IgG 0.28, second-sample IgM 7.58/IgG 3.13. For all subsequent analyses, patients showing a primary infection pattern were considered as a single group. Median index values for sera exhibiting seroconversion pattern 3 (secondary infection) were as follows: first-sample IgM 0.54/IgG 4.28, second-sample IgM 4.36/IgG 8.48.
Table 1.
Dengue virus IgM and IgG seroconversion patterns by interval between collection of first and second specimens
| Results pattern | Seroconversion fora: |
No. of patients by days between samplesb |
|||
|---|---|---|---|---|---|
| First sample | Second sample | <30 days | ≥30 days | Total | |
| Primary infection | |||||
| Pattern 1 | IgM-neg/IgG-neg | IgM-pos/IgG-pos | 28 (20) | 12 (8) | 40 (28) |
| Pattern 2 | IgM-pos/IgG-neg | IgM-pos/IgG-pos | 27 (21) | 15 (13) | 42 (34) |
| Secondary infection | |||||
| Pattern 3 | IgM-neg/IgG-pos | IgM-pos/IgG-pos | 58 (38) | 5 (1) | 63 (39) |
neg, negative; pos, positive.
Numbers in parentheses indicate the number of patients with second-draw specimens available for IgG avidity testing.
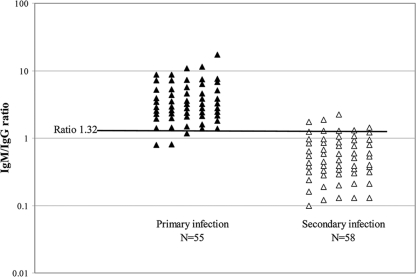
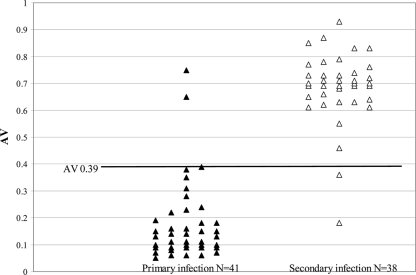
To identify the IgM/IgG ratio and IgG AV that best distinguish primary and secondary infections in our laboratory, second-specimen ratio and AV results for primary- and secondary-infection patients whose second specimens were collected <30 days after the first specimens were subjected to ROC curve analysis. Figure 1 shows the distribution of the IgM/IgG ratio values and identifies the discriminatory value as 1.32; 52/55 (95%) primary infections exhibited ratios of >1.32, whereas 54/58 (93%) secondary infections exhibited ratios of ≤1.32. Figure 2 shows the distribution of IgG AVs and identifies the discriminatory value as 0.39; 39/41 (95%) primary-infection sera available for IgG avidity testing exhibited AVs of ≤0.39, whereas 36/38 (95%) secondary-infection sera exhibited AVs of >0.39.
Fig. 1.
Distribution of second-specimen DV IgM/IgG ratios for patients with primary or secondary DV infections as defined by seroconversion patterns in a comparison of two sera collected <30 days apart. Results are shown using a logarithmic scale and in 5 columns per group to enable better visualization of individual values. The dark horizontal line indicates the discriminatory ratio of 1.32 as determined by ROC curve analysis.
Fig. 2.
Distribution of second-specimen DV IgG AVs for patients with primary or secondary DV infections as defined by seroconversion patterns in a comparison of two sera collected <30 days apart. The dark horizontal line indicates the discriminatory AV of 0.39 as determined by ROC curve analysis.
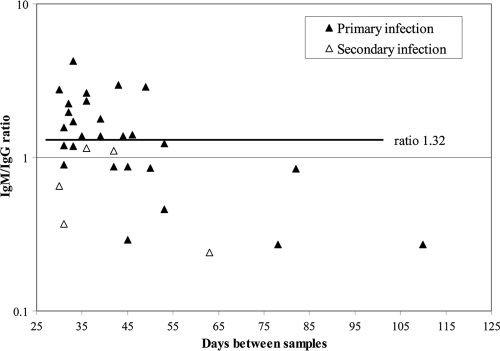
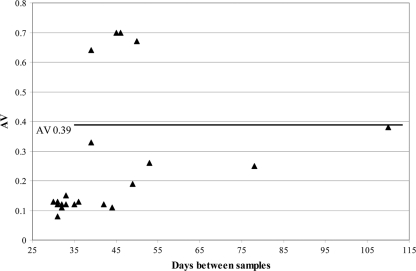
We next investigated the efficacy of the discriminatory IgM/IgG ratio when using second specimens collected ≥30 days after the first specimens from patients with primary or secondary infections. Figure 3 presents second-specimen IgM/IgG ratios plotted as a function of days between first and second samples for 27 primary-infection and 5 secondary-infection patients; only 15/27 (56%) sera from primary-infection patients exhibited a ratio of >1.32, indicating primary infection. This percentage was significantly lower than the 95% observed for primary-infection patients whose second specimens were collected <30 days after the first specimens (P = 0.0001). All 5 sera from secondary-infection patients with ≥30 days between samples exhibited IgM/IgG ratios of <1.32 (Fig. 3).
Fig. 3.
Distribution of second-specimen DV IgM/IgG ratios for patients with primary or secondary DV infections as defined by seroconversion patterns in a comparison of two sera collected ≥30 days apart. The dark horizontal line indicates the discriminatory ratio for sera collected <30 days apart (refer to Fig. 1).
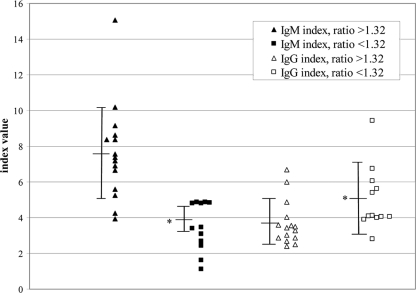
The IgM and IgG indices among primary-infection patients whose second samples were collected ≥30 days after the first samples are shown in Fig. 4. For the 12 primary-infection patients with IgM/IgG ratios of <1.32, the mean IgM index was significantly low and the mean IgG index was significantly high compared to the respective means for the 15 primary-infection patients with ratios of >1.32. Thus, the lower ratios reflected both decreased IgM levels and increased IgG levels.
Fig. 4.
Second-specimen DV IgM and IgG index values for the 27 patients in the primary-infection ≥30-day group, segregated on the basis of the IgM/IgG ratio. Three horizontal bars connected by a vertical bar represent the group mean ± 1 standard deviation. An asterisk indicates that the mean value was significantly different from that of the >1.32-ratio group of the same antibody class.
Figure 5 presents the results for studies investigating the efficacy of the discriminatory IgG AV when using second specimens collected ≥30 days after the first specimens from primary-infection patients. Of 21 available second specimens, 17 (81%) exhibited IgG AVs of ≤0.39, consistent with primary infection; this percentage was not significantly different from the 95% observed for primary-infection patients whose second specimens were collected <30 days after the first specimens (P = 0.21). This proportion of primary-infection patients in the ≥30-day group with AVs of ≤0.39 (81%) was higher than the proportion of patients in this group with an IgM/IgG ratio of >1.32 (56%), but the difference did not reach statistical significance (P = 0.12). Only one serum sample from a secondary-infection patient with ≥30 days between samples was available for IgG avidity testing; the sample was collected 31 days after the first specimen and had an AV of 0.62, consistent with secondary infection.
Fig. 5.
Distribution of second-specimen DV IgG AVs for patients with primary DV infections as defined by seroconversion patterns in a comparison of two sera collected ≥30 days apart. The dark horizontal line indicates the discriminatory AV for sera collected <30 days apart (refer to Fig. 2).
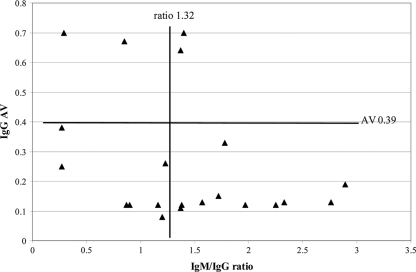
Figure 6 shows the relationship between the IgM/IgG ratio and IgG AV for the 21 patients in the ≥30-day primary infection group with available results for both analytes. Overall agreement between the methods was 57% (12/21). Of the 17 patients correctly classified as having primary infections based on an AV of ≤0.39, 10 (59%) were also correctly classified based on a ratio of >1.32, and 7 (41%) were incorrectly classified based on a ratio of ≤1.32. From another viewpoint, AV correctly classified 7 of 9 (78%) primary-infection patients who were incorrectly classified as secondary-infection patients based on an IgM/IgG ratio of ≤1.32. Of the 4 patients incorrectly classified as having secondary infections based on an AV of >0.39, 2 (50%) were also incorrectly classified based on a ratio of ≤1.32.
Fig. 6.
Relationship between second-specimen DV IgM/IgG ratio and IgG AV for 21 patients with primary DV infections as defined by seroconversion patterns in a comparison of two sera collected ≥30 days apart. The vertical line indicates the discriminatory ratio for sera collected <30 days apart (refer to Fig. 1), and the horizontal line indicates the discriminatory AV for sera collected <30 days apart (refer to Fig. 2). Quadrants: top left, secondary infection by IgG AV and IgM/IgG ratio; bottom left, primary infection by IgG AV, secondary infection by IgM/IgG ratio; top right, secondary infection by IgG AV, primary infection by IgM/IgG ratio; bottom right, primary infection by IgG AV and IgM/IgG ratio.
DISCUSSION
In a reference laboratory setting, clinical information typically does not accompany specimens submitted for testing. Thus, defining our laboratory's IgM/IgG ratio and IgG AV cutoffs for distinguishing primary and secondary DV infections using sera collected at known time points following disease onset was not an option. We reasoned, however, that using time windows based on the number of days between the collection of two sera demonstrating distinctive primary and secondary seroconversion patterns could serve as a reliable alternative approach. A major assumption inherent to this reasoning is that the first of the two samples was collected during the acute phase of infection (i.e., within the first week after symptom onset). During this time period, one would expect patients with primary infection to be either negative for both DV IgM and IgG or positive for IgM only, whereas patients with secondary infection would be positive for IgG only (8, 10, 12, 20, 21, 26). Our cutoffs for IgM/IgG ratio and IgG AV correctly classified ≥93% of primary and secondary infections using second samples collected <30 days after the first sample; thus, these cutoffs can be interpreted as indicators of primary and secondary infection <37 days (i.e., within approximately 5 weeks) after disease onset.
Our cutoff IgM/IgG ratio of 1.32 was consistent with the published (5, 6, 11, 13, 22) range of cutoff ratios (1.2 to 2.0) and was particularly similar to the 1.4 cutoff used by Kuno et al. (13), whose assays were most similar to ours (IgM capture ELISA, indirect IgG ELISA). The proportions of primary or secondary infections in the <30-day group that were classified correctly using our ratio cutoff (95% and 93%, respectively) were also consistent with published values (5, 6, 11, 13, 22). Our cutoff AV of 0.39 was consistent with the range of published (4–6, 14, 15) cutoff AVs (0.24 to 0.50), as were the proportions of primary and secondary infections in the <30-day group classified correctly (95% for both) (4–6, 14, 15).
The major focus of our study was to determine the efficacy of cutoffs established for the <30-day group when applied to second samples collected from primary-infection patients ≥30 days after the first sample. Scant information is available for such specimens; in the only relevant publication identified, Kuno et al. (13) reported that sera collected >66 days after disease onset from two patients with primary DV infection no longer exhibited IgM/IgG ratios indicative of primary infection. The primary infections in the ≥30-day group misclassified by ratio had significantly low IgM indices and significantly high IgG indices compared to correctly classified samples; visual examination of Fig. 4 suggests that the impact of decreased IgM indices was more marked than the increase in IgG indices. These findings indicate that some primary-infection patients begin showing shifts in DV IgM and IgG levels sooner than others and that these shifts have a major effect on the IgM/IgG ratio. Of the 9 primary-infection patients in the ≥30-day group misclassified by ratio and also tested with the IgG avidity assay, 7 (78%) were correctly classified by AV. Thus, although the proportion of primary-infection patients in the ≥30-day group correctly classified by avidity versus ratio did not reach statistical significance, at the individual patient level, IgG AV was clearly better than the IgM/IgG ratio for classifying patients in this group.
The 2 primary infections in the <30-day group and the 4 primary infections in the ≥30-day group that were misclassified by AV all had AVs of >0.60, well above the cutoff of 0.39. It is possible that these cases represent primary DV infections in individuals previously exposed (via infection or vaccination) to other flaviviruses, such as yellow fever virus and Japanese encephalitis virus. Domingo et al. (6) showed that such patients produce high-avidity flavivirus-specific IgG in response to primary DV infection. Also of interest is that 3 of these 6 patients were also misclassified by the IgM/IgG ratio, consistent with a blunted IgM response and anamnestic production of flavivirus IgG following primary DV infection.
A limitation of our study is the small number of secondary-infection patients in the ≥30-day group; only 5 with ratio data and 1 with AV data were available. We assume that most sera from such patients would exhibit ratios of ≤1.32 and AVs of >0.39, since these parameters would not be expected to revert to values typical of primary infections; however, testing of additional samples is required to validate this assumption.
Another study limitation is that the small number of sera collected >50 days after the first sample prevented us from estimating how long IgG AV remains a reliable discriminator of primary versus secondary DV infections. Further, the relationship between the expected gradual increase in AV and IgM persistence following primary infection has not been characterized. Is there a time window where IgG AV is ≥0.39 but IgM is still detectable following primary infection, or does IgM decrease to undetectable levels before IgG AVs of ≥0.39 are reached? Long-term follow-up studies of newly identified primary and secondary DV infections are needed to address these questions.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Alcon S., et al. 2002. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blacksell S. D., et al. 2008. Evaluation of the Panbio dengue virus nonstructural 1 antigen detection and immunoglobulin M antibody enzyme-linked immunosorbent assays for the diagnosis of acute dengue infections in Laos. Diagn. Microbiol. Infect. Dis. 60:43–49 [DOI] [PubMed] [Google Scholar]
- 3. Branch S. L., Levett P. N. 1999. Evaluation of four methods for detection of immunoglobulin M antibodies to dengue virus. Clin. Diagn. Lab. Immunol. 6:555–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Souza V. A. U. F., et al. 2004. Use of an immunoglobulin G avidity test to discriminate between primary and secondary dengue virus infections. J. Clin. Microbiol. 42:1782–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Souza V. A. U. F., et al. 2007. Sensitivity and specificity of three ELISA-based assays for discriminating primary from secondary acute dengue virus infection. J. Clin. Virol. 39:230–233 [DOI] [PubMed] [Google Scholar]
- 6. Domingo C., et al. 2009. Molecular and serologic markers of acute dengue infection in naïve and flavivirus-vaccinated travelers. Diagn. Microbiol. Infect. Dis. 65:42–48 [DOI] [PubMed] [Google Scholar]
- 7. Groen J., Koraka P., Velzing J., Copra C., Osterhaus A. D. 2000. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin. Diagn. Lab. Immunol. 7:867–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gubler D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gutierrez J., Maroto C. 1996. Are IgG antibody avidity assays useful in the diagnosis of infectious diseases? A review. Microbios 87:113–121 [PubMed] [Google Scholar]
- 10. Guzman M. G., Kouri G. 1996. Advances in dengue diagnosis. Clin. Diagn. Lab. Immunol. 3:621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Innis B. L., et al. 1989. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am. J. Trop. Med. Hyg. 40:418–427 [DOI] [PubMed] [Google Scholar]
- 12. Koraka P., et al. 2001. Kinetics of dengue virus-specific immunoglobulin classes and subclasses correlate with clinical outcome of infection. J. Clin. Microbiol. 39:4332–4338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuno G., Gomez I., Gubler D. J. 1991. An ELISA procedure for the diagnosis of dengue infections. J. Virol. Methods 33:101–113 [DOI] [PubMed] [Google Scholar]
- 14. Matheus S., et al. 2005. Discrimination between primary and secondary dengue virus infection by an immunoglobulin G avidity test using a single acute-phase serum sample. J. Clin. Microbiol. 43:2793–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matheus S., et al. 2005. Use of four dengue virus antigens for determination of dengue immune status by enzyme-linked immunosorbent assay of immunoglobulin G avidity. J. Clin. Microbiol. 43:5785–5786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monath T. P. 1994. Dengue: the risk to developed and developing countries. Proc. Natl. Acad. Sci. U. S. A. 91:2395–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morens D. M., Fauci A. S. 2008. Dengue and hemorrhagic fever. A potential threat to public health in the United States. JAMA 299:214–216 [DOI] [PubMed] [Google Scholar]
- 18. Prince H. E., Yeh C., Lapé-Nixon M. 2008. Development of a more efficient algorithm for identifying false-positive reactivity results in a dengue virus immunoglobulin M screening assay. Clin. Vaccine Immunol. 15:1304–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rothman A. L., Ennis F. A. 1999. Immunopathogenesis of dengue hemorrhagic fever. Virology 257:1–6 [DOI] [PubMed] [Google Scholar]
- 20. Sa-Ngasang A., et al. 2006. Specific IgM and IgG responses in primary and secondary dengue virus infections determined by enzyme-linked immunosorbent assay. Epidemiol. Infect. 134:820–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schilling S., Ludolfs D., An L. V., Schmitz H. 2004. Laboratory diagnosis of primary and secondary dengue infection. J. Clin. Virol. 31:179–184 [DOI] [PubMed] [Google Scholar]
- 22. Shu P-Y., et al. 2003. Comparison of capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) and nonstructural protein S1 serotype-specific IgG ELISA for differentiation of primary and secondary dengue virus infections. Clin. Diagn. Lab. Immunol. 10:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solomon T., Mallewa M. 2001. Dengue and other emerging flaviviruses. J. Infect. 42:104–115 [DOI] [PubMed] [Google Scholar]
- 24. Vaughn D. W., et al. 1997. Dengue in the early febrile phase: viremia and antibody responses. J. Infect. Dis. 176:322–330 [DOI] [PubMed] [Google Scholar]
- 25. Vaughn D. W., et al. 1999. Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am. J. Trop. Med. Hyg. 60:693–698 [DOI] [PubMed] [Google Scholar]
- 26. Vazquez S., et al. 2007. Kinetics of antibodies in sera, saliva, and urine samples from adult patients with primary or secondary dengue 3 virus infections. Int. J. Infect. Dis. 11:256–262 [DOI] [PubMed] [Google Scholar]