Abstract
In the Global Polio Eradication Initiative, laboratory diagnosis plays a critical role by isolating and identifying poliovirus (PV) from the stool samples from acute flaccid paralysis (AFP) cases. In recent years, reestablishment of PV circulation in countries where PV was previously eliminated has occurred because of decreased herd immunity, possibly due to poor vaccination coverage. To monitor the vulnerability of countries to PV circulation, surveillance of neutralizing-antibody titers against PV in susceptible populations is essential in the end game of the polio eradication program. In this study, we have developed a PV neutralization test with type 1, 2, and 3 PV pseudoviruses to determine the neutralizing-antibody titer against PV in human serum samples. With this test, the neutralizing-antibody titer against PV could be determined within 2 days by automated interpretation of luciferase signals without using infectious PV strains. We validated the pseudovirus PV neutralization test with 131 human serum samples collected from a wide range of age groups (ages 1 to >60 years) by comparison with a conventional neutralization test. We found good correlation in the neutralizing-antibody titers determined by these tests. These results suggest that a pseudovirus PV neutralization test would serve as a safe and simple procedure for the measurement of the neutralizing-antibody titer against PV.
INTRODUCTION
In the Global Polio Eradication Initiative, laboratory diagnosis plays a critical role by isolating and identifying poliovirus (PV) from the stool samples from acute flaccid paralysis (AFP) cases for surveillance of PV circulation. In the World Health Organization (WHO) Global Polio Laboratory Network, PV isolation and identification have been performed at WHO national polio laboratories in a cell culture system (18, 19), followed by differentiation of the isolates into oral PV vaccine (OPV)-related PV, vaccine-derived PV (VDPV), and wild-type PV isolates by several methods at WHO regional reference laboratories (12, 19). Surveillance of PV is essential for monitoring the progress of PV eradication in countries where PV is endemic (4 countries as of 2011) and for the maintenance of the polio-free status of countries where PV is not endemic by preventing circulation of imported PVs or VDPVs from countries where PV is endemic through proper vaccination campaigns.
In the end game of the eradication program, surveillance of seroprevalence against PV in susceptible populations is essential for monitoring vulnerability to PV circulation in PV-free countries to sustain their PV-free status and the seroconversion rates in countries where PV is endemic to evaluate the effectiveness of vaccination strategies. In laboratories, the neutralizing-antibody titer has been determined by a conventional PV neutralization test (cPNT) using a susceptible cell culture system and infectious challenge virus (20). Characteristic requirements for a cPNT are as follows: (i) use of infectious virus (usually OPV strains are used), (ii) expertise of personnel (for observation of cytopathic effect [CPE] in inoculated cells), and (iii) extended time for results (5 to 7 days of culture). In Japan, surveillance of neutralizing antibody against PV has been performed every 2 or 3 years since 1974 for serum samples from healthy volunteers (about 1,100 to 1,800 individuals in 6 to 8 prefectures) in a wide range of ages (0 to >40 years) based on cPNTs in prefectural laboratories (http://idsc.nih.go.jp/yosoku/Polio/Year-P2009.html) (9). Considering the biosafety and expertise required for the test, a PV neutralization test that is safer and simpler than cPNT would be desirable in the end game of the eradication program.
In the present study, we have developed a novel PV neutralization test using non-self-proliferating PV pseudovirus, which encapsidated luciferase-encoding PV replicons with PV capsid proteins (2). In a pseudovirus PV neutralization test (pPNT), the neutralizing-antibody titer was determined based on the luciferase signals in inoculated cells within 2 days. The results suggested that pPNT would serve as a safe and simple procedure for the measurement of the neutralizing-antibody titer against PV.
MATERIALS AND METHODS
Cells, viruses, and human sera.
RD cells (human rhabdomyosarcoma cells) and HEK293 cells (human embryonic kidney cells) were cultured as monolayers in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS). Vero cells (African green monkey kidney cells) were cultured as monolayers in Eagle's minimum essential medium (EMEM) supplemented with 0.11% bovine serum albumin (BSA) (fraction V; Sigma). RD cells were used for the titration of PV and for the pPNT. Vero cells were used for the pPNT. HEK293 cells were used for production of PV pseudoviruses. PV pseudoviruses, which encapsidated luciferase-encoding PV replicons with PV capsid proteins derived from PV1Mahoney, PV2MEF-1, and PV3Saukett A, were prepared as reported previously (2) (see below for the construction of type 2 and 3 PV capsid protein expression vectors and preparation of PV pseudoviruses). Human sera were collected from healthy volunteers (aged 1 to 76 years) with informed consent. The experiments performed in the present study were approved by the Committee for Ethical Regulation of the National Institute of Infectious Diseases, Japan.
General methods of molecular cloning.
Escherichia coli strain XL10gold (Stratagene) was used for the preparation of plasmids. Ligation of DNA fragments was performed using a quick ligation kit (New England BioLabs). PCR was performed using KOD Plus DNA polymerase (Toyobo). Reverse transcription-PCR (RT-PCR) was performed using a ReverTra Plus kit (Toyobo). DNA sequencing was performed using a BigDye Terminator v3.0 cycle-sequencing ready-reaction kit (Applied Biosystems) and then analyzed with a 3130 genetic analyzer (Applied Biosystems).
Construction of type 2 and 3 PV capsid expression vectors.
For the construction of expression vectors of type 2 and 3 PV capsid proteins, we fused the enhanced green fluorescent protein (EGFP) gene to a PV capsid protein coding region of PV2MEF-1 or of PV3Saukett A by PCR to measure the expression levels of PV capsid proteins. EGFP coding regions were amplified by PCR with primers 5′TGGTAAGCTTACCATGGGAGCTCTGAGCAA3′ and 5′ATGAGACTTGGGCGCCGTAGGTGGTCAGGC3′ (for fusion with the type 2 PV capsid protein coding region) or primers 5′TGGTAAGCTTACCATGGGAGCTCTGAGCAA3′ and 5′CGAAACTTGAGCTCCGTAGGTGGTCAGGCC3′ (for fusion with the type 3 PV capsid protein coding region) (HindIII sites in the primers are underlined) using pIRES2-EGFP (Clontech) as the template. The type 2 PV capsid protein coding region was amplified by RT-PCR with primers 5′GCCTGACCACCTACGGCGCCCAAGTCTCAT3′ and 5′TTAATCTAGATTAATAGGTTGTCAAGCCTT3′ (the XbaI site is underlined) using viral genomic RNA of PV2MEF-1 as the template. The type 3 PV capsid protein coding region was amplified by RT-PCR with primers 5′GGCCTGACCACCTACGGAGCTCAAGTTTCG3′ and 5′TTAAGGATCCTTAATAGGTGGTCAAACCTT3′ (the BamHI site is underlined) using viral genomic RNA of PV3Saukett A as the template. Next, these DNA fragments of EGFP and PV capsid protein coding regions were fused by PCR using the primers 5′TGGTAAGCTTACCATGGGAGCTCTGAGCAA3′ and 5′TTAATCTAGATTAATAGGTTGTCAAGCCTT3′ (for type 2 PV) (the HindIII and XbaI sites are underlined) or primers 5′TGGTAAGCTTACCATGGGAGCTCTGAGCAA3′ and 5′TTAAGGATCCTTAATAGGTGGTCAAACCTT3′ (for type 3 PV) (the HindIII and BamHI sites are underlined). The fused DNA fragments were then cloned into the pKS435 expression vector (a kind gift from Koji Sakai, AIDS Research Center, National Institute of Infectious Diseases, Tokyo, Japan) (2), following digestion by HindIII and XbaI (for type 2 PV) or by HindIII and BamHI (for type 3 PV). The resultant plasmids were named pKS435-EGFP-PV2(MEF-1) and pKS435-EGFP-PV3(Saukett A), respectively, and used as PV capsid protein expression vectors for production of PV pseudoviruses (1, 2).
Preparation of PV pseudovirus.
PV pseudoviruses were prepared as reported previously with modifications (2). Briefly, a six-well plate (Falcon) with a 10% confluent monolayer of HEK293 cells was transfected with 2 μg of corresponding PV capsid expression vectors per well using a Lipofectamine 2000 reagent (Invitrogen), and the cells were incubated at 37°C in 2 ml DMEM supplemented with 10% FCS per well for 48 h. RNA transcripts of the PV replicon were obtained by using a RiboMax large-scale RNA production system-T7 kit (Promega) with DraI-linearized DNA of pPV-Fluc mc, which includes a PV replicon based on PV1Mahoney that has a firefly luciferase gene instead of the capsid-coding region, as the template. RNA transcripts were transfected into the monolayer of HEK293 cells transiently expressing PV capsid proteins at 48 h posttransfection using a Lipofectamine RNAiMax reagent (Invitrogen). Cells were harvested at 25 h posttransfection of the RNA transcripts, when most of the cells showed CPE, and then stored at −20°C.
cPNT.
cPNTs were performed according to the standard procedure recommended by the WHO with modifications (20). First, 200 μl of human serum samples and standard anti-PV sera (positive control) were diluted 4-fold with EMEM (1/4 dilution), and then 2-fold dilution series were prepared with EMEM supplemented with 0.11% BSA, resulting in 1/4 to 1/1,024 dilution, and then 50 μl of diluted sera or EMEM supplemented with 0.11% BSA was added to three 96-well plates (2 wells for one dilution in 1 plate; a total of 6 wells for one dilution in 3 plates). Next, 50 μl of type 1, 2, or 3 PV Sabin strains (100 50% cell culture infective doses [CCID50]) was added to each well of the plates (1 plate for each serotype of PV; a total of 3 plates) and then incubated at 37°C for 3 h. After incubation, 100 μl of Vero cell suspension in EMEM supplemented with 0.11% BSA (1.0 × 105 to 2.0 × 105 cells) was added to each well of the plates, and then the plates were incubated at 37°C for 7 days. The neutralizing-antibody titer of the serum against each type of PV was determined as the 50% endpoints of the serum that inhibited the PV infection observed by CPE of inoculated cells.
pPNT.
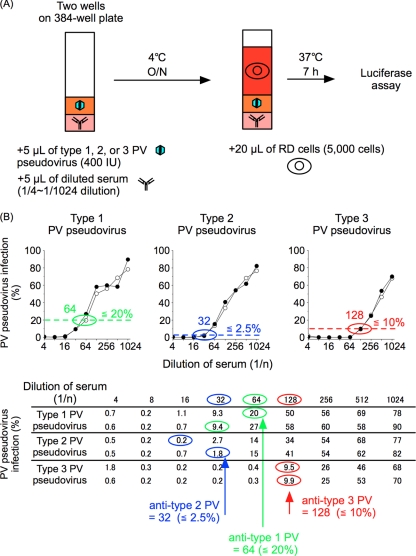
For pPNT, first, 25 μl of human serum samples and standard anti-PV sera (positive control) was diluted with DMEM supplemented 4-fold with 1% FCS (1/4 dilution); 2-fold dilution series were made, resulting in 1/4 to 1/1,024 dilution; and then 5 μl of diluted sera or DMEM supplemented with 1% FCS (mock treatment for the negative control) was added to three 384-well plates (2 wells for one dilution in 1 plate; a total of 6 wells for one dilution in 3 plates) (catalog no. 781080; Greiner Bio-One). Next, 5 μl of type 1, 2, or 3 PV pseudovirus solution (400 infectious units [IU]) was added to each well of the plates (1 plate for each serotype of PV pseudovirus; a total of 3 plates), and then the plates were subjected to centrifugation (700 × g; 10 s; PlateSpin; Kubota). After centrifugation, the plates were sealed with a film (MicroAmp; Applied Biosystem) and then incubated at 4°C overnight. After incubation, 20 μl of RD cell suspension in DMEM supplemented with 5% FCS (5.0 × 103 cells) was added to each well of the plates, and then the plates were incubated at 37°C for 7 h. The luciferase activity of the infected cells was measured at 7 h postinfection (p.i.) with the Steady-Glo Luciferase Assay System (Promega) using a 2030 ARVO X luminometer (PerkinElmer) according to the manufacturer's instructions. PV pseudovirus infection was calculated as a percentage of the luciferase activity of the infected cells, where the luciferase activity in mock-treated cells was taken as 100% (mean signals, 1.4 × 105 to 1.8 × 105 cps; standard deviations, about 20% of the means). The neutralizing antibody titers of the serum against type 1, 2, and 3 PV were determined as the highest dilution of the serum that inhibited each type of PV pseudovirus infection at ≤20%, 2.5%, and 10%, respectively.
RESULTS
Development of a pseudovirus PV neutralization test for measurement of the neutralizing-antibody titer against PV in human sera.
To establish a PV neutralization test using PV pseudovirus, we produced type 1, 2, and 3 PV pseudoviruses, which have a luciferase-encoding PV replicon in type 1, 2, and 3 PV capsid proteins, respectively. In our previous study, we established a production system for type 1 PV pseudovirus with PV1Mahoney capsid proteins (2). In the present study, we constructed PV capsid expression vectors that express capsid proteins of PV2MEF-1 or PV3Saukett A. With these expression vectors and a luciferase-encoding PV replicon, we obtained type 2 and 3 PV pseudoviruses with high titers (2.4 × 107 and 1.1 × 107 IU per ml, respectively) that were comparable to that of type 1 PV pseudovirus (4.4 × 107 IU per ml) (2). We performed serial passages of the type 2 and 3 PV pseudoviruses produced in an attempt to isolate infectious virus that might have emerged in the preparation (from 6.3 × 106 IU of PV pseudoviruses and 3 passages in HEp-2c cells, as previously performed for type 1 PV pseudovirus [2]), but no infectious virus was isolated from type 2 and 3 PV pseudoviruses (data not shown).
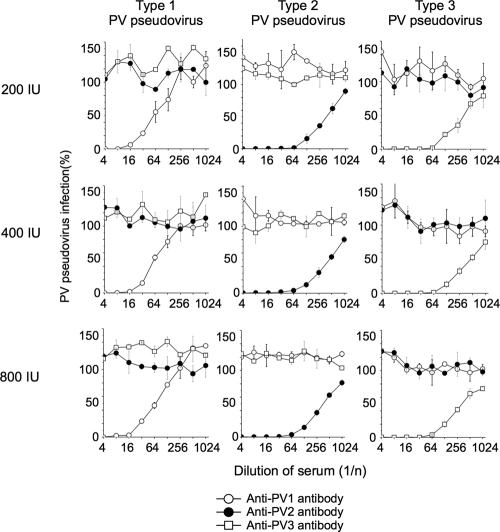
We analyzed the specificity of neutralization of PV pseudoviruses by using type-specific standard anti-PV sera and optimized the titer of PV pseudovirus for the neutralization test (Fig. 1). Different titers of PV pseudoviruses (200, 400, and 800 IU) were incubated with standard anti-PV sera (monkey antisera containing 13 units of neutralizing-antibody titer in 5 μl against type 1, 2, or 3 PV Sabin strains). Neutralization was performed at 4°C overnight according to the conditions used in cPNT. Standard anti-PV sera showed specific neutralization of corresponding types of PV pseudovirus in the range of titers of PV pseudoviruses. Similar neutralization curves were observed in a range of titers of PV pseudoviruses for all the serotypes. The neutralizing activity of the standard serum against type 1 PV pseudovirus was lower than those against type 2 and type 3 PV pseudoviruses. Luciferase signals in the inoculated cells with 200, 400, and 800 IU of PV pseudoviruses were 1.2 × 107 to 1.5 × 107 cps, 2.7 × 107 to 3.3 × 107 cps, and 4.7 × 107 to 5.4 × 107 cps, with standard deviations of 13 to 15%, 7.5 to 14%, and 8.4 to 12% of the means, respectively. In this range of titers of PV pseudoviruses (200 to 800 IU), consistent results were obtained in the specificity and neutralization curves. Therefore, we determined that 400 IU of PV pseudoviruses was the optimal titer to give consistent results in pPNT. A summary of the procedure of pPNT with interpretation is shown in Fig. 2.
Fig. 1.
Optimization of the PV pseudovirus titer for the pseudovirus PV neutralization test. Neutralization curves of PV pseudoviruses (200, 400, and 800 IU) with standard anti-PV sera are shown. The error bars indicate standard deviations.
Fig. 2.
Procedure for pPNT. (A) Schematic representation of the pPNT procedure. O/N, overnight. (B) Interpretation of pPNT. (Top) Neutralization curve of PV pseudoviruses with a human serum sample. The threshold levels for determination of the neutralizing-antibody titer are shown by the dotted lines (≤20%, 2.5%, and 10% for type 1, 2, and 3 PV, respectively), with neutralizing-antibody titers circled (64, 32, and 128 for type 1, 2, and 3 PV, respectively). (Bottom) Original data for the neutralization curve shown at the top. The wells that showed the highest dilution below the threshold values in two lines of measurement are circled. The wells that showed the highest dilutions below the threshold values in the measurement are indicated by arrows for each type of PV pseudovirus.
Measurement of the neutralizing-antibody titer against PV in human sera by cPNT and pPNT.
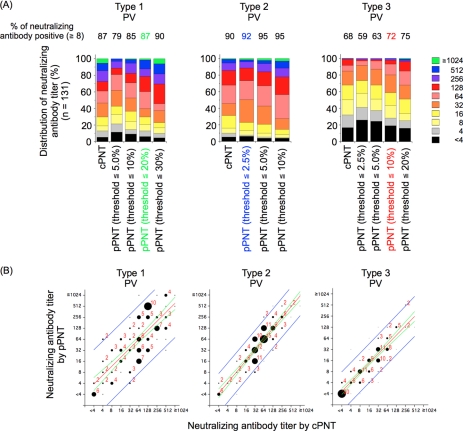
We performed pPNT with 131 human serum samples and compared the results with those obtained by cPNT. In pPNT, the reciprocal of the highest dilution of serum that showed a value equal to or less than the threshold values was determined to be the neutralizing antibody titer (e.g., 64 for type 1 in Fig. 2B). Threshold values for each type of PV were determined by comparison with the results obtained by cPNT to give the anti-PV neutralizing-antibody-positive rates of samples (the percentage of samples that showed neutralizing-antibody titers of ≥8) closest to those determined by cPNT (Fig. 3A). The background signals in the tests corresponded to approximately 0.83% PV pseudovirus infection (standard deviation, 0.72%). Therefore, we could not set the threshold values below 2.5% PV pseudovirus infection for type 2 PV because of the high fluctuation near the background level. According to these criteria, threshold values for type 1, 2, and 3 PV were set at 20, 2.5, and 10% PV pseudovirus infection, respectively. With these threshold values, we observed good correlations in the neutralizing-antibody titers determined by cPNT and pPNT (R = 0.77, 0.90, and 0.88 for type 1, 2, and 3 PV, respectively) (Fig. 3B).
Fig. 3.
Determination of threshold values for each type of PV in pPNT for human sera. (A) Distribution of neutralizing-antibody titers in human serum samples (n = 131) determined by pPNT in a range of threshold values. The results were compared with those obtained by cPNT. The neutralizing-antibody-positive rates of the samples were determined for each threshold value and are shown above the graphs. The threshold values that showed neutralizing-antibody-positive rates closest to those obtained by cPNT are highlighted with color for each type of PV (green, 20%; blue, 2.5%; and red,10% for type 1, 2, and 3 PV, respectively). (B) Scatter plots of neutralizing-antibody titers obtained by cPNT and pPNT. The threshold values used in pPNT were 20%, 2.5%, and 10% for type 1, 2, and 3 PV, respectively. The numbers of the serum samples in the corresponding spots are visualized by the sizes of the circles, along with the number of the sample for each spot when more than one sample was in the same spot. The regression line (red), 95% confidence interval (green), and 95% prediction interval (blue) of the analyses are shown.
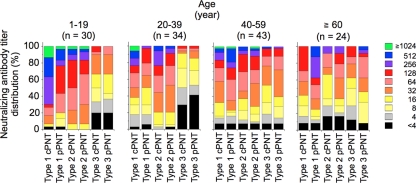
Finally, we compared the neutralizing-antibody titers determined by cPNT and pPNT among different age groups (Fig. 4). For type 2 and 3 PV, the distributions of neutralizing-antibody titers were consistent between cPNT and pPNT for all age groups. However, the distribution of titers for type 1 PV showed different profiles in cPNT and pPNT: lower titers in the young age group (1 to 19 years old) and higher titers in the elderly age group (over 60 years old) by pPNT. This suggested that anti-type 1 PV antibody has different properties among the age groups.
Fig. 4.
Comparison of the neutralizing-antibody titers obtained by cPNT and pPNT in different age groups. The distributions of neutralizing-antibody titers obtained by cPNT and pPNT in the indicated age groups are shown. The numbers of serum samples examined for each age group are shown above the graphs in parentheses. The threshold values for pPNT were 20%, 2.5%, and 10% for type 1, 2, and 3 PV, respectively.
DISCUSSION
In this study, we developed a novel PV neutralization test using PV pseudoviruses instead of infectious PV strains. Pseudotyped viruses, especially enveloped viruses that require a high biosafety level for containment (e.g., severe acute respiratory syndrome coronavirus [SARS-CoV], lyssaviruses, Nipah virus, and highly pathogenic avian influenza A viruses), have been widely used in neutralization tests because of their safety (6, 7, 16, 17, 21). The advantages of pPNT compared to cPNT are as follows: (i) biosafety (pPNT uses non-self-proliferating PV pseudovirus versus infectious PV strains), (ii) simplicity of procedures (automated luciferase assay and interpretation versus observation requiring expertise), and (iii) shortened time to results (2 days versus 5 to 7 days). Moreover, pPNT requires less human serum than is required for cPNT (25 μl versus 200 μl) and thus could contribute to saving precious human serum resources to be used for biological tests. In the end game of the polio eradication program, biosafety and reduced burden in laboratory tests would have more importance than in areas where polio is endemic.
We observed good correlation in the overall results obtained by cPNT and pPNT for all types of PV. For pseudovirus neutralization tests, threshold values arbitrarily set in a range of 10 to 50% generally gave results that had good correlation with those obtained by conventional tests, especially for determining the neutralization-antibody-positive rates (6, 16, 17). Fine-tuning of threshold values might have benefit for linking the results obtained by different neutralization methods (e.g., suppression of CPE that could arise from 1 infectious unit of virus versus overall suppression of pseudovirus infection to threshold values). Therefore, we set the threshold values of pPNT in a range of the values examined for each type of PV to give antibody-positive rates closest to those obtained by cPNT (Fig. 3). PV pseudoviruses with capsid proteins derived from different strains in the same serotype (e.g., capsid proteins of the Sabin strain versus those of wild-type strains) might have different fine-tuned threshold values, in part depending on the variation in antigenicity and on the properties of the set of human sera examined without an absolute standard neutralizing antibody. The herd immunity required for PV elimination has been predicted to be about 80 to 97% from a circulation model, depending on the level of hygiene (5), or more than 70% from the emergence of circulating vaccine-derived PV in the Dominican Republic in 2000 and 2001, assuming there is no pocket of poor vaccination coverage (20 to 30%) in the country (10). A neutralizing-antibody titer of ≥8 is considered to have a protective effect against paralytic poliomyelitis (reviewed in reference 13). Therefore, a wide range of threshold values (5 to 30% for type 1 PV, 2.5 to 10% for type 2, and 5.0 to 20% for type 3) in pPNT could be practically acceptable to estimate antibody-positive rates with an accuracy within 10% of those determined by cPNT.
We used type 1, 2, and 3 PV pseudoviruses that have capsid proteins of PV1Mahoney, PV2MEF-1, and PV3Saukett A, respectively, for pPNT. These PV strains are virulent and are currently used as antigens of inactivated PV vaccine (IPV) (11). PV consists of 3 serotypes (14), although some antigenic variation could occur within each serotype after long circulation (for >10 years) (3). Therefore, to evaluate the neutralizing antibodies to wild-type PV strains, these PV pseudoviruses would have more benefit than using infectious PV vaccine strains. For neutralizing-antibody titers for type 1 PV, the overall results were consistent between cPNT and pPNT (Fig. 3). However, there was an age-dependent difference in the distribution of the titers: lower titers for the younger group and higher titers for the elderly group by pPNT (Fig. 4). In Japan, poliomyelitis outbreaks caused by wild-type strains mainly occurred before 1962 (>90% of the total reported cases from 1947 to date), before the introduction of OPV in 1961. Therefore, it is plausible that sera from the elderly group (≥60 years old; birth years before 1950) have higher neutralization activity against wild-type strains than vaccine strains. The low titers against type 1 PV observed by pPNT in the younger group suggested that antibodies induced by OPV apparently have higher titers for the vaccine strain than the parental PV1Mahoney strain or other type 1 attenuated strains (CHAT) (4, 8). Standard serum (monkey antisera raised against each Sabin strain) also showed relatively low neutralizing activity against type 1 PV pseudovirus compared to those against type 2 and 3 PV pseudoviruses (Fig. 1). Different levels of neutralizing-antibody titer against type 1 PV strains might have arisen in part from differences in the epitopes of these strains recognized by the antibodies, as reported for those induced by OPV and IPV (antigenic sites 3 and 1, respectively) (15). Nevertheless, it is enigmatic that the distribution of neutralizing-antibody titers for a young age group (20 to 39 years old) showed only slight differences in the results obtained by cPNT and pPNT, in contrast to that of the 0-to-19-year age group. These age groups belong to the post-OPV introduction era, and thus, the properties of the antigen seem not to be the major determinant that caused this difference. This suggested that a transition in antibody properties occurred in long-term immune memory.
In summary, we developed a pseudovirus neutralization test for measurement of the neutralizing-antibody titer against PV in human serum. The pPNT would serve as a safe, simple, and rapid test for serosurveillance of PV.
ACKNOWLEDGMENTS
We are grateful to Junko Wada for her excellent technical assistance.
This study was supported in part by Grants-in-Aid for the Promotion of Polio Eradication and Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor and Welfare.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Arita M., Ami Y., Wakita T., Shimizu H. 2008. Cooperative effect of the attenuation determinants derived from poliovirus Sabin 1 strain is essential for attenuation of enterovirus 71 in the NOD/SCID mouse infection model. J. Virol. 82:1787–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arita M., Nagata N., Sata T., Miyamura T., Shimizu H. 2006. Quantitative analysis of poliomyelitis-like paralysis in mice induced by a poliovirus replicon. J. Gen. Virol. 87:3317–3327 [DOI] [PubMed] [Google Scholar]
- 3. Blomqvist S., et al. 2004. Characterization of a highly evolved vaccine-derived poliovirus type 3 isolated from sewage in Estonia. J. Virol. 78:4876–4883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eggers M., et al. 2010. Immunity status of adults and children against poliomyelitis virus type 1 strains CHAT and Sabin (LSc-2ab) in Germany. BMC Infect. Dis. 10:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fine P. E. 1993. Herd immunity: history, theory, practice. Epidemiol. Rev. 15:265–302 [DOI] [PubMed] [Google Scholar]
- 6. Fukushi S., et al. 2006. Evaluation of a novel vesicular stomatitis virus pseudotype-based assay for detection of neutralizing antibody responses to SARS-CoV. J. Med. Virol. 78:1509–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han D. P., Kim H. G., Kim Y. B., Poon L. L., Cho M. W. 2004. Development of a safe neutralization assay for SARS-CoV and characterization of S-glycoprotein. Virology 326:140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iwai M., et al. 2006. Assessment of efficacy of a live oral poliovirus vaccine for virulent Sabin-like poliovirus 1 strains in Japan. Acta Virol. 50:139–143 [PubMed] [Google Scholar]
- 9. Iwai M., et al. 2008. Evaluation of a two-dose administration of live oral poliovirus vaccine for wild and virulent vaccine-derived poliovirus type 1, 2, 3 strains in Japan. Scand. J. Infect. Dis. 40:247–253 [DOI] [PubMed] [Google Scholar]
- 10. Kew O., et al. 2002. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 296:356–359 [DOI] [PubMed] [Google Scholar]
- 11. Kew O. M., Sutter R. W., de Gourville E. M., Dowdle W. R., Pallansch M. A. 2005. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 59:587–635 [DOI] [PubMed] [Google Scholar]
- 12. Kilpatrick D. R., et al. 2009. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J. Clin. Microbiol. 47:1939–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nathanson N. 2005. David Bodian's contribution to the development of poliovirus vaccine. Am. J. Epidemiol. 161:207–212 [DOI] [PubMed] [Google Scholar]
- 14. National Foundation for Infantile Paralysis Committee on Typing. 1951. Immunologic classification of poliomyelitis viruses. I. A cooperative program for the typing of one hundred strains. Am. J. Hyg. 54:191–204 [PubMed] [Google Scholar]
- 15. Rezapkin G., et al. 2010. Repertoire of antibodies against type 1 poliovirus in human sera. J. Virol. Methods 169:322–331 [DOI] [PubMed] [Google Scholar]
- 16. Tamin A., et al. 2009. Development of a neutralization assay for Nipah virus using pseudotype particles. J. Virol. Methods 160:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang W., et al. 2008. Establishment of retroviral pseudotypes with influenza hemagglutinins from H1, H3, and H5 subtypes for sensitive and specific detection of neutralizing antibodies. J. Virol. Methods 153:111–119 [DOI] [PubMed] [Google Scholar]
- 18. Wood D. J., Hull B. 1999. L20B cells simplify culture of polioviruses from clinical samples. J. Med. Virol. 58:188–192 [PubMed] [Google Scholar]
- 19. World Health Organization. 2004. Polio laboratory manual, 4th ed., WHO/IVB/04.10, and supplement to the WHO polio laboratory manual. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 20. World Health Organization. 1995. Manual of laboratory methods for potency testing of vaccines used in the WHO Expanded Programme on Immunization. WHO publication no. WHO/BLG/95.1. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 21. Wright E., et al. 2008. Investigating antibody neutralization of lyssaviruses using lentiviral pseudotypes: a cross-species comparison. J. Gen. Virol. 89:2204–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]