Abstract
Caprine tuberculosis (TB) has increased in recent years, highlighting the need to address the problem the infection poses in goats. Moreover, goats may represent a cheaper alternative for testing of prototype vaccines in large ruminants and humans. With this aim, a Mycobacterium caprae infection model has been developed in goats. Eleven 6-month-old goats were infected by the endobronchial route with 1.5 × 103 CFU, and two other goats were kept as noninfected controls. The animals were monitored for clinical and immunological parameters throughout the experiment. After 14 weeks, the goats were euthanized, and detailed postmortem analysis of lung lesions was performed by multidetector computed tomography (MDCT) and direct observation. The respiratory lymph nodes were also evaluated and cultured for bacteriological analysis. All infected animals were positive in a single intradermal comparative cervical tuberculin (SICCT) test at 12 weeks postinfection (p.i.). Gamma interferon (IFN-γ) antigen-specific responses were detected from 4 weeks p.i. until the end of the experiment. The humoral response to MPB83 was especially strong at 14 weeks p.i. (13 days after SICCT boost). All infected animals presented severe TB lesions in the lungs and associated lymph nodes. M. caprae was recovered from pulmonary lymph nodes in all inoculated goats. MDCT allowed a precise quantitative measure of TB lesions. Lesions in goats induced by M. caprae appeared to be more severe than those induced in cattle by M. bovis over a similar period of time. The present work proposes a reliable new experimental animal model for a better understanding of caprine tuberculosis and future development of vaccine trials in this and other species.
INTRODUCTION
Tuberculosis (TB) in the domestic goat (Capra hircus), mainly caused by Mycobacterium caprae (1), is an endemic disease in the Iberian Peninsula. M. caprae is widespread in goat herds and is an emerging infectious agent in cattle (15, 33). Infected goat herds can constitute a reservoir of TB-inducing mycobacteria in the field, posing a risk of infection to cattle and wildlife (17, 33). Furthermore, caprine TB not only may hamper the eradication campaigns against bovine TB in affected areas but may be also responsible for cases of TB in humans (11, 21, 30, 32).
In the last decade, interest in vaccines against bovine TB has been renewed as a tool for controlling infection in cattle and in wildlife (5) in areas where eradication by the test-and-slaughter scheme alone is not considered feasible. Moreover, ruminant and porcine models of TB may be useful for screening prototype vaccines for humans, due to their similar lesional patterns and immunological responses to mycobacteria (7, 14, 18). Standardization of the goat as a model of TB would improve our understanding of TB in the species, which in turn could help in developing new strategies to combat the disease in goat flocks. Similarly, it could be used as an animal model for TB vaccine development in humans.
Caprine and bovine TB are closely related in regard to the immune response and pathological characteristics. In natural infections, TB in goats, as in cattle, is primarily a lower respiratory tract disease, with lesions in the lungs and associated lymph nodes (LN). Occasionally, tuberculous lesions may also be found in the upper respiratory tract lymph nodes and other organs, like the spleen, liver, or mesenteric lymph nodes (12, 31). Histologically, the lesions are similar to those observed in cattle and humans. Typical tuberculous granulomatous necrotizing lesions are observed, characterized by central caseous necrosis, often with some mineralization, surrounded by macrophages, foamy macrophages, numerous giant cells, lymphocytes, and a fibrotic capsule. Acid-fast bacilli are usually present inside the caseous necrosis, but in very low numbers (11).
Several TB diagnostic tests currently available for use in cattle, such as the tuberculin skin test or the gamma interferon (IFN-γ) assay, can be also applied, with minor modifications, for diagnosis of TB in goats (19, 22). Refinement of the specificity of these tests has been achieved in recent years for use in humans, based on the detection in peripheral blood of effector T cells reacting to antigens secreted by actively growing bacilli, such ESAT-6 and CFP-10, which are not induced by Mycobacterium bovis BCG vaccination (27). As has been observed previously in cattle (37), we have recently shown that an IFN-γ–ESAT-6-specific response also occurs in goats naturally infected with M. caprae, which is positively correlated with the severity of the pathological changes (14). A peptide cocktail containing ESAT-6 and CFP-10 has also been successfully used for diagnosis of TB in naturally infected goats (2). In cattle, it has been shown that the route of challenge can have a significant influence on infection outcome (29).
The endobronchial route of inoculation has been used successfully in several experimental models of TB infection in cattle (13), brushtail possums (4), and European badgers (10) because of its capacity to mimic the natural infection. In adult goats, an infection model of transthoracic inoculation of M. caprae has been previously described (3), demonstrating the potential of the species as a research model for TB.
Qualitative and semiquantitative scoring systems for gross lesions have been used to assess the efficacy of vaccines, based on lesion distribution and extent. Improvement in this scoring system to produce a more precise quantitative system would be of benefit to allow better comparison between treatment groups and experiments. Recently, magnetic resonance imaging (MRI) has been used to measure the disease burden in macaques experimentally infected with M. tuberculosis (34, 35) with promising results. The aim of the present work was to experimentally reproduce TB infection in young goats by inoculation with M. caprae by the endobronchial route, to characterize the immune response, and to standardize methods for quantifying pathological changes in target tissues, including the assessment of multidetector computed tomography (MDCT) to measure the magnitude of lesions in pulmonary tuberculosis. To our knowledge, this is the first study aimed at comprehensively characterizing the effect of endobronchial infection with M. caprae on goats.
MATERIALS AND METHODS
Experimental animals.
Thirteen 6-month-old female Murciano-Granadina goats obtained from an officially TB-free herd were used. The goats were negative in the single intradermal comparative cervical tuberculin (SICCT) test and the IFN-γ assay (Bovigam, Prionics, Schlieren, Switzerland), as well as seronegative for paratuberculosis (Paratub.Serum-Sl Institut Pourquier, Montpellier, France). The herd was not vaccinated against paratuberculosis.
Eleven goats were housed in appropriate containment accommodations for a week prior to infection with M. caprae. Two additional goats were kept uninfected in an outdoor box throughout the experiment. All experimental procedures with animals were in accordance with the European Union laws for protection of experimental animals and were approved by the Animal Welfare Committees of the Universitat Autònoma de Barcelona and the Generalitat de Catalunya.
M. caprae cultures and experimental infection.
The M. caprae SB0416 (http://www.mbovis.org) field strain used as an inoculum was originally isolated from a tuberculous goat from Catalonia. The isolate was subcultured in Middlebrook 7H11 solid medium (BD Diagnostics, Sparks MD), and the bacteria were resuspended in brain heart infusion broth with 20% glycerol at a concentration of 2 × 106 CFU/ml (calculated by plating dilutions on Middlebrook 7H11 medium). The suspension was stored at −80°C in 0.5-ml aliquots. The inoculum was prepared at the required final concentration by diluting the suspension with sterile phosphate-buffered saline (PBS).
For infection, goats were preanesthetized with 0.05 mg of acepromacin (Calmo Neosan)/kg of body weight and 0.2 mg/kg of butorphanol tartrate (Torbugesic) coadministered by intramuscular injection; after 30 min, a catheter was placed into the left cephalic vein, and 4 to 6 mg/kg of propophol (Propofol Lipuro) and 0.2 mg/kg of midazolam (Dormicum) were both administered intravenously. The goats were then intubated with an endotracheal tube and were placed in right lateral decubitus. A plastic cannula (3.3-mm outer diameter) was passed through the endotracheal tube to the level of the carina. For inoculation, a thinner cannula (2.1-mm outer diameter) was passed through the thicker one to a bronchus, and then 0.5 ml of M. caprae inoculum was injected into the inner cannula, followed by flushing with 5 ml of 0.9% saline. The inoculum was titrated after the inoculation in duplicate by 10-fold serial dilution in Middlebrook 7H11 solid medium; accordingly, each goat received 1.5 × 103 CFU of M. caprae. The animals recovered from anesthesia in sternal decubitus.
Clinical signs and sampling.
Before and during the experimental infection, the goats were observed for clinical signs. The rectal temperature was measured weekly, and weight every 2 weeks. Blood samples were collected every 2 weeks from the jugular vein in heparinized blood tubes for immunological studies and isolation of mycobacteria. Also, two nasal swabs were collected from each animal at the same time points; one was decontaminated and subsequently cultured, and the other was submerged in ultrapure water for 1 h at 75°C for mycobacterium inactivation and stored at −80°C until a specific M. tuberculosis complex (MTC) PCR assay was performed.
Antigens and peptides.
Bovine (purified protein derivative B [PPD-B]) and avian (PPD-A) tuberculins were obtained from CZ Veterinaria (Porriño, Galicia, Spain). ESAT-6/CFP-10 and Rv3615c peptide cocktails synthesized as described previously (36, 38) were received from H. M. Vordermeier (Veterinary Laboratories Agency, Weybridge, United Kingdom). Recombinant MPB83 was obtained from Lionex (Braunschweig, Germany). Phytohemagglutinin (PHA) (Sigma-Aldrich, Steinheim, Germany) was used as a positive control.
Skin test.
A SICCT test was performed on all goats at 12 weeks postinfection (p.i.) (2 weeks before sacrifice) by inoculating 0.1 ml of both PPD-B and PPD-A on the left and the right side of the neck, respectively. The preinoculation skin fold thickness was recorded before PPD injection, and the skin fold thickness was measured again after 72 h. The goats were considered positive if the increase in skin fold thickness after PPD-B application was greater than 2 mm and greater than the increase after PPD-A application.
Whole-blood IFN-γ assay.
Blood samples were collected at the time points described above, preserved at room temperature, and processed less than 2 h after collection. One milliliter of whole blood was stimulated in 96-well cell culture plates with PPD-A, PPD-B, and PHA at a final concentration of 10 μg/ml or with peptide cocktails, each one at a final concentration of 5 μg/ml. PBS was used as a nonstimulated control. Plasma supernatants were collected after 24 h of culture at 37°C and 5% CO2 and were stored at −20°C and thawed just before performing the Bovigam IFN-γ enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions. ELISA results are reported as the optical density at 450 nm (OD450). Specific reaction was expressed as the ΔOD450 (the OD450 of the antigen-stimulated sample minus the OD450 of a nonstimulated control). A sample was classified as positive when the PPD-B ΔOD450 was higher than 0.05 and the OD450 of PPD-B was higher than the OD450 of PPD-A, according to the manufacturer's interpretation.
Serology.
Plasma samples from all animals were analyzed in duplicate for antibodies to mycobacteria using ELISA, as described previously (18) with minor modifications. The 96-well plates were coated with PPD-B (2 μg/ml) or MPB83 (1 μg/ml) diluted in carbonate/bicarbonate buffer and incubated overnight at 4°C. After blockade for 45 min at 37°C with PBS containing 0.05% Tween 20 (PBS-T20) with 0.5% casein, plasma samples (at 1/200 dilution in PBS-T20 with 1% casein) were added in duplicate and incubated for 1 h at 37°C. After washing, a combination of protein A and protein G conjugated with peroxidase (Sigma-Aldrich, Steinheim, Germany) was added at final concentrations of 50 ng/ml and 100 ng/ml, respectively. The plates were then read in a spectrophotometer, and the ΔOD450 was calculated as the sample OD450 minus the background OD450 (nonspecific absorbance in wells where antigen had not been added). A sample was classified as positive when the ΔOD450 was higher than the cutoff point, calculated as the mean of the background OD450 plus 3 standard deviations (SD).
Postmortem examination.
All goats were euthanized at 14 weeks p.i. by intravenous injection of sodium pentobarbital and carefully examined in order to evaluate the extension of tuberculous lesions in lungs and respiratory LN.
(i) Lungs. Lung gross lesions were recorded first by palpation and external observation of the different lobes. LN were removed for bacteriological investigation, taking special care not to incise the pleural surface. The heart and pericardium were removed, and then the whole lungs were fixed with 10% buffered formalin by pouring the fixative into the trachea while holding the lungs in a vertical position until the trachea was filled with fixative. After that, the trachea was tied, and the formalin-flooded whole lungs were immersed in a container with formalin as previously described (18) for 2 months. After complete fixation, the lungs were scanned by using a high-resolution 64-slice MDCT scanner (Brillance CT 64 channel; Philips Medical Systems, Cleveland, OH). MDCT data were analyzed and postprocessed on a workstation (Aquarius Station; TaraRecon, Foster City, CA). Tuberculous lesions were defined as the following 4 lesion types in respect to their density patterns: calcified lesions, cavitary lesions, solid lesions, and complex lesions. The total pulmonary volume and the volume of the lesions were measured.
MDCT quantification of lesions was compared with conventional visual inspection with the aid of image analysis software. For that purpose, the lungs were sliced at 4- to 5-mm intervals. Each slice was photographed, and the gross lesions were subsequently quantified in pictures with the aid of an image analyzer (ImageJ 1.43u; National Institutes of Health). The approximate volume of granulomas was calculated for each slice (area of lesion × slice thickness). The total volume of granulomas for each lobe was calculated by adding partial slice volumes. The data obtained by applying both the MDCT and visual direct scoring were compared in order to evaluate the correspondence between the two methods. Representative sections of gross lesions were also processed for histopathological examination (hematoxylin-eosin staining and Ziehl-Neelsen staining for acid-fast bacilli) to confirm the tuberculous nature of the lesions.
(ii) LN. The number and diameters of macroscopic lesions were recorded in cranial mediastinal LN, tracheobronchial LN, and caudal mediastinal LN, as well as both right and left retropharyngeal LN. The approximate volumes of gross lesions were calculated as 4/3 × π × r3, assuming that most lesions showed fairly spherical morphology. The same pathologist performed all evaluations in order to ensure homogeneous application of the scoring criteria. After pathological measurement, each LN was processed entirely for bacterial enumeration.
Culture of M. caprae.
(i) Lymph nodes. To calculate the bacterial load (CFU/g) of each LN, the weight was recorded before homogenization. Then, the LN was mechanically sliced using dissection scissors and automatically homogenized in 10 ml of sterile distilled water in a Masticator (IUL Instruments, Barcelona, Catalonia, Spain). The homogenate was decontaminated with a final concentration of 0.35% (wt/vol) hexadecylpyridinium chloride (HPC) (9) for 15 min with orbital shaking, after which it was centrifuged at 2,471 × g for 30 min. The supernatant was discarded, and the pellet was resuspended in 10 ml of PBS containing 0.05% Tween 80. Enumeration of viable bacteria was performed by plating 0.1 ml of 10-fold serial dilutions of LN homogenates on Middlebrook 7H11 agar and incubating them at 37°C for 28 days.
(ii) Peripheral blood. Whole blood (5 ml) from each goat was inoculated into BacT/Alert MB flasks (bioMérieux España, Madrid, Spain) at the time points described above and incubated for 30 days before being considered negative, as recommended by the manufacturer.
(iii) Nasal swabs. Nasal swabs were decontaminated for 30 min with 0.35% wt/vol) HPC and subsequently cultured on Coletsos and pyruvate-enriched Löwenstein-Jensen media (bioMérieux España, Madrid, Spain); cultures were incubated for 60 days before being considered negative.
DNA amplification.
The DNA from inactivated samples from nasal swabs was extracted using a DNA purification kit (Promega Biotech Iberica, Madrid, Spain). A seminested PCR was run under standard conditions. Two consecutive PCRs were performed using oligonucleotide primers described previously (IS-F, 5′-CCTGCGAGCGTAGGCGTCGG-3′; IS-R1, 5′-TCAGCCGCGTCCACGCCGCCA-3′) (28), adding another reverse primer for the second reaction (IS-R2, 5′-CTCGTCCAGCGCCGCTTCGG-3′) (16). These primers are specific for the MTC-IS6110 insertion sequence.
Data analysis.
Differences in the mean rectal temperature between weekly measures were compared by employing analysis of variance (ANOVA) with a Student-Newman-Keuls multiple-comparison test and are reported with the 95% confidence intervals (CI). Comparison of bacterial loads (log10 CFU/g) between pulmonary LN, as well as comparison of IgG or IFN-γ ELISA absorbance values (ΔOD450) between antigens within the infected group, were analyzed by a nonparametric Friedman test with the post hoc Mann-Whitney-Wilcoxon test. Correlation between MDCT and direct visual measurement of macroscopic lesions was performed by a nonparametric Spearman rank test. Immune responses and log10-transformed pathological and bacteriological data were compared by applying linear regression or by the nonparametric Spearman rank test, depending on whether experimental units passed the Shapiro-Wilk normality test. Analysis of the data was performed using the SPSS statistical package version 17.0.
RESULTS
Clinical observations.
Few clinical signs were observed in goats infected with M. caprae throughout the experiment. A significant increase in mean rectal temperature was detected at 4 weeks p.i. (39.6°C; 39.5 to 39.7, 95% CI) compared with the rest of the time points (mean, 39°C; 39.0 to 39.1, 95% CI) (P < 0.05). Coughing was observed in 3 out of 11 (27%) infected goats at 6 weeks p.i.; the majority of goats (9/11) showed coughing at the end of the experiment (14 weeks p.i.). One goat also showed tachypnea.
Immunological response.
The immunological response to infection with M. caprae was characterized using both cell-mediated and humoral immunological tests. Goats were subjected to the SICCT test at 12 weeks p.i. The mean increases in skin fold thickness 72 h after PPD-B and PPD-A application were 21.3 mm (19.4 to 23.2 mm, 95% CI) and 10.9 mm (9 to 12.7 mm, 95% CI), respectively. All infected goats were positive for PPD-B, according to the official interpretation criteria described above.
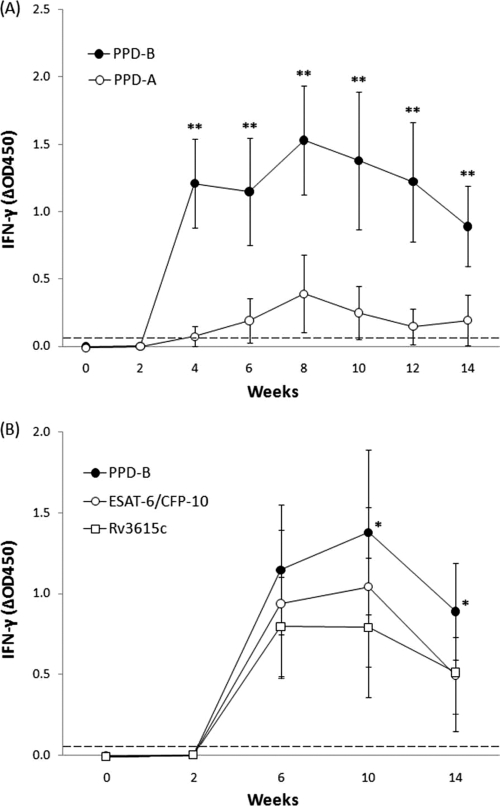
Cell-mediated immunity (CMI) was measured throughout the course of the experiment by the release of IFN-γ from whole blood stimulated with PPD-B, PPD-A, ESAT-6/CFP-10, or Rv3615c (Fig. 1). According to the standard interpretation of the Bovigam assay (considering the PPD-B-stimulated well), all infected goats were negative from the day of infection to the 2nd week p.i., but all goats became positive at 4 weeks p.i. (individual data not shown) and remained positive throughout the experiment, with the exception of two goats that were negative at 10 weeks p.i. (and were again positive at 12 and 14 weeks p.i.). The peak mean value of PPD-B stimulation was reached at 8 weeks p.i., and from that point on, a progressive decrease in the mean PPD-B absorbance was observed until the end of the experiment (Fig. 1A). Uninfected goats remained negative throughout the experiment (individual data not shown). Production of IFN-γ in blood cultures in response to stimulation with PPD-A was also first detectable at 4 weeks p.i., and it was maintained until 14 weeks p.i.; however, the mean ΔOD450 was significantly lower than that observed in cultures stimulated with PPD-B at 4 weeks p.i. (P < 0.005) (Fig. 1A). As expected, no avian reactors (Bovigam readout) were observed at any time point.
Fig. 1.
Kinetics of IFN-γ responses in infected goats. The release of IFN-γ was measured by ELISA after in vitro stimulation of whole blood with different antigens. The results are expressed as mean ΔOD450 responses with 95% CI. The dashed horizontal line is the cutoff point for positivity. (A) PPD-B and PPD-A. **, P < 0.005; significant differences were determined by a nonparametric Mann-Witney-Wilcoxon test. (B) PPD-B, ESAT-6/CFP-10, and Rv3615c. *, P < 0.05; significant differences were determined by a nonparametric Friedman test with a post hoc Mann-Whitney-Wilcoxon test.
The release of IFN-γ in response to both ESAT-6/CFP-10 and Rv3615c peptide cocktails followed similar kinetics but was also significantly weaker than that in response to PPD-B at 10 weeks p.i. and 14 weeks p.i. (P < 0.05) (Fig. 1B). If the standard cutoff point for positivity of the Bovigam assay is used for these antigens (OD450 of the stimulated sample − OD450 of the unstimulated control > 0.05), all goats reacted positively from 4 weeks p.i. onward, with the exception of the same two goats that were negative for PPD-B at 10 weeks p.i., whose results were also negative at the same time point using both peptide cocktails. Moreover, one of these goats was also negative at 14 weeks p.i., and in addition, another goat was negative at 14 weeks p.i. using Rv3615c (individual data not shown). Therefore, considering the three time points analyzed between 6 and 14 weeks p.i., 29/33 samples from infected goats were positive for the two peptide cocktails (sensitivity, 88%), whereas 31/33 samples were positive for PPD-B at the same time points (sensitivity, 94%). The two uninfected goats remained negative in all IFN-γ tests during the trial (data not shown).
To analyze the IgG response to infection with M. caprae, plasma samples from all goats were tested by ELISA every 2 weeks after infection in plates coated with PPD-B or the mycobacterial antigen MPB83. All goats were seronegative to both antigens before M. caprae infection, and uninfected goats remained seronegative throughout the experiment. After infection, the goats remained seronegative to PPD-B at all time points before the tuberculin boost at 12 weeks p.i., but all seroconverted at 14 weeks p.i. (at 13 days after the SICCT test) (individual data not shown). In contrast, seropositivity to MBPB83 after infection appeared earlier in some animals, but it was weak and inconstant, with a total of 7/11 goats positive at 8, 10, or 12 weeks p.i. (Table 1). All infected goats showed strong responses to MBPB83 after the SICCT test; indeed, pronounced differences in the mean ΔOD450 were found between the two antigens used, with much higher IgG responses to MBPB83 than to PPD-B (P < 0.005) (Fig. 2).
Table 1.
Detection of antibodies to MPB83 in plasma of goats infected or not with M. caprae
| Group | Goat | Result at wk postinfectiona |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | ||
| Infected | 572 | − | − | − | − | − | − | − | + |
| 605 | − | − | − | + | + | + | − | + | |
| 571 | − | − | − | − | + | + | − | + | |
| 567 | − | − | − | − | − | − | + | + | |
| 607 | − | − | − | + | + | − | − | + | |
| 563 | − | − | − | − | + | + | + | + | |
| 597 | − | − | − | + | + | + | + | + | |
| 568 | − | + | − | − | − | − | + | + | |
| 562 | − | − | − | − | − | + | + | + | |
| 565 | − | − | − | + | + | + | + | + | |
| 577 | − | − | + | + | + | + | + | + | |
| Noninfected | 162 | − | − | − | − | − | − | − | − |
| 187 | − | − | − | − | − | − | − | − | |
ELISA results at different time points postinfection (week 0 represents the day of infection) are expressed according to the cutoff described in the text as positive (+) or negative (−). The goats were subjected to a SICCT test 13 days before the blood sampling at 14 weeks p.i.
Fig. 2.
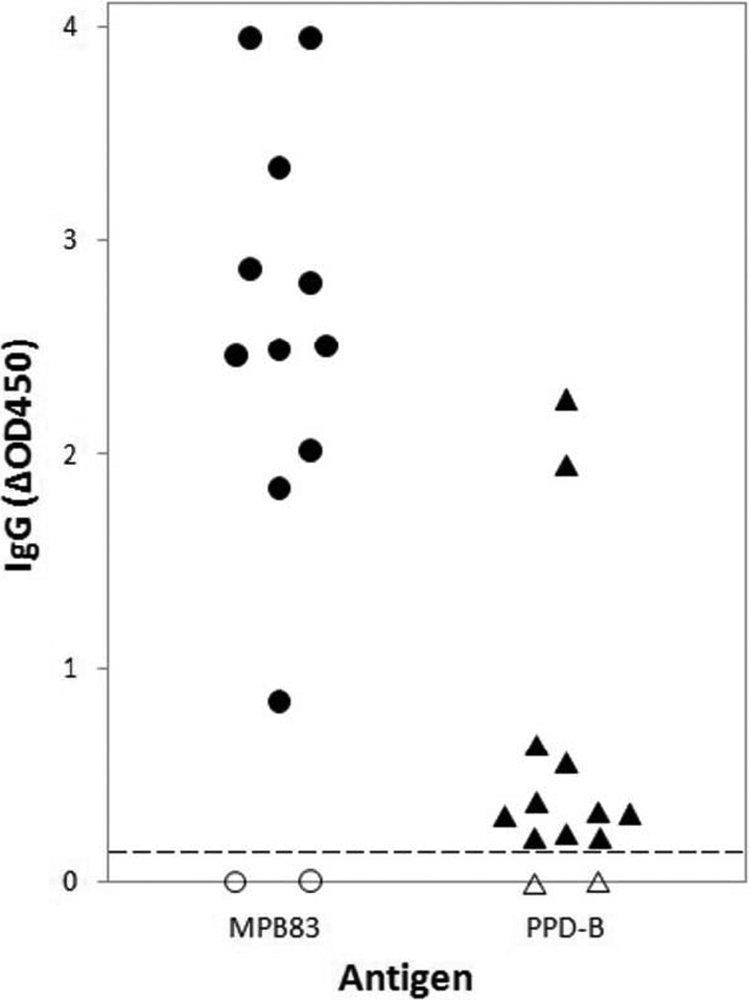
Humoral responses to MBP83 and PPD-B at 14 weeks postinfection. Shown are the OD450 absorbances of total IgG to MPB83 and PPD-B from individual goats infected or not with M. caprae. The results are expressed as ΔOD450 (OD450 of antigen-stimulated wells minus OD450 of nonstimulated wells). Filled symbols, infected animals; open symbols, noninfected control animals; dashed horizontal line, cutoff point for positivity.
Pathology.
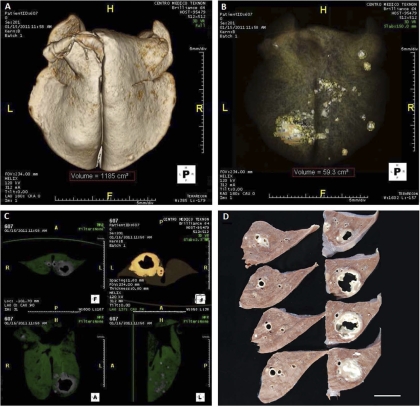
The pathological findings were mainly restricted to the thoracic cavity. All the infected goats showed granulomatous caseous necrotizing lesions in the lungs and in lung-associated lymph nodes. With few exceptions, the majority of lung lesions were located in the right lobes, and the right diaphragmatic lobe was affected in most goats. Seven out of 11 goats showed well-developed cavitary lesions. MDCT scan technology allowed a three-dimensional (3D) representation of the lungs and a cross-sectional visualization and analysis of lesions (Fig. 3). A comparison of the volumes of granulomatous necrotizing lesions in the lungs measured by MDCT and by image analysis of photographs of lung sections (direct observation) is shown in Table 2. By MDCT, the volumes of TB lesions for each goat ranged from 18.8 to 182 cm3, with a mean of 60 cm3. MDCT allowed calculation of the percentages of lung volume occupied by TB lesions (Table 2), which ranged from 1.1 to 14.3% (mean, 5.3%). The extent of lesions in the lung was also measured by recording the number of affected lung lobes in each infected goat (visual inspection), and these values are also shown in Table 2.
Fig. 3.
Gross pathology analysis of a goat case. (A) MDTC-3D representation of the whole lung after excluding air (H, head; F, foot, L, left, R, right). The total volume of the lung was calculated in cm3 and is shown in the red dashed box at the bottom. (B) Volume-rendering image of the lung showing different tissue densities discriminated by color: water, gray; air, black; calcium, white. The volume of affected lung is also shown. (C) Pathological areas identified by segmentation in axial (top; A, anterior; P, posterior), coronal, and sagittal (bottom) planes. (D) Formalin-fixed, 5-mm sections of the left diaphragmatic lobe showing a large cavitary lesion. Cranial to caudal sections are represented as bottom up and left-right. Bar = 3 cm.
Table 2.
Quantification of gross lesions in lungs in goats infected with M. capraea
| Goat | 64-slice MDCT |
Direct visual observation |
||
|---|---|---|---|---|
| Vol of lesions in lung (cm3) | Vol ratio lesion/lung (%) | Vol of lesions in lung (cm3) | No. of lobes with lesions/total | |
| 572 | 26.8 | 1.8 | 5.4 | 1/7 |
| 605 | 97.1 | 8.3 | 39.5 | 2/7 |
| 571 | 35.5 | 3.8 | 17.2 | 2/7 |
| 567 | 100 | 10.6 | 108.9 | 6/7 |
| 607 | 59.3 | 5 | 25 | 4/7 |
| 563 | 22.3 | 1.9 | 3.2 | 1/7 |
| 597 | 24.1 | 2.5 | 9.8 | 3/7 |
| 568 | 66.3 | 6.5 | 58.3 | 6/7 |
| 562 | 182 | 14.3 | 104.8 | 6/7 |
| 565 | 31 | 1.1 | 9.3 | 4/7 |
| 577 | 18.8 | 2.4 | 0.9 | 2/7 |
The total volumes of lesions and the percentages of lungs affected were calculated using 64-slice MDCT and were compared to direct visual quantification by slicing, photography, and image analysis. Means (95% CI) were as follows: volume of lesions in lung (cm3), 60.3 (30.8–89.8); volume ratio lesion/lung (%),5.3 (2.8–7.8); volume of lesions in lung (cm3), 34.7 (11.4–58.1); no. of lobes with lesions/total, 3.4/7 (2.3–4.4/7).
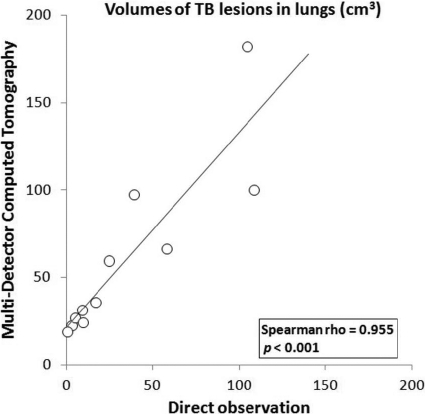
Significant positive correlation was observed between volume values obtained by MDCT and by direct observation (Spearman rho = 0.955; P < 0.001) (Fig. 4), although the volumes were higher for MDCT data (Table 2).
Fig. 4.
Correlation between the volumes of lesions in lungs measured by two quantitative methods. Visible lesions in lungs were calculated by MDCT and by image analysis of photographs of lung sections (direct observation) in infected goats (n = 11). Statistical analysis was conducted with a nonparametric Spearman rank test.
Pulmonary LN involvement was also extensive. All infected animals presented gross lesions in caudal mediastinal LN, whereas 10/11 and 8/11 goats presented lesions in tracheobronchial and cranial mediastinal LN, respectively. Also, two animals showed lesions in retropharyngeal LN. TB lesions in mesenteric LN were also recorded in the other two animals, with one of them also showing TB lesions in the spleen. In total, 4/11 goats showed extrapulmonary TB lesions. The volumes of gross lesions in LN, as well as the number of affected LN in each goat, are shown in Table 3.
Table 3.
Pathological findings and bacterial loads in pulmonary LN of infected goats
| Goat | Bacterial load in LN (log10 CFU) |
Vol of lesions in LN (cm3) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cranial mediastinal |
Tracheobronchial |
Caudal mediastinal |
Total |
Cranial mediastinal | Tracheobronchial | Caudal mediastinal | Total | |||||
| Per gram tissue | Whole LN | Per gram tissue | Whole LN | Per gram tissue | Whole LN | Per gram tissue | Whole LN | |||||
| 572 | 2.4 | 3.2 | 3 | 4.1 | 3.1 | 4.2 | 0 | 0.3 | 5.8 | 6.1 | ||
| 605 | 3.2 | 3.8 | 2.1 | 2.7 | 3.1 | 4.4 | 3.5 | 4.5 | 3.6 | 0.003 | 50.1 | 53.7 |
| 571 | 2.5 | 3.2 | 3 | 4 | 3.3 | 4.2 | 3.5 | 4.4 | 0.3 | 1.7 | 4.7 | 6.8 |
| 567 | 2.9 | 4.3 | 4 | 5.3 | 4 | 5.3 | 0 | 4.6 | 15.7 | 20.2 | ||
| 607 | 2.8 | 4 | 2.5 | 3.6 | 3 | 4.1 | 0.05 | 0 | 2.5 | 2.5 | ||
| 563 | 2.8 | 3.8 | 2.8 | 3.8 | 0 | 0.001 | 1.3 | 1.3 | ||||
| 597 | 3.3 | 3.6 | 2.4 | 3.3 | 3.6 | 4.6 | 3.8 | 4.6 | 0.07 | 0.9 | 2.8 | 3.8 |
| 568 | 3.3 | 3.9 | 2.6 | 3.5 | 3 | 4.1 | 3.5 | 4.4 | 0.08 | 0.6 | 3.4 | 4.1 |
| 562 | 2.9 | 3.4 | 2.5 | 3.4 | 3.4 | 4.8 | 3.6 | 4.8 | 0.2 | 1.2 | 57.5 | 58.9 |
| 565 | 3.4 | 4.3 | 3.2 | 4.2 | 3.3 | 4.5 | 3.8 | 4.8 | 1.2 | 6.8 | 8 | 16 |
| 577 | 3 | 3.3 | 2.4 | 3.2 | 4.1 | 5.2 | 4.1 | 5.2 | 0.1 | 0.1 | 4.2 | 4.5 |
| Mean (95% CI) | 2 (1–2.9) | 2.3 (1.2–3.4) | 2.4 (1.9–2.9) | 3.3 (2.6–4) | 3.3 (3–3.6) | 4.4 (4.1–4.7) | 3.5 (3.3–3.8) | 4.6 (4.3–4.8) | 0.5 (0–1.2) | 1.5 (0.2–2.8) | 14.2 (2.3–26) | 16.2 (3.9–28.4) |
Bacteriology.
M. caprae was isolated from postmortem tissue samples from all inoculated animals, but it was not detected by PCR or mycobacterial isolation from any of the nasal swabs or blood samples taken during the experiment.
Mycobacteria were recovered from caudal mediastinial LN in all goats, from tracheobronchial LN of 10/11 goats, and from cranial mediastinial LN of 7/11 goats. In contrast, mycobacteria were isolated from retropharyngeal LN in only two goats. M. caprae was also isolated in all TB lesions observed in extrarespiratory organs. The bacterial load of M. caprae per gram of cultured pulmonary LN tissue and the total bacterial load of each LN are also shown in Table 3.
The mean bacterial load (log10 CFU/g) in the cultured pulmonary LN was 3.5 log10 CFU/g (3.3 to 3.8, 95% CI), with a range between animals of 2.8 to 4.1 log10 CFU/g. Some differences were also found among the bacterial loads of each pulmonary LN. In caudal mediastinal LN, the bacterial load was 3.3 log10 CFU/g (3 to 3.6, 95% CI), significantly higher than in cranial mediastinal LN (2 log10 CFU/g; 1 to 2.9, 95% CI) (P < 0.05) and in tracheobronchial LN (2.4 log10 CFU/g; 1.9 to 2.9, 95% CI) (P < 0.01). When the total bacterial count of respiratory LN was considered, this value ranged from 3.8 to 5.3 log10 CFU, with a mean value of 4.6 (4.3 to 4.8, 95% CI). Also, the whole bacterial load was higher for the caudal mediastinal LN than for other LN (Table 3).
Cross-sectional analysis.
Association between cellular and humoral immune responses, pathology, and bacteriology were evaluated transversally, combining data obtained from all experimental goats (n = 13). In LN, a positive correlation was found between pathology (the volume of lesions as log10 mm3) and the bacterial load (log10 CFU/g) (Pearson r = 0.858; P < 0.001). Positive correlations were also found between the bacterial load in LN and IFN-γ specific responses to PPD-B (Pearson r = 0.528; P = 0.032) and to ESAT-6/CFP-10 (Pearson r = 0.579; P = 0.019), but not to Rv3615c (Spearman rho = 0.296; P = 0.163), at 14 weeks p.i. However, only IFN-γ responses to PPD-B at 14 weeks p.i. were correlated significantly with the volume of gross lesions in lungs determined by MDCT (Pearson r = 0.540; P = 0.028).
Humoral immune responses to MPB83 at 14 weeks p.i. correlated positively with both the bacterial load in LN (Pearson r = 0.775; P = 0.001) and the volume of gross lesions in lungs determined by MDCT (Pearson r = 0.685; P = 0.007), whereas IgG responses to PPD-B did not correlate significantly with the bacterial load (Spearman rho = 0.322; P = 0.141) and were slightly positively correlated with the volume of gross lesions in lungs (Spearman rho = 0.481; P = 0.048).
DISCUSSION
Recent interest in the development of TB vaccines in domestic ruminants and wildlife, such as badgers and wild boar, has driven research to standardize infection models in domestic animals, like ruminants (7) and pigs (18). Modeling TB in goats may be of great value in increasing our knowledge of infection in the species, and at the same time, the model can be used for research on TB in cattle. With these aims, we have established an efficient experimental goat model of TB, with slight clinical signs (coughing at the end of the experiment) and a relatively fast progression of lesions, very similar to natural disease. Gross TB lesions were reproduced in all the infected goats, which is an advantage over the previous model in adult goats (3). It is well known from experiments in calves and other models that the route and dose of challenge can be very relevant to the pathological outcome of infection (see reference 29 for a review). A high challenge dose (higher than 106 CFU) by nonnatural routes (such as intravenous or subcutaneous) may lead to systemic dissemination of infection, with lesions that are not representative of natural field cases (40). Using a relative low challenge dose of 1.5 × 103 CFU by the endobronchial route, we have been able to reproduce typical granulomatous caseous necrotizing lesions in the lung and lung-associated LN in 11 out of 11 experimentally infected goats, resembling those observed in naturally infected goats (12, 31), and as seen sometimes in natural cases, the majority of our goats had liquefactive necrosis and cavernous lung lesions, which are features of tuberculosis in humans. In a previous study in goats experimentally infected with M. caprae (3), adult goats were infected transthoracically with 102 to 103 CFU, achieving infection in all 6 infected goats (as demonstrated by mycobacterial culture) but with absence of macroscopic lesions in lung parenchyma in two goats, in spite of a much longer duration of infection (9 months). This difference could be due to the use of 6-month-old goats in our study compared to adult animals and is clearly an advantage over a model with adult goats. Extension of the infection with production of gross lesions in extrapulmonary sites is often included in scoring systems to assess vaccine efficacy, and therefore, an inoculation route that conveys the challenge dose to a circumscribed area, mimicking natural infection, should be preferred to models that disperse mycobacteria into different systems or mucosal surfaces. In this respect, transthoracic inoculation drops inoculated mycobacteria directly into the lung parenchyma but may also cause pleuritis (according to our personal observations) and local infection of the thoracic wall at the inoculation point, with mycobacteria draining into regional LN, like the axillary nodes, and thus complicating the assessment of extrapulmonary dissemination. In our study, four animals had extension of the infection from the thoracic primary focus to extrapulmonary tissues, like medial retropharyngeal or mesenteric lymph nodes, and to the spleen (in one case, indicative of systemic circulation of mycobacteria). Probably, pulmonary lesions allow dissemination to the upper respiratory/head and mesenteric lymph nodes by mycobacterial shedding in tracheobronchial secretions and subsequent ingestion. Similarly to results in calves inoculated with a dose of 104 CFU of M. bovis (26), nasal shedding of M. caprae was not detected in our study, showing that, if nasal shedding occurs, it happens at a very low load or intermittently. As expected, blood culture was also negative throughout the experiment in all goats, indicating that bacteremia is not a feature of TB in goats, at least in the early phase of the infection.
The data obtained here strengthen the hypothesis that young goats are highly susceptible to infection by M. caprae. Pathological and bacteriological findings point to fast progression of lesions, which reached relatively large size in some animals (more than 5% of the lung affected). In natural cases of TB, such large lesions with liquefactive necrosis and caverna formation are usually associated with a long period of lesion progression in herds not subjected to eradication (12, 14, 31). In contrast, in trials carried out in other species, like badgers (10) and calves (39), using the endobronchial route of infection, lesions progressed slowly, resembling what is observed in natural cases of TB in these species. Particularly in the low-challenge-dose experiments in calves, large coalescent lesions were not usually found; in contrast, our model appears to have faster progression of lesions, which can be considered an advantage.
Assessment of vaccine efficacy in experimental trials by nonimmunological parameters has used semiquantitative scoring systems based on the number of pulmonary lobes affected and the size of lesions in the lung and pulmonary LN, as well as the bacterial load in LN in cattle (37), in rabbits (20), and in macaques (23). A drawback of these scoring systems is that the intrapulmonary extension of lesions to one or more lobes may be strongly influenced by the inoculation procedure, and consequently, this may also influence the extension to lymph nodes (which depends on the drainage of the lobes affected). Clear evidence of this in our study is the direction of the inoculum to the right lung by inoculation of goats in the right decubitus position. To avoid this drawback and to increase the usefulness of the pathological assessment, in our study, we attempted to express the severity of lung lesions in a quantitative way to allow better comparisons between treatment groups and different experiments. The use of high-resolution 64-slice MDCT can resolve the whole burden of lung lesions to a volume, and the ratio of affected lung can be calculated. Additionally, we made an effort to compare the results obtained with MDCT with a conventional visual inspection of a sliced lung, photography, and calculation of the area of lesions in each picture by image analysis. If sections of lung are similar in thickness, an approximate volume of lesions can be obtained by adding the volumes of lesions in each slice. This is a time-consuming task, although it also provides an approximate total volume of lesions per lung. We have shown that the results of visual inspection had a strong correlation with those obtained by MDCT, although in general they were lower. Interestingly, MDCT seems to have the capacity to detect small changes in density patterns due to inflammatory reactions around the granuloma that may not be visible by direct macroscopic observation. This, together with error introduced by the use of the same thickness for all lung slices for calculation, could explain the slightly higher but homogeneous animal-to-animal volume values obtained by using MDCT comparison to direct observation measures. Therefore, MDCT can be a far more precise method than the pathology-scoring systems usually applied to assess the severity of lesions or their reduction in future vaccine efficacy assays. A similar approach to the measurement of lung lesions was followed recently by Sharpe et al. in macaques (34). The authors measured the total volume of lung lesions in relation to the whole lung volume after fixation by immersion in formalin by using MRI stereology. They concluded that the ratio of lung lesions to the whole volume was superior to thoracic radiography or pathology scores for measuring disease burden. Also, in their aerosol model of infection, the total volume of lesions accurately reflected differences in challenge doses in different groups.
Methodologically, irrespective of whether MRI or MDCT technology is used, it is worth stressing the importance of insufflation of the lung with formalin to distend the lungs to approximately the same volume they would have in the pulmonary cavity. This renders the ratio of the lesion volume to the total lung volume comparable between different experiments and research groups. The use of this ratio also corrects for slight differences that could exist in the sizes of the animals and of the lungs, even in age-matched animals. We believe that this very precise quantitative data set offers the possibility of a better assessment of vaccine efficacy in TB studies. The same conclusion has been drawn by Sharpe et al. (34), who stressed the benefits of MRI stereology as an accurate and quantifiable assessment that is easy to standardize and comparable between laboratories, suggesting that it will be an essential component of pathology assessment in vaccine efficacy studies.
Our experimental model may be useful for assessing the performance of diagnosing techniques in caprine TB. The infection was detected satisfactorily at 12 weeks p.i. with the SICCT test, the official antemortem diagnostic test currently used for bovine TB eradication campaigns, and all infected goats were also positive in the standard IFN-γ assay from 4 weeks p.i., confirming the usefulness of these techniques as well for diagnosis of caprine TB, as described previously by others (19, 22).
Intriguingly, the kinetics of cell-mediated immune responses to infection with M. caprae, measured as anamnestic IFN-γ secretion, was slightly different from that described previously in the calf model. In goats, for all antigens used, the levels of specific IFN-γ were not appreciable until 4 weeks p.i., while experimental infections in cattle with a similar mycobacterial dose usually showed a significant specific IFN-γ response at 2 weeks p.i., especially in samples stimulated with PPD-B (6, 37, 39). Nevertheless, the appearance of detectable levels of IFN-γ a week later has also been reported in cattle infected with a low dose of M. bovis (13). Unexpectedly, a decrease in IFN-γ responses seemed to occur in infected goats at 10 weeks p.i., whereas in a long-term cattle infection, these responses maintained their intensity for at least 20 weeks (6). This phenomenon, if confirmed in further long-term studies, could correlate with the fast progression of infection in our goat model, as deduced from the extent of lesions observed, coincident with a decline in the activity of effector IFN-γ-producing cells.
Peptide cocktails of ESAT-6/CFP-10 and Rv3615c are being considered new differentiation of infected and vaccinated animal (DIVA) reagents for use in cattle (36, 38). The usefulness of ESAT-6/CFP-10 has been successfully demonstrated in the field, showing high sensitivity and specificity in comparison to tuberculins in cattle that had been naturally infected with M. bovis (8) and more recently in goats infected naturally with M. caprae (2). Interestingly, the sensitivity obtained in our study for the two peptide cocktails (88%) would increase to 91% if the results obtained for the two cocktails were combined, the same theoretical sensitivity that was reported previously for cattle infected with M. bovis (36). Moreover, the IFN-γ response to ESAT-6/CFP-10, but not to Rv3615c, correlates positively with the bacterial burden in LN, although an even higher correlation has been described previously for IFN-γ responses to ESAT-6 and the bacterial burden in M. bovis-infected cattle (24). These findings are consistent with the concept that the bacterial load in infected tissues is proportional to host IFN-γ responses to antigens secreted by actively growing mycobacteria, such ESAT-6 and CFP-10 (27), but these responses became lower at the end of the experiment, so the correlation should be considered at each stage of the disease. The capacity of ESAT-6/CFP-10 to predict the disease status, the increase in sensitivity when the two peptide cocktails are used, and their DIVA capabilities in animals vaccinated with M. bovis BCG could make them useful tools for vaccine trials to distinguish vaccinated/protected and infected animals.
Serology is another important tool for assessing infection or exposure to mycobacteria and could be another useful biomarker to determine disease status, although it is not yet clear whether antibody responses play a role in controlling TB. In recent years, serological tests have been assessed in trials in cattle and wild mammals, and most of them have concluded that MPB70, and especially MPB83, is serodominant, being recognized in early stages of infection (24, 25, 41). The serodominance of MPB83 described in other species is also consistent with our findings, as most of the goats (10/11) were seropositive at least at one time point before the boost effect of the SICCT test (12 weeks p.i.). Moreover, 2 weeks after boosting with PPDs, all animals reacted strongly, increasing dramatically the sensitivity of ELISA, as has been shown in cattle (39). In contrast, the IgG ELISA with PPD-B as an antigen failed to detect any positive animals before the boost effect of the SICCT test, after which antibody responses were positive, although very weak in comparison to MPB83 IgG ELISA. This result suggests that serology to MPB83 could be a useful tool to detect infected animals on farms, as well as to monitor the progression of the infection in experimental trials.
In summary, our goat TB infection model may be useful in TB research for understanding the pathogenesis of TB in goats and for testing of therapeutic and immunoprophylactic treatments and of new diagnostic tools. The use of MDCT for quantification of the volume of lesions and their ratio to the whole lung volume may serve for a quantitative evaluation of pathology in vaccination trials. Research in human TB can also benefit from large-animal models different from nonhuman primates, due to the similarities to the human disease and their lower cost (39, 42).
Also, reports of caprine TB have increased in recent years, and studies are needed to validate whether control measures used in cattle can be applied to goat herds. Vaccination based on BCG has been developed for use in wild species that act as reservoirs of M. bovis and could represent a control tool for caprine TB and to limit its transmission to cattle and humans.
ACKNOWLEDGMENTS
This study was funded by European Union projects NADIR (FP7-INFRA-2008-1.1.2, no. 228394) and TB-STEP (FP7-KBBE-2007-1.3.04, no. 212414).
We thank the Radiology Service of the Centro Médico Teknon for providing its facilities for performing the MDCT scan analysis. We give special thanks to Félix García, Ana Andaluz, and Xavier Moll from the Department of Animal Medicine and Surgery of the Universitat Autònoma de Barcelona for their help with the experimental mycobacterial inoculation. We also thank Xavier Abad and Raúl Núñez for their assistance with M. caprae inoculum preparation and with bacterial cultures. We express our appreciation to the staff of the Level 3 Biocontainment Unit of CReSA for their technical assistance. MTC peptides were kindly supplied by the Veterinary Laboratories Agency.
We declare that we have no competing interests.
B.P.D.V. and M.D. conceived and designed the experiments, analyzed the data, and drafted the manuscript. M.D., S.L.-S., M.N., and D.S. kept the necropsy and pathological records; B.P.D.V. and M.M. performed the immunological and bacteriological assays; D.S. performed the skin tests; and N.R. and M.E. performed MDCT and analyzed the resulting data. M.H.V., B.V.-R., and P.-J.C. contributed substantively to scientific discussion of the results. All of the authors have read and approved the final manuscript.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Aranaz A., Cousins D., Mateos A., Dominguez L. 2003. Elevation of Mycobacterium tuberculosis subsp. caprae Aranaz et al. 1999 to species rank as Mycobacterium caprae comb. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 53:1785–1789 [DOI] [PubMed] [Google Scholar]
- 2. Bezos J., et al. 2011. Assessment of in vivo and in vitro tuberculosis diagnostic tests in Mycobacterium caprae naturally infected caprine flocks. Prev. Vet. Med. 100:187–192 [DOI] [PubMed] [Google Scholar]
- 3. Bezos J., et al. 2010. Experimental infection with Mycobacterium caprae in goats and evaluation of immunological status in tuberculosis and paratuberculosis co-infected animals. Vet. Immunol. Immunopathol. 133:269–275 [DOI] [PubMed] [Google Scholar]
- 4. Buddle B. M., Aldwell F. E., Pfeffer A., de Lisle G. W. 1994. Experimental Mycobacterium bovis infection in the brushtail possum (Trichosurus vulpecula): pathology, haematology and lymphocyte stimulation responses. Vet. Microbiol. 38:241–254 [DOI] [PubMed] [Google Scholar]
- 5. Buddle B. M., Wedlock D. N., Denis M. 2006. Progress in the development of tuberculosis vaccines for cattle and wildlife. Vet. Microbiol. 112:191–200 [DOI] [PubMed] [Google Scholar]
- 6. Buddle B. M., et al. 2003. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect. Immun. 71:6411–6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buddle B., et al. 2005. Cattle as a model for development of vaccines against human tuberculosis. Tuberculosis 85:19–24 [DOI] [PubMed] [Google Scholar]
- 8. Cockle P. J., Gordon S. V., Hewinson R. G., Vordermeier H. M. 2006. Field evaluation of a novel differential diagnostic reagent for detection of Mycobacterium bovis in cattle. Clin. Vaccine Immunol. 13:1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corner L. A., Trajstman A. C. 1988. An evaluation of 1-hexadecylpyridinium chloride as a decontaminant in the primary isolation of Mycobacterium bovis from bovine lesions. Vet. Microbiol. 18:127–134 [DOI] [PubMed] [Google Scholar]
- 10. Corner L. A. L., et al. 2007. Experimental tuberculosis in the European badger (Meles meles) after endobronchial inoculation of Mycobacterium bovis: I. Pathology and bacteriology. Res. Vet. Sci. 83:53–62 [DOI] [PubMed] [Google Scholar]
- 11. Cvetnic Z., et al. 2007. Mycobacterium caprae in cattle and humans in Croatia. Int. J. Tuberc. Lung Dis. 11:652–658 [PubMed] [Google Scholar]
- 12. Daniel R., et al. 2009. Outbreak of tuberculosis caused by Mycobacterium bovis in golden Guernsey goats in Great Britain. Vet. Rec. 165:335–342 [DOI] [PubMed] [Google Scholar]
- 13. Dean G. S., et al. 2005. Minimum infective dose of Mycobacterium bovis in cattle. Infect. Immun. 73:6467–6471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Domingo M., et al. 2009. Effectiveness and safety of a treatment regimen based on isoniazid plus vaccination with Mycobacterium tuberculosis cells' fragments: field-study with naturally Mycobacterium caprae-infected goats. Scand. J. Immunol. 69:500–507 [DOI] [PubMed] [Google Scholar]
- 15. Duarte E. L., Domingos M., Amado A., Botelho A. 2008. Spoligotype diversity of Mycobacterium bovis and Mycobacterium caprae animal isolates. Vet. Microbiol. 130:415–421 [DOI] [PubMed] [Google Scholar]
- 16. Eisenach K. D., Cave M. D., Bates J. H., Crawford J. T. 1990. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J. Infect. Dis. 161:977–981 [DOI] [PubMed] [Google Scholar]
- 17. Erler W., et al. 2004. Molecular fingerprinting of Mycobacterium bovis subsp. caprae isolates from Central Europe. J. Clin. Microbiol. 42:2234–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gil O., et al. 2010. Granuloma encapsulation is a key factor for containing tuberculosis infection in minipigs. PLoS One 5:e10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gutiérrez M., Tellechea J., García Marín J. F. 1998. Evaluation of cellular and serological diagnostic tests for the detection of Mycobacterium bovis-infected goats. Vet. Microbiol. 62:281–290 [DOI] [PubMed] [Google Scholar]
- 20. Jassal M., Nedeltchev G., Osborne J., Bishai W. 2011. A modified scoring system to describe gross pathology in the rabbit model of tuberculosis. BMC Microbiol. 11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kubica T., Rusch-Gerdes S., Niemann S. 2003. Mycobacterium bovis subsp. caprae caused one-third of human M. bovis-associated tuberculosis cases reported in Germany between 1999 and 2001. J. Clin. Microbiol. 41:3070–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liébana E., Aranaz A., Urquía J. J., Mateos A., Dominguez L. 1998. Evaluation of the gamma-interferon assay for eradication of tuberculosis in a goat herd. Aust. Vet. J. 76:50–53 [DOI] [PubMed] [Google Scholar]
- 23. Lin P. L., et al. 2009. Quantitative comparison of active and latent tuberculosis in the Cynomolgus macaque model. Infect. Immun. 77:4631–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lyashchenko K., et al. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72:2462–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lyashchenko K. P., et al. 2008. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Vet. Microbiol. 132:283–292 [DOI] [PubMed] [Google Scholar]
- 26. McCorry T., et al. 2005. Shedding of Mycobacterium bovis in the nasal mucus of cattle infected experimentally with tuberculosis by the intranasal and intratracheal routes. Vet. Rec. 157:613–618 [DOI] [PubMed] [Google Scholar]
- 27. Pai M., Riley L. W., Colford J. M. 2004. Interferon-γ assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761–776 [DOI] [PubMed] [Google Scholar]
- 28. Plikaytis B. B., Eisenach K. D., Crawford J. T., Shinnick T. M. 1991. Differentiation of Mycobacterium tuberculosis and Mycobacterium bovis BCG by a polymerase chain reaction assay. Mol. Cell Probes 5:215–219 [DOI] [PubMed] [Google Scholar]
- 29. Pollock J. M., Rodgers J. D., Welsh M. D., McNair J. 2006. Pathogenesis of bovine tuberculosis: the role of experimental models of infection. Vet. Microbiol. 112:141–150 [DOI] [PubMed] [Google Scholar]
- 30. Prodinger W. M., et al. 2005. Characterization of Mycobacterium caprae isolates from Europe by mycobacterial interspersed repetitive unit genotyping. J. Clin. Microbiol. 43:4984–4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quintas H., Reis J., Pires I., Alegria N. 2010. Tuberculosis in goats. Vet. Rec. 166:437–438 [DOI] [PubMed] [Google Scholar]
- 32. Rodríguez E., et al. 2009. Human tuberculosis due to Mycobacterium bovis and M. caprae in Spain, 2004–2007. Int. J. Tuberc. Lung Dis. 13:1536–1541 [PubMed] [Google Scholar]
- 33. Rodríguez S., et al. 2011. Mycobacterium caprae infection in livestock and wildlife, Spain. Emerg. Infect. Dis. 17:532–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharpe S. A., et al. 2009. Determination of lesion volume by MRI and stereology in a macaque model of tuberculosis. Tuberculosis 89:405–416 [DOI] [PubMed] [Google Scholar]
- 35. Sharpe S. A., et al. 2010. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin. Vaccine Immunol. 17:1170–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sidders B., et al. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect. Immun. 76:3932–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vordermeier H. M., et al. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vordermeier H. M., et al. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vordermeier H. M., et al. 2009. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77:3364–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Waddington F. G., Ellwood D. C. 1972. An experiment to challenge the resistance to tuberculosis in B.C.G. vaccinated cattle in Malawi. Br. Vet. J. 128:541–552 [DOI] [PubMed] [Google Scholar]
- 41. Waters W. R., et al. 2006. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin. Vaccine Immunol. 13:648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young D. 2009. Animal models of tuberculosis. Eur. J. Immunol. 39:2011–2014 [DOI] [PubMed] [Google Scholar]