Abstract
To develop a novel, effective HBV therapeutic vaccine, we constructed two HBV DNA immunogens that contained PreS1, HBSS1, and HBCS1. Several delivery methods, such as intramuscular (i.m.) injection, intramuscular injection plus electroporation (i.m.-EP), and intradermal injection plus electroporation (i.d.-EP) were used in a murine model to analyze and compare the immune responses that were induced by the DNA immunogens. We found that i.d.-EP accelerated specific antibody seroconversion and produced high antibody (anti-PreS1, anti-S, and anti-C antibody) titers after HBSS1 and HBCS1 immunization. Combining the HBSS1 and HBCS1 DNA immunogens with i.d.-EP produced the strongest multiantigen (PreS1, S, and C)-specific cellular immune response and the highest specific PreS1 antibody levels. The results indicated that DNA immunization using HBSS1 and HBCS1 might be an ideal candidate, with its ability to elicit robust B and T cell immune responses against multiantigen when combined with optimized delivery technology. The present study provides a basis for the design and rational application of a novel HBV DNA vaccine.
INTRODUCTION
Hepatitis B virus (HBV) causes acute and chronic hepatitis and is associated with cirrhosis and liver cancer. Although the hepatitis B vaccine has been in use for over 20 years, HBV is still one of the most widespread pathogens (14). The most widely used HBV vaccine is a subunit vaccine containing the full-length S particle that is expressed by yeast or CHO cells (34). The HBV vaccine is very effective for mass immunization; however, 5% to 10% of the population do not produce protective anti-HBV surface (HBs) antigen antibodies, and in recent years, the S variant strains have increased in prevalence (5). The drugs that are currently used for the clinical treatment of hepatitis B include interferon and nucleoside analogues, such as lamivudine (5). Due to the limited effect of this drug treatment and the adverse reactions to their long-term application, new treatments for HBV, such as therapeutic HBV vaccines, are clearly needed (5).
The direct injection of HBV DNA immunogens stimulates strong, long-lasting humoral and cellular immune responses in small-animal and chimpanzee models (11, 18, 22, 25, 27) and has become the focus of research on therapeutic HBV vaccines in recent years. However, applying these results to a larger population does not work well for small-animal models, and a large amount of DNA is needed to stimulate an effective immune response (17). Therefore, scientists are exploring ways to enhance the immunogenicity of DNA vaccines (1, 16, 30, 32). Currently, electroporation is the most effective method for DNA vaccine delivery (16, 30, 32). Electroporation increases antigen (Ag) expression in muscle and skin 10- to 100-fold more than a direct injection, which leads to increased immunogenicity, a more durable response, and a reduced efficient dose in sheep, pigs, and other large animals.
Another strategy for boosting the immunogenicity of DNA vaccines is to fuse exogenous B or T cell epitopes to virus-like particle (VLP) vectors to increase exposure after expression (26). After expression, a number of viral structural proteins automatically assemble into virus-like particles and can carry modified foreign epitopes without altering the particle's structure. For example, the widely used HBV and human papillomavirus (HPV) vaccines are virus-like particle immunogens. HBs antigen (29) and HBV core (HBc) antigen (10) were two of the first VLP antigens that were used to carry foreign epitopes (8, 9, 13, 15, 20, 23, 28, 31, 33).
Previous data have shown that the HBCS1 that is expressed in Escherichia coli, containing core (amino acids [aa] 1 to 144) and PreS1 (aa 1 to 42), can form virus-like particles (33). Moreover, the HBSS1 that is expressed in CHO cells, containing S (aa 1 to 223) and PreS1 (aa 21 to 47), form stable, secreted virus-like particles with an equivalent molar ratio of PreS1 to S antigens (28). The two novel virus-like particle protein antigens can stimulate fast, effective humoral immune responses in animal models. Protein vaccines have a long life cycle and a complicated purification process and are expensive. Moreover, they are not effective in eliciting T cell immune responses and usually require adjuvants. In contrast, DNA immunogens are relatively fast to produce, are inexpensive, and can stimulate stronger cellular immune responses than protein immunogens (16). In the present study, we constructed two DNA plasmids that contain the PreS1 particle-like antigen, i.e., pVRC-HBSS1 and pVRC-HBCS1, containing the S and C gene sequences, respectively, as described previously (28, 33). The routes of administration of the two DNA immunogens, with and without electroporation, were compared by corresponding cell-mediated and humoral immune responses. Our results demonstrate that an intradermal injection plus electroporation with a combination of core-PreS1 and S-PreS1 can synergistically enhance the effects of the DNA immunogen. Therefore, this method is a potential strategy for the development of a novel HBV vaccine with therapeutic effect.
MATERIALS AND METHODS
Plasmid construction and expression in vitro.
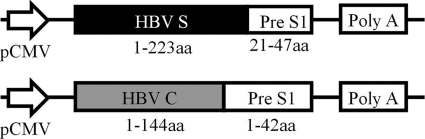
To construct the HBV particle-like DNA immunogen plasmids, pVRC-HBSS1 and pVRC-HBCS1, the pVRC8301 plasmid (31) was digested with the restriction enzymes SalI and BamHI. The HBc (aa 1 to 144) and Pre S1 (aa 1 to 42) fusion gene fragment was amplified using PCR with the pET-HBCS1 plasmid (33) and was then digested with SalI and BamHI. The HBCS1 gene was purified from a gel after electrophoresis and was cloned into the SalI and BamHI sites of the pVRC8301 vector, resulting in pVRC-HBCS1. We used the same approach to amplify HBSS1 from the pcHBSS1 plasmid (S antigen, aa 1 to 223; Pre S1 antigen, aa 21 to 47) to create pVRC-HBSS1 (Fig. 1). The HBV DNA immunogen plasmids were transformed in E. coli (Top 10 strain) and were propagated in LB medium containing kanamycin. The plasmid DNA was isolated and verified using restriction enzyme analysis and sequencing. For the DNA immunization, each DNA plasmid was first amplified in E. coli (Top 10; Invitrogen) and was purified using the Endofree Plasmid Giga kit (Qiagen). The purity of the DNA preparations was determined by reading the optical density at 260 and 280 nm.
Fig. 1.
Schematic diagram of the HBV DNA vaccines that contain PreS1 and the S or C fusion gene. The protein boxes are shown to scale (in amino acid residues).
The expression of the HBV antigen from the DNA immunogen plasmid was confirmed in 293T cells that were transiently transfected with pVRC-HBSS1 and pVRC-HBCS1. The transfected cells were maintained for 48 h at 37°C with 5% CO2 and were then fixed with 50% methanol. The expressed HBV recombinant fusion proteins were detected using indirect immunofluorescence (IF) staining and rabbit antisera against HBcAg or HBsAg. The levels of the fusion proteins in the supernatant and 293T cell lysate, harvested after 48 to 72 h, were determined using Western blot analysis (28, 33).
Animals and DNA immunization.
Female BALB/c (H-2d) mice between 6 and 8 weeks of age (Animal Care Center, Chinese Academy of Medical Science, Beijing, China) were randomly assigned to 10 groups (Table 1). In accordance with the Institutional Animal Care and Use Committee (IACUC)-approved protocol, all mice were immunized at weeks 0, 3, and 6 and bled at weeks 0, 2, 5, and 10. The mice were anesthetized and primed with phosphate-buffered saline (PBS) or the plasmid DNA using an injection into the tibialis anterior (TA) muscles (intramuscular [i.m.]; 50 μg/100 μl) or an intradermal (i.d.) injection into the lower dorsal side (10 μg/30 μl).
Table 1.
Vaccination groups and administration strategy for HBV DNA vaccines
| Group | Vaccine or reagent | Dose | Route |
|---|---|---|---|
| A1 | PBS | 50 μl | i.m. |
| A2 | pVRC-SS1 | 50 μg | i.m. |
| A3 | pVRC-CS1 | 50 μg | i.m. |
| B1 | PBS | 50 μl | i.m.-EPa |
| B2 | pVRC-SS1 | 50 μg | i.m.-EP |
| B3 | pVRC-CS1 | 50 μg | i.m.-EP |
| C1 | PBS | 50 μl | i.d.-EPb |
| C2 | pVRC-SS1 | 10 μg | i.d.-EP |
| C3 | pVRC-CS1 | 10 μg | i.d.-EP |
| C4 | pVRC-SS1 + pVRC-CS1 | 10 μg + 10 μg | i.d.-EP |
EP parameters: i.m. (pulse generator [ECM830; BTX] with a two-array needle electrode), 27.5 v/mm for 20 ms, 8 times, with a 1-s interval between the EPs.
EP parameters: i.d. (square-wave pulse generator [ECM830; BTX] with a caliper electrode consisting of two brass plate electrodes, 1 by 1 cm), 10 v/mm for 15 ms, 3 times, followed by reverse electroporation at 7.5 v/mm for 20 ms, 3 times, with a 1-s interval between the EPs.
The gene delivery using in vivo electroporation (EP) was performed as previously described (1, 30, 32). In brief, for the i.m. immunization, the DNA plasmids in PBS (50 μl per site; 50 μg/dose) were injected into the TA muscle and were immediately pulsed with electricity using a two-needle array electrode (ECM830; BTX) with needles that were 5 mm apart. For the i.d. immunization, the DNA plasmids in PBS (25 μl per site; 10 μg/dose) were injected intradermally into the lower dorsal side. After 20 to 30 s, an in vivo EP was applied using a square-wave pulse generator (ECM830; BTX) with a caliper electrode that consisted of two brass plate electrodes (1 by 1 cm). The EP parameters were as follows: i.m., 27.5 v/mm, 20 ms, 8 times, with a 1-s interval between EPs; i.d., 10 v/mm, 15 ms, 3 times, followed by a reverse electrode at 7.5 v/mm, 20 ms, 3 times, with a 1-s interval between EPs.
Serum samples were collected as scheduled, and the separated serum was stored at −70°C. The mice were sacrificed 4 weeks after the last immunization. The spleens of the mice were removed, and a single-cell suspension of the splenocytes was prepared as previously described. The final splenocyte preparations contained 2 × 106 or 5 × 106 cells/ml in R10 medium (RPMI 1640 medium with 10% fetal bovine serum [FBS] and 1% penicillin-streptomycin).
ELISA.
The mouse sera were tested for an HBV antigen-specific IgG antibody (anti-PreS1, anti-S, and anti-C) response using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (WanTai Co.) according to the manufacturer's instructions (9). The optical density was read using a 450-nm ELISA reader (Bio-Rad). The end titration titer was determined when the reading at the last serum dilution was 2-fold greater than that of the negative-control wells that contained normal mouse sera.
The determination of the IgG subclass was conducted as described previously (22). The serum samples from the immunized mice were diluted 1:100, and the IgG subclass was determined using a murine antibody-isotyping ELISA kit (Sigma). Biotinylated rat anti-mouse IgG1 (1:1,000) or biotinylated rat anti-mouse IgG2a and IgG2b (1:2,000) were used as controls.
ELISpot analysis of antigen-specific T cells.
Enzyme-linked immunospot (ELISpot) assays were performed on fresh mouse splenocytes, as previously described (9). Multiscreen Immobilon P filtration plates (Millipore) were coated with 5 μg/ml of purified rat anti-mouse gamma interferon (IFN-γ) IgG1 (clone R4-6A2; BD Biosciences) in PBS, and incubated at 4°C overnight. Afterward, these plates were washed 3 times with PBS, and each plate was blocked with 200 μl of R10 medium per well for 2 to 3 h at 37°C. The peptides that were used in the present study were synthesized using an Applied Biosystems (Foster City, CA) 430A peptide synthesizer and 9-fluorenylmethyl carbonate (Fmoc) chemistry. After synthesis, the peptides were cleaved from the resin, and the protecting groups were removed. The peptides were purified using reverse-phase high-performance liquid chromatography (HPLC) to a purity of >95% and were characterized using mass spectrometry. The HBV PreS1 antigen-relevant peptides used were an 8-peptide array of 9-mers (GQNLSTSNP, STSNPLGFF, LGFFPDHQL, DHQLDPAFR, DPAFRANTA, RANTANPDW, NPDWDFNPN, and NPNKDTWPD). The HBV S antigen-relevant peptides used were separated into two pools of peptides: S-1 (VLQAGFFL, IPQSLDSWWTSL, and FLGGTPVCL, selected from amino acids 13 to 49 of S Ag) and S-2 (LLDYQGMLP, GLSPTVWLS, and SILSPFIPLL, selected from amino acids 97 to 215 of S Ag). The HBV core antigen-relevant peptides used were a 4-peptide array (FLPSDFFPSV, YVNVNWGLK, CLTFGRETV, and TPPATRPPNAPLL). The HIV Env V3 (IGPGRAFYT) peptide was used as the nonrelevant negative control. The peptides (final concentration, 4 μg/ml) were added to the wells with 100 μl of freshly isolated splenocytes (100,000 or 500,000 cells/well in R10 medium) in duplicate. The plates were incubated for 20 to 24 h overnight at 37°C in 5% CO2. The plates were then washed, incubated with 100 μl biotinylated rat anti-mouse IFN-γ IgG1 (clone XMG1.2; BD Biosciences; 1 μg/ml in PBS with 0.005% Tween 20 and 5% FBS), and incubated at 4°C overnight. After additional washes, 100 μl of alkaline phosphatase-conjugated streptavidin complex (BD Bioscience) was added to each well and incubated for 2 h at room temperature. The plates were washed, and any spots representing individual IFN-γ-producing cells were detected after a 7-min color reaction using 1-Step nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP) (Pierce). The IFN-γ spot-forming cells (SFCs) were counted. The results are expressed as the number of SFCs per 106 input cells. The number of peptide-specific IFN-γ-secreting T cells was calculated by subtracting the background (no-peptide) control value from the established SFC count.
Statistical analysis.
The statistical analyses were performed using analysis of variance (ANOVA), a Student-Newman-Keuls test, and a Pearson correlation analysis using SSPS 10.0. Differences with a P value of <0.05 were considered to be statistically significant.
RESULTS
Construction, expression, and identification of the DNA immunogen.
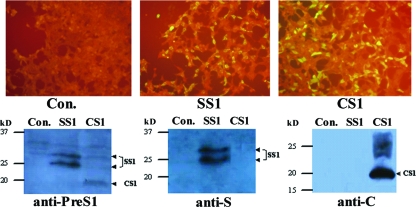
Our previous studies have shown that the fusion of amino acids 21 through 47 of HBV PreS1 (which may be the receptor-binding domain) to the C-terminal domain of the truncated S antigen (aa 1 to 223) results in the expression plasmid pCHBSS1. This plasmid can form a stable VLP structure antigen in CHO cells and can stimulate an immune response after in vitro immunization (9, 28). Moreover, the fusion of amino acids 1 through 42 of the HBV PreS1 protein to the C terminus of the truncated core antigen (aa 1 to 144) resulted in the expression plasmid pET-HBCS1, which can also form VLP antigens in E. coli (8, 33). Therefore, in the present study, we used PCR and restriction enzyme digestion to clone the two PreS1 antigens into the DNA vaccine vector pVRC and to form two candidate DNA immunogens, pVRC-HBSS1 and pVRC-HBCS1 (Fig. 1). The in vitro transient transfection of the 293T cells confirmed the effective expression of the two fusion antigens by IF assay (IFA) and Western blot analysis using Ag-specific antibody (Fig. 2). CS1 antigen was produced as 20 kDa. The SS1 antigen was detected as two protein species (molecular masses, 24 kDa and 27 kDa), presumably reflecting both nonglycosylated and glycosylated forms of SS1. These results are consistent with those of previous reports (28, 33).
Fig. 2.
Expression of the HBV SS1 and CS1 antigen-derived DNA vaccine constructs in 293T cells. The cells were transfected with the DNA vectors as follows: Con, pVRC8301 as a control; SS1, pVRC-HBSS1 expressing PreS1 and S fusion protein; CS1, pVRC-HBCS1 expressing the PreS1 and C fusion protein. (Top) IFA. Transfected Th cells were fixed, permeabilized, stained with rabbit anti-PreS1 antibody and fluorescein isothiocyanate (FITC) conjugated with a secondary antibody and were then visualized using fluorescence microscopy. (Bottom) Western blot analysis of HBV antigen expression. The cell lysates were run on a 12.5% SDS polyacrylamide gel and analyzed using Western blotting with antibodies for the individual antigens, as indicated. The expression bands of the SS1 and CS1 proteins are indicated by arrowheads.
Electroporation can accelerate the process of seroconversion and enhance the antibody response.
To improve the immunogenicity of the DNA immunogen and to optimize the immunization program, we divided the mice into four groups, with 6 mice in each group (Table 1). The three immunization methods that were performed were i.m, intramuscular injection plus electroporation (i.m.-EP) (using a two-array needle), and intradermal injection plus electroporation (i.d.-EP) (using a caliper electrode). The DNA-immunizing dose for the i.d. group was 10 μg, which was approximately one-fifth that of the i.m. group (50 μg).
Two weeks after single or double HBV DNA immunization, the eyes and sera of the mice were sampled and diluted 1:10. Next, an ELISA was used to determine whether seroconversion with the specific antibodies (anti-PreS1, anti-S, and anti-C) had occurred. No seroconversion with anti-S or anti-C antibody was observed in the single-dose injection group, whereas seroconversion with anti-PreS1 antibodies was observed in the electroporation group. In the pVRC-HBSS1 immunogen group, the anti-PreS1 seroconversion rate of the i.d.-EP group (5/6) was significantly higher than that of the i.m.-EP group (1/6 or 2/6). Immune seroconversion (1/6 or 2/6) with anti-S or anti-C antibody was observed after the i.m. injection in the double-immunization group; however, the amount of seroconversion was significantly lower than that in the injection plus electroporation group (3/6 to 6/6). The i.m.-EP group exhibited the highest seroconversion rate (5/6 or 6/6) of all groups.
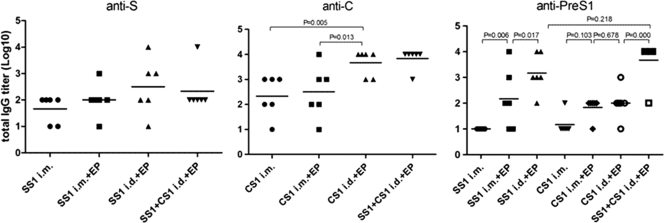
After three immunizations with the DNA immunogen, the specific antibody titer was determined using an ELISA (Fig. 3). There was no statistically significant difference between the anti-S antibody titers within each group; however, the dose that was required to induce antibodies in the i.d.-EP group was one-fifth of that of the i.m. group or the i.m.-EP group. Additionally, the average antibody titer was slightly higher in the i.d.-EP group than in either of the other two groups. The anti-C antibody titer of the i.d.-EP group was significantly higher than those of the direct i.m. and i.m.-EP groups (P < 0.05). After immunization with pVRC-HBSS1, the anti-PreS1 antibody titer in the i.m.-EP group was significantly higher than that in the direct i.m. group (P < 0.05). Furthermore, the antibody titer in the i.d.-EP group was significantly higher than that in the i.m.-EP group (P < 0.05). There was no significant difference between the three groups after pVRC-HBCS1 immunization. The mean antibody titers of the two electroporation groups were slightly higher than that of the direct i.m. group, and that in the i.m.-EP group that was immunized with both plasmids was higher than that in the group immunized with individual plasmids, likely due to the supplementary effect of the PerS1 antigen encoded by both plasmids.
Fig. 3.
Antibody responses elicited by HBV DNA vaccines that were administered using an injection or an injection plus EP. Each group of mice (6 mice/group) was immunized using the plasmid at either 50 μg (i.m.) or 10 μg (i.d.) in PBS three times at 3-week intervals. The antisera were collected at 28 days postimmunization, and the total IgG titers that were specific for the HBV antigen were determined using an ELISA and plotted as described in Materials and Methods. The symbols represent the titers of the sera from the individual mice. The horizontal lines represent the means (n = 6). The experiments were repeated at least three times, and similar results were obtained.
In short, these results indicate that the electroporation of a DNA immunogen can accelerate seroconversion and increase the level of the antibody response. Moreover, an intradermal injection plus electroporation is superior to an intramuscular injection plus electroporation, as it induces higher antibody levels and accelerated seroconversion with specific antibodies.
The IgG subtype after immunization with several HBV DNA immunogens.
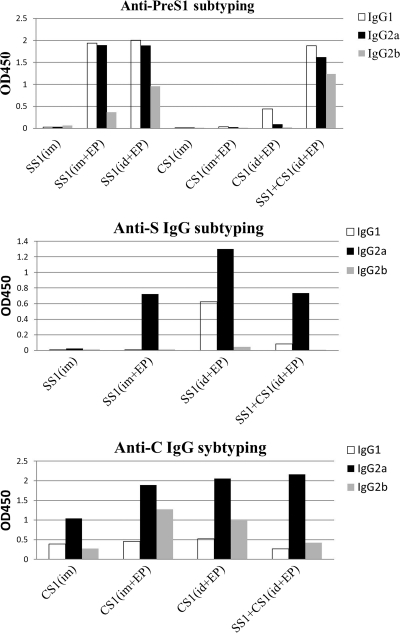
To further understand the immune response of each group after immunization, we also analyzed the IgG subtype that was induced in each group with 1:100-diluted blood samples that were collected 4 weeks after the last immunization (Fig. 4). The IgG subtypes of the antibodies against the S and core antigens that were induced by the DNA immunogen were mainly composed of IgG2a, whereas the anti-PreS1 antibody subtypes were mixtures of IgG1, IgG2a, and IgG2b. There was a slightly larger amount of IgG1 antibodies than of IgG2a antibodies against PreS1, and the IgG2b subtype antibodies were observed in all of the groups that contained pVRC-HBSS1. In summary, the DNA immunogen induced a Th1-type immune response against the C and S antigens; however, the immune response against the PreS1 antigen was diverse and was slightly biased in favor of the Th2-type response.
Fig. 4.
Subtype analysis of the HBV antigen-specific IgGs in mouse sera that were exposed to the HBV DNA vaccines. The antigen-specific IgG1 and IgG2a or IgG2b were measured using an IgG-isotyping ELISA, as described in Materials and Methods. The mouse sera were collected 4 weeks after the 3rd DNA immunization and were diluted 1:100. The bars indicate the average optical density at 450 nm (OD450) of the IgG subtype-derived mixed sera of each group.
A combination of the two HBV DNA immunogens that were administered using intradermal electroporation can synergistically enhance the antigen-specific cellular immune response.
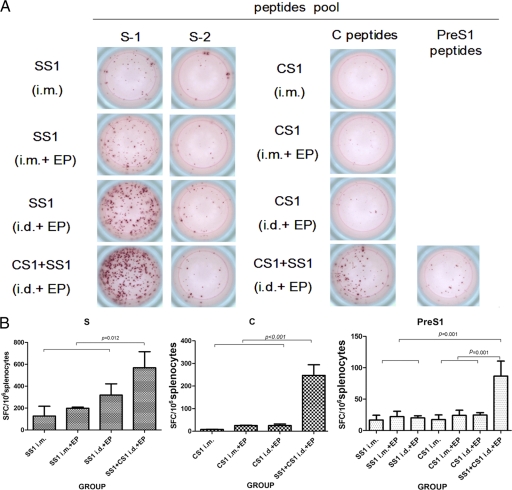
The cellular immune response is the most critical assessment index of the effectiveness of therapeutic vaccines. Therefore, we used IFN-γ ELISpot to detect the antigen-specific IFN-γ-secreting cells in the spleen cells of mice 4 weeks after they received three DNA immunizations. The peptide library that was used to stimulate the spleen cells is described above, and the results are shown in Fig. 5.
Fig. 5.
ELISpot analysis of IFN-γ secretion in mouse splenocytes immunized with HBV DNA vaccines as indicated. (A) Sample wells with spots with mock or HBV peptide stimulation. (B) Frequencies of HBV peptide-specific spots per million splenocytes in HBSS1, HBCS1, or combined vaccine (HBSS1- HBCS1)-immunized mice. The data represent the average numbers of SFCs per million splenocytes from 6 mice/group plus standard deviations. The splenocytes were collected 4 weeks after the 3rd DNA immunization.
Of the cells that secreted IFN-γ specifically in response to the S, C, and PreS1 antigens, the S-specific SFC readings were the highest. The reading after stimulation with the S-1-specific peptide pool was significantly higher than that from the S-2-specific peptide pool (Fig. 5A). The S antigen-specific SFC readings among all groups were significant; however, the number of IFN-γ-secreting cells in response to C and PreS1 after immunization with a single plasmid (pVRC-HBSS1 or pVRC-HBCS1) was low (<50 SFC/106 spleen cells) and was not statistically different. Interestingly, after immunization with both plasmids (pVRC-HBSS1 and pVRC-HBCS1) and intradermal electroporation, the PreS1- and C-specific SFC levels were higher than 50 SFC/106 spleen cells, which was significantly higher than that of the single-plasmid DNA immunogen groups. The S antigen-specific SFC levels were higher than 500 SFC/106 spleen cells in the group with both plasmids with intradermal electroporation. Therefore, the dual-plasmid DNA immunogens with intradermal electroporation can synergistically enhance the antigen-specific cellular immune responses.
DISCUSSION
Traditional vaccines are designed to induce high titers of neutralizing antibodies. However, a number of intracellular pathogens, including HBV, can still cause a persistent infection (34). In addition to neutralizing antibodies, the specific cellular immune responses support antibody production and are also crucial to the control and clearance of infections (5, 34). Many immunological studies (34) have shown that strong cellular immune responses to specific HBV proteins (including Th cells and cytotoxic T lymphocytes [CTL]) are detected during the acute phase of an HBV infection in recovering patients, but in patients with chronic persistent HBV infection, the cellular immune responses are very weak or undetectable. The animal experiment results show that the secreted Th1 cytokines, including IFN and tumor necrosis factor alpha (TNF-α), can eliminate a liver HBV infection in transgenic mice. Therefore, the simultaneous induction of strong humoral and cellular immune responses to multiple antigens is the goal for the development of a new therapeutic HBV vaccine (5). Most of the therapeutic HBV vaccines in ongoing clinical trials have used envelope proteins as the target antigen, but other antigens (such as core, PreS, and polymerase) are also specific targets of the immune response during self-limiting hepatitis B. As previously reported, HBVc antigen contains multiple Th and CTL epitopes (9, 15), and the HBc-specific cellular immune response might cause a self-limiting HBV infection (34). Truncated HBc antigen-specific heterologous epitopes may be used as effective VLP vectors and can significantly enhance the heterologous epitope immunogenicity and immune response of Th1 cells (8, 20). In addition, the HBs antigen that is expressed in CHO cells or yeast can self-assemble into 22-nm spherical VLPs and has been used to provide effective protection for the commercially available HBV vaccine (34). A novel immunization approach, based on the use of a combination of recombinant HBsAg and HBcAg (which both form virus-like particles), known as “NASVAC,” is currently being developed for both the prevention and cure of hepatitis B (2). The vaccine was found to be safe and immunogenic, eliciting anti-HBc and anti-HBs seroconversion in a phase I/II trial (3, 6).To our knowledge, PreS1 is the priority target antigen for the new HBV vaccines, because PreS1 has a greater number of immunogenic T cell and B cell epitopes than the S protein (9, 12, 15), including a strong T cell epitope (aa 21 to 28) and an effective B cell epitope (aa 12 to 32 and 32 to 53). The PreS1 protein has binding sites for liver cells (aa 21 to 47) that play an important role in viral attachment and entry (13, 15). Neurath et al. have shown that direct immunization with a PreS1 polypeptide (aa 21 to 47) can protect against HBV infection in chimpanzees (21). Additionally, the PreS1 antibody is produced earlier than the S antibody, and the PreS1-specific cell-mediated immunity may help overcome immune unresponsiveness to the S protein in certain individuals (19, 24). Therefore, the PreS1 antigen may play an important role in the neutralization, blockade, and clearance of HBV infections. In the present study, we constructed two kinds of particle-like structures expressing plasmids containing S and PreS1 antigens that contained HBV-neutralizing epitopes and a C antigen to induce a Th1-type response. Our results suggest that DNA immunogens that contain HBSS1 and HBCS1 can generate powerful cellular immune responses, and the PreS1 antibody was indeed produced earlier than the S antibody. Furthermore, this response was specific to multiple antigens (PreS1, S, and C) and was biased in favor of the Th1-type response, as indicated by the production of IgG2a anti-C and anti-S antibodies.
The major current challenge associated with the use of DNA immunogens is to determine ways to enhance the response of the immune system (16). Recently, reports (1, 30, 32) have indicated that in vivo electroporation can significantly enhance the cellular uptake of exogenous DNA and that the expression of exogenous protein can lead to the aggregation of local inflammatory factors. Thus, the humoral and cellular immune responses that are induced by DNA immunogens can be simultaneously increased. As a result, the effective dose of the DNA immunogen can be greatly reduced in the large-animal model (4). Furthermore, many preclinical and clinical studies have indicated that in vivo electroporation is a safe, effective, and acceptable method in animals and human beings (7). Therefore, in the present study, in addition to a conventional direct intramuscular injection, we applied two immunization methods with in vivo electroporation. Our results indicate that an intradermal injection plus electroporation increases the amount of specific antibody seroconversion. Moreover, small doses (10 μg) resulted in humoral and cellular immune responses that were as robust as those achieved by high doses (50 μg) of a direct immunization. Interestingly, we found that the core- and PreS1-specific immune responses induced by separate HBCS1 or HBSS1 plasmids were weak; however, a combined HBSS1/HBCS1 DNA immunogen using i.d.-EP induced the most powerful immune responses to PreS1, S, and C antigens. Immunization with DNA immunogens that contain the weak antigen CS1 and the strong antigen SS1 can synergistically enhance the cellular immune response. Therefore, the specific molecular mechanism that is responsible for this phenomenon deserves further study.
In conclusion, our study indicated that the combined DNA immunogen and immunization program is an important first step in the development of a new and effective therapeutic HBV vaccine and is worthy of further evaluation and application in large animals and humans. We also believe that with the further integration of molecular adjuvants with vaccine and various boosting approaches, this novel immunization program may become the best candidate strategy for research into and development of a new therapeutic HBV vaccine.
ACKNOWLEDGMENTS
We thank Gary Nabel (VRC, NIAID, NIH) for the pVRC plasmid.
The present study was supported by the China Mega-Projects of Science Research for the Research and Development of New Drugs (no. 2009ZX09102-237).
Footnotes
Published ahead of print on 7 September 2011.
REFERENCES
- 1. Abdulhaqq S. A., Weiner D. B. 2008. DNA vaccines: developing new strategies to enhance immune responses. Immunol. Res. 42:219–232 [DOI] [PubMed] [Google Scholar]
- 2. Aguilar J. C., et al. 2004. Development of a nasal vaccine for chronic hepatitis B infection that uses the ability of hepatitis B core antigen to stimulate a strong Th1 response against hepatitis B surface antigen. Immunol. Cell Biol. 82:539–546 [DOI] [PubMed] [Google Scholar]
- 3. Akbar S. M., et al. 2010. A therapeutic nasal vaccine combining both HBsAg and HBcAg was safe, has antiviral potential and induced antigen-specific immunity in patients with chronic hepatitis B. Hepatol. Int. 4:159 [Google Scholar]
- 4. Babiuk S., et al. 2002. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine 20:3399–3408 [DOI] [PubMed] [Google Scholar]
- 5. Bertoletti A., Gehring A. 2009. Therapeutic vaccination and novel strategies to treat chronic HBV infection. Expert Rev. Gastroenterol. Hepatol. 3:561–569 [DOI] [PubMed] [Google Scholar]
- 6. Betancourt A. A., et al. 2007. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int. J. Infect. Dis. 11:394–401 [DOI] [PubMed] [Google Scholar]
- 7. Bodles-Brakhop A. M., Heller R., Draghia-Akli R. 2009. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol. Ther. 17:585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen X., Li M., Le X., Ma W., Zhou B. 2004. Recombinant hepatitis B core antigen carrying preS1 epitopes induce immune response against chronic HBV infection. Vaccine 22:439–446 [DOI] [PubMed] [Google Scholar]
- 9. Chen H., et al. 2010. Impact of different adjuvants on immunogenicity of HBV particle vaccine containing the S+PreS1 fusion antigen in Balb/C mice. Chinese J. Biotechnol. 26:74–78 [PubMed] [Google Scholar]
- 10. Clarke B. E., et al. 1987. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature 330:381–384 [DOI] [PubMed] [Google Scholar]
- 11. Davis H. L., McCluskie M. J., Gerin J. L., Purcell R. H. 1996. DNA vaccine for hepatitis B: evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc. Natl. Acad. Sci. U. S. A. 93:7213–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrari C., et al. 1989. The preS1 antigen of HBV is highly immunogenic at T-cell level in man. J. Clin. Invest. 84:1314–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hui J., et al. 1999. Immunization with a plasmid encoding a modified hepatitis B surface antigen carrying the receptor-binding site for hepatocytes. Vaccine 17:1711–1718 [DOI] [PubMed] [Google Scholar]
- 14. Leachy D. 2005. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J. Clin. Virol. 34:S1–S3 [DOI] [PubMed] [Google Scholar]
- 15. Livingston B. D., et al. 1999. Immunization with the HBV core 18-27 epitope elicits CTL responses in humans expressing different HLA-A2 supertype molecules. Hum. Immunol. 60:1013–1017 [DOI] [PubMed] [Google Scholar]
- 16. Lu S., Wang S., Grimes-Serrano J. M. 2008. Current progress of DNA vaccine studies in humans. Expert Rev. Vaccines 7:175–191 [DOI] [PubMed] [Google Scholar]
- 17. Mancini-Bourgine M., Fontaine H., Brechot C., Pol S., Michel M. L. 2006. Immunogenicity of a hepatitis B DNA vaccine administered to chronic HBV carriers. Vaccine 24:4482–4489 [DOI] [PubMed] [Google Scholar]
- 18. Michel M. L., et al. 1995. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc. Natl. Acad. Sci. U. S. A. 92:5307–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Milich D. R., Melachlan A., Thormton G. B. 1987. T-cell recognition of preS regions of HbsAg can bypass nonresponse to the S region. Adv. Exp. Med. Biol. 225:233–239 [DOI] [PubMed] [Google Scholar]
- 20. Milich D. R., et al. 1995. The hepatitis nucleocapsid as a vaccine carrier moiety. Ann. N. Y. Acad. Sci. 754:187–201 [DOI] [PubMed] [Google Scholar]
- 21. Neurath A. R., Seto B., Strick N. 1989. Antibodies to synthetic peptides from PreS1 region of hepatitis B virus (HBV) envelop (env) protein is virus-neutralizing and protective. Vaccine 7:234–236 [DOI] [PubMed] [Google Scholar]
- 22. Oka Y., Fazle Akbar S. M., Horiike N., Joko K., Onji M. 2001. Mechanism and therapeutic potential of DNA-based immunization against the envelope proteins of hepatitis B virus in normal and transgenic mice. Immunology 103:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pumpens P., Grens E. 2001. HBV core particles as a carrier for B cell/T cell epitopes. Intervirology 44:98–114 [DOI] [PubMed] [Google Scholar]
- 24. Pride M. W., Bailey C. R., Muchmore E., Thanavala Y. 1998. Evaluation of B and T-cell response in chimpanzees immunized with hepagene, a hepatitis B vaccine containing PreS1, PreS2 and S gene products. Vaccine 16:543–550 [DOI] [PubMed] [Google Scholar]
- 25. Rottinghaus S. T., Poland G. A., Jacobson R. M., Barr L. J., Roy M. J. 2003. Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination. Vaccine 21:4604–4608 [DOI] [PubMed] [Google Scholar]
- 26. Roy P., Noad R. 2008. Virus-like particles as a vaccine delivery system: myths and facts. Hum. Vaccin. 4:5–12 [DOI] [PubMed] [Google Scholar]
- 27. Shata M. T., et al. 2006. Attempted therapeutic immunization in a chimpanzee chronic HBV carrier with a high viral load. J. Med. Primatol. 35:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tian S. F., et al. 2002. The physicochemical and biological properties of SS1 fusion antigen of HBsAg secreted by mammalian cells. Chinese J. Virol. 18:312–316 [Google Scholar]
- 29. Valenzuela P., et al. 1985. Antigen engineering in yeast: synthesis and assembly of hybrid hepatitis B surface antigen-herpes simplex 1 gD particles. Nat. Biotechnol. 3:323–326 [Google Scholar]
- 30. Widera G., et al. 2000. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 164:4635–4640 [DOI] [PubMed] [Google Scholar]
- 31. Yang Z. Y., et al. 2004. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 428:561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang L., Nolan E., Kreitschitz S., Rabussay D. P. 2002. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim. Biophys. Acta 1572:1–9 [DOI] [PubMed] [Google Scholar]
- 33. Zhao Y. Q., Zhan M. Y. 2001. Study on the immunogenicity of fusion protein CS1 of hepatitis B virus. Chinese J. Virol. 17:333–337 [Google Scholar]
- 34. Zhou Y. H., Wu C., Zhuang H. 2009. Vaccination against hepatitis B: the Chinese experience. Chin Med. J. 122:98–102 [PubMed] [Google Scholar]