Abstract
Inhalation of Yersinia pestis causes pneumonic plague, which rapidly progresses to death. A previously licensed killed whole-cell vaccine is presently unavailable due to its reactogenicity and inconclusive evidence of efficacy. The present study now shows that vaccination intranasally (i.n.) with inactivated Y. pestis CO92 (iYp) adjuvanted with interleukin-12 (IL-12) followed by an i.n. challenge with a lethal dose of Y. pestis CO92 prevented bacterial colonization and protected 100% of mice from pneumonic plague. Survival of the vaccinated mice correlated with levels of systemic and lung antibodies, reduced pulmonary pathology and proinflammatory cytokines, and the presence of lung lymphoid cell aggregates. Protection against pneumonic plague was partially dependent upon Fc receptors and could be transferred to naïve mice with immune mouse serum. On the other hand, protection was not dependent upon complement, and following vaccination, depletion of CD4 and/or CD8 T cells before challenge did not affect survival. In summary, the results demonstrate the safety, immunogenicity, and protective efficacy of i.n. administered iYp plus IL-12 in a mouse model of pneumonic plague.
INTRODUCTION
Plague, which is caused by the Gram-negative bacterium Yersinia pestis, is one of the deadliest diseases of humans and animals (27). The cause of more than 200 million human deaths, Y. pestis is considered to be a category A biothreat by the Centers for Disease Control and Prevention (12, 27). Primary pneumonic plague, caused by inhalation of infectious Y. pestis aerosols, is the most dangerous of all forms of plague, can be transmitted person-to-person (37), and rapidly progresses to death in 2 to 4 days if antibiotic treatment is delayed after onset of symptoms (7). A killed whole-cell vaccine was previously licensed for use in the United States but was found to be reactogenic and did not protect against pneumonic plague; thus, it is no longer available (18, 19, 29, 33, 34). However, two studies have reported that protection against pneumonic plague can be induced in experimental animals using inactivated Y. pestis (iYp). In the first study by Ehrenkranz and Meyer (9), extremely high doses of killed Y. pestis strain Yerka delivered multiple times subcutaneously (s.c.) or intramuscularly (i.m.) were found to protect rhesus monkeys from intratracheal (i.t.) challenge with Y. pestis strain 195/P, but lower doses that would be appropriate for human use failed to induce protection. Another study by Baca-Estrada et al. (3) showed that mice could be partially protected against i.n. Y. pestis strain GB challenge when liposome-encapsulated killed organisms were delivered i.n. following s.c. priming. However, this s.c. prime–i.n. boost procedure has not been fully evaluated for safety, immunogenicity, or efficacy.
Whole-cell vaccines display arrays of multiple antigens in a way that is believed to best mimic a pathogen. Antigens present on such vaccines include many known and likely novel targets that may represent future subunit vaccine candidates. Moreover, whole-cell vaccines contain pathogen-associated molecular patterns such as lipopolysaccharides that elicit innate immunity and help shape adaptive immune responses.
Vaccination by the i.n. route is an attractive strategy as it is noninvasive and elicits both local and systemic immunity (8, 25, 26). In the present work, we have investigated the safety, immunogenicity, and protective efficacy of an i.n. delivered iYp CO92 vaccine. Interleukin-12 (IL-12) was used as an adjuvant since our previous studies have demonstrated this cytokine to be highly effective as an i.n. vaccine adjuvant against lethal viral and bacterial infections, including those with influenza virus (2), Streptococcus pneumoniae (1), and Francisella tularensis (4). Studies by Yamanaka et al. (42, 43) have also suggested that DNA coexpressing IL-12 and Y. pestis antigen can be used to protect against pneumonic plague. We further investigated the mechanism for protection, including the potential roles of antibodies, Fc receptors (FcRs), complement, and CD4 and CD8 T cells. The results of our study show that iYp can be used safely and effectively for protection of mice against pneumonic plague.
MATERIALS AND METHODS
Mice.
Wild-type (WT) BALB/c and C57BL/6 mice (FcRγ+/+ and C3+/+), 8 to 12 weeks old, were purchased from Charles River Laboratories (Wilmington, MA) through a contract with the National Cancer Institute. C57BL/6 FcRγ−/− and Rag1−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). C3+/− mice were purchased from the Jackson Laboratory and bred in-house to obtain homozygous C3−/− mice. Absence of complement in C3−/− mice was confirmed by enzyme-linked immunosorbent assay (ELISA). Vaccinated mice were housed in microisolator cages at Albany Medical College and infections were performed in a CDC-certified animal biosafety level 3 (ABSL-3) facility. All experimental procedures were performed according to a protocol approved by the Institutional Animal Care and Use Committee as well as the Institutional Biosafety Committee.
Y. pestis growth conditions.
Y. pestis strain CO92 was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository. Cultures of Y. pestis CO92 were grown on Congo red agar (CRA) plates and incubated for 48 h in 5% CO2 at 28°C. Five colonies were inoculated into heart infusion broth (Oxoid Ltd., Hempshire, England) and incubated with shaking at 37°C for 18 h. Aliquots were stored frozen at −80°C. Numbers of CFU were monitored in thawed culture aliquots before mouse challenges.
Preparation of iYp.
For inactivation of Y. pestis CO92, the bacteria were exposed to 0.25% paraformaldehyde in calcium- and magnesium-free phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA) for 1 h at 37°C. After being washed in PBS to remove the fixative, the bacteria were resuspended in PBS and stored in aliquots at −80°C. In vitro confirmation of inactivation was assessed by culturing on CRA and blood agar plates at 37°C for 7 days. In vivo confirmation of inactivation was also performed by intraperitoneal (i.p.) inoculation of BALB/c mice with 106 to 108 CFU equivalents followed by 7 days of observation for survival. A dose of 103 CFU live Y. pestis CO92 is typically lethal for BALB/c mice when inoculated i.p. Lungs and spleens from the mice were also plated on CRA plates for confirmation of sterility.
Determination of iYp toxicity.
iYp was previously shown to be reactogenic when used for vaccination (15, 18, 19, 21, 38), and it was thus necessary to assess the potential toxicity of our vaccination approach. BALB/c mice (8/group) were anesthetized by i.p. injection of ketamine hydrochloride (KetaVed; Vedco, St. Joseph, MO) and xylazine hydrochloride (Phoenix Scientific, St. Joseph, MO) in sterile PBS. The mice were then inoculated i.n. with PBS containing 1% normal mouse serum (NMS) as carrier, 105 to 108 CFU equivalents of iYp, and 0.5 μg of IL-12 as the adjuvant (total inoculation volume of 30 μl/mouse). Body weight was measured daily for 10 days after primary vaccination. In addition, coat appearance, activity, and overt signs of discomfort such as malaise and hunched posture were monitored after primary and secondary vaccinations. Finally, lung pathology was measured on days 3, 5, and 7 postinoculation (see below). The levels of tumor necrosis factor alpha (TNF-α) in lung homogenates were measured 1, 3, and 5 days after primary and secondary vaccinations by using the BD OptEIA mouse TNF ELISA set per the manufacturer's protocol.
i.n. vaccination and bacterial challenge.
Vaccination was performed by i.n. inoculation of 107 CFU of iYp plus 0.5 μg of IL-12, iYp alone, or vehicle alone in 30 μl of PBS containing 1% NMS on days 0 and 21. The mice were then challenged i.n. approximately 8 weeks later with 103 CFU of live Y. pestis CO92. The inoculum dose was confirmed by plating on CRA plates. Survival was then monitored daily for 21 days postchallenge.
Determination of Y. pestis CO92-specific antibody titers.
Y. pestis CO92-specific IgG and IgA antibodies expressed in sera and bronchoalveolar lavage (BAL) fluids were quantified using ELISA essentially as outlined by Baron et al. (4). Briefly, microtiter plates were coated with 50 μl of 2.5 × 106/ml of iYp overnight at 4°C in carbonate buffer followed by washing and addition of serial dilutions of mouse sera or lung homogenates. Biotin-conjugated goat anti-mouse IgG or IgA (Caltag, Burlingame, CA) was added, followed by streptavidin-horseradish peroxidase conjugate (Biosource, Camarillo, CA) and TMB substrate solution (BD OptEIA, BD Biosciences, CA) for color development. The plates were read at 450 and 650 nm using a PowerWave HT microplate reader (BioTek, Winooski, VT). Antibody titers were determined as the reciprocal of the test sample dilution that gave a 0.1 optical density (OD) value above the background.
Measurement of bacterial burden and pulmonary cytokines.
Lungs, livers, and spleens were collected from infected mice 24, 48, and 72 h postinfection. The tissues were homogenized in a Mini-Beadbeater-96 (BioSpec, Bartlesville, OK) and diluted 10-fold in Dulbecco's minimal essential medium (DMEM)–5% fetal calf serum (FCS). For determination of bacterial burden, the homogenates were incubated on CRA plates at 28°C and bacterial colonies were counted after 48 h. Levels of pulmonary cytokines in lung homogenates were determined 72 h after Y. pestis CO92 infection using a multiplex bead-based immunoassay (BD cytometric bead array kit; BD) and the results were analyzed using a BD FACSArray (BD Biosciences, San Jose, CA).
Pulmonary histopathology.
Lungs were collected 3, 5, 7, and 35 days after vaccination and 3 days after i.n. challenge with Y. pestis CO92. After fixation in 10% buffered formalin, 5-μm lung sections were prepared, mounted on glass slides, and stained with hematoxylin and eosin (H&E). Photomicrographs were acquired using an Olympus BX-41 light microscope equipped with an Olympus DP72 digital camera and the images were analyzed using Olympus cellSens software.
Passive serum transfer.
Pooled immune mouse serum (IMS) was prepared from vaccinated BALB/c mice or on day 21 postchallenge. All sera were incubated at 56°C for 30 min to inactivate complement, diluted 4-fold in PBS, and i.p inoculated into recipient mice (0.2 ml/mouse). After i.n. infection with 103 CFU of Y. pestis CO92, survival was monitored for 10 or 14 days.
In vivo depletion of CD4 and CD8 T cells.
Rat anti-mouse CD4 monoclonal antibody (MAb) (clone GK1.5) and rat anti-mouse CD8 MAb (clone 53-6.72) (both from BioXCell, West Lebanon, NH) were used to deplete T-cell subsets before bacterial challenge of vaccinated mice. Seventy days following vaccination, mice received 500 μg each of GK1.5 and 53-6.72 MAbs i.p. on days −10, −7, −4, and −1 before i.n. Y. pestis CO92 challenge and 3 days postchallenge. Control mice received rat IgG or PBS-1% NMS vehicle alone. In vivo depletion of CD4 and CD8 T cells was established in a group of mice by flow cytometry on lung and spleen cells stained with anti-CD4-APC (clone RM4-5; BD Pharmingen) and anti-CD8-Tricolor (clone CT-CD8a; Caltag) antibodies.
Statistical analysis.
Survival curves were compared using the log rank (Mantel-Cox) test. Means of weight loss, antibody levels, and TNF-α levels by ELISA were compared by two-way analysis of variance (ANOVA). Means of the bacterial burdens and multiplex bead-array cytokine analyses were compared using the nonparametric Mann-Whitney test. GraphPad Prism version 5 and GraphPad InStat version 3 (San Diego, CA) were used for the statistical analyses of the experimental data. A P value of <0.05 was considered statistically significant.
RESULTS
Safety of iYp plus IL-12 i.n.
For this study, it was first necessary to determine a safe and nonreactogenic vaccine dose that was free of side effects when administered by the i.n. route. It was found that mice vaccinated i.n. with 107 CFU equivalents of iYp plus IL-12 lost approximately 8% of body weight by 2 days after vaccination compared to mice vaccinated with 107 CFU iYp alone (P < 0.05) (Fig. 1A). However, this loss of body weight was transient and the mice regained their body weights by day 3 postvaccination. Moreover, the animals displayed behavior comparable to that of PBS-treated control mice. On the other hand, a 10-fold-higher vaccine dose (108 CFU of iYp plus IL-12) resulted in not only up to 17% reduction in weight but also inactivity and development of a hunched posture with ruffled fur. After primary vaccination, the levels of pulmonary TNF-α were significantly higher in mice vaccinated with 107 CFU of iYp plus IL-12 than those seen for mice vaccinated with iYp alone on day 1 postvaccination (Fig. 1B). However, the levels of TNF-α among the vaccinated mice were not significantly different on days 3 and 5. Similarly, after booster vaccination on day 21, TNF-α levels did not change. Thus, inclusion of IL-12 during vaccination had minimal effect on TNF-α expression. In all cases, vaccination with iYp with or without IL-12 resulted in high TNF-α levels compared to what was seen for PBS-treated or naïve mice. Nevertheless, none of the vaccinated or control mice at any time displayed overt signs of distress, such as ruffled coat, malaise, or hunched posture. On day 3 postvaccination, lung histopathology revealed more perivascular and peribronchial cellular infiltration in the parenchyma of 107 CFU iYp-plus-IL-12–vaccinated mice compared to mice vaccinated with 107 CFU iYp alone (Fig. 1C). The intensity of cellular infiltration in the lung parenchyma subsequently decreased by day 7 postvaccination. PBS-inoculated mice did not develop any cellular infiltrations or inflammatory changes in their lungs. Vaccine doses of less than 107 CFU iYp did not affect the body weights of the mice. The results suggest that a vaccine dose of 107 iYp CFU or less, combined with IL-12 as an adjuvant, is a safe dose for use in mice i.n.
Fig. 1.
Delivery of 107 CFU iYp plus IL-12 i.n. does not cause adverse reactions in BALB/c mice. (A) Body weights of mice (8/group) after i.n. vaccination. *, P < 0.05 compared to the iYp group. (B) Pulmonary TNF-α levels measured in lung homogenates by ELISA (3 mice/group). *, P < 0.05 (iYp plus IL-12 compared to the iYp, PBS, and naïve groups); **, P < 0.01; ***, P < 0.001 (iYp plus IL-12 compared to PBS and naïve groups). ns, not significant. Arrows indicate the times of primary and secondary vaccinations. (C) H&E staining of lung sections showing perivascular and peribronchiolar cellular infiltrates (black arrows) after i.n. vaccination (3 mice/group). Bar = 200 μm. Error bars in graphs represent the means ± standard deviation (SD). These results are representative of a single experiment.
Protection against pulmonary Y. pestis infection by iYp-plus-IL-12 i.n. vaccination.
Several studies have reported that iYp is not fully effective against pneumonic plague when given parenterally (18, 29, 34, 38). Since it is known that i.n. vaccination generates immunity not only at the site of inoculation but also at other mucosal sites and systemically (23, 35, 41), it was of interest to determine if an i.n. prime-boost vaccination strategy using iYp-plus-IL-12 could generate efficacious immunity against lethal primary pneumonic plague. All vaccinated mice were challenged i.n. with Y. pestis CO92 approximately 8 weeks following the final vaccination and observed daily for survival thereafter. Control PBS-treated mice all succumbed to infection with a median survival time of 4 days (Fig. 2B). Four of six mice vaccinated with iYp alone survived, while all mice vaccinated with iYp plus IL-12 remained alive on day 21 postchallenge (P < 0.001 compared to PBS-treated mice). Thus, iYp given i.n. was able to generate protective immunity against Y. pestis CO92 pneumonic plague, although complete protection was observed only in the presence of IL-12 as a mucosal adjuvant. Mice treated with IL-12 alone showed no protection against lethal Y. pestis CO92 challenge (data not shown).
Fig. 2.
Efficacy of i.n. iYp-plus-IL-12 vaccination against pneumonic plague. (A) Experimental protocol. (B) Survival comparison of vaccinated and control BALB/c mice (6/group). **, P < 0.001, compared to PBS group. This survival curve is representative of three independent experiments. ns, not significant.
Vaccination with iYp plus IL-12 enhances expression of local and systemic anti-Y. pestis antibodies.
Levels of serum IgG anti-Y. pestis antibodies were measured weekly by ELISA. It was found that i.n. vaccination with iYp plus IL-12 increased serum antibody levels on days 7, 14, and 21, but these levels were not significantly greater than those of animals vaccinated with iYp alone (Fig. 3A). After booster vaccination on day 21, serum IgG antibody levels increased in mice vaccinated with iYp plus IL-12 and these levels were significantly greater (P < 0.001) than those for the iYp group. Expression of IgG antibody in BAL fluid was likewise increased by vaccination, although there were no significant differences between the iYp-plus-IL-12 and iYp groups (Fig. 3B). Nevertheless, levels of IgA in BAL fluid were statistically different between these two groups (P < 0.05) (Fig. 3C). The levels of IgA in serum were increased but similar in both vaccinated groups (Fig. 3C). IgG antibody levels were similar in the sera of mice following Y. pestis CO92 challenge (Fig. 3D).
Fig. 3.
Levels of Y. pestis CO92-specific antibodies in BALB/c mice. (A) Serum IgG titers were higher in iYp-plus-IL-12–vaccinated mice (4/group). ***, P < 0.001, compared to the iYp group. The arrows represent times of priming and boosting, respectively. (B) Serum and BAL IgG titers on day 28 postvaccination (3 mice/group). ***, P < 0.001, iYp plus IL-12 compared to the iYp and PBS groups. (C) IgA titers in serum and BAL on day 28 postvaccination (3 mice/group). **, P < 0.01, iYp-plus-IL-12 group compared to iYp group. (D) Serum IgG titers on day 21 postchallenge with 103 CFU of Y. pestis CO92 in vaccinated mice (3 mice/group). Hatched lines represent limits of antibody detection by ELISA. Error bars represent the means ± SD. The serum IgG determinations were repeated two times.
Vaccination with iYp plus IL-12 prevents colonization of Y. pestis and generates sterilizing immunity.
Since i.n. inoculation of iYp plus IL-12 resulted in 100% protection, it was of interest to determine if this treatment influenced systemic bacterial spread. Bacteria could not be detected in lungs, livers, or spleens of mice vaccinated with iYp plus IL-12 at any time point tested (Fig. 4A to C). After 24 h of i.n. challenge, lungs of PBS-treated mice had an average of 7 × 103 CFU (Fig. 4A), which increased to approximately 3.5 × 107 CFU by 48 h postchallenge and to nearly 109 by 72 h. In the liver, no bacteria were detected in PBS-treated mice at 24 h but 104 CFU were found at 48 h and 3 × 107 at 72 h, indicating systemic spread (Fig. 4B). Similarly, 3 of 4 mice had no detectable bacteria in their spleens 24 h after infection, but by 48 h, there was an average of 4.5 × 104 CFU and by 72 h, 7 × 107 CFU (Fig. 4C). On the other hand, at none of these time points were any detectable bacteria present in the lungs, livers, or spleens of iYp-plus-IL-12–vaccinated mice. At 21 days postchallenge, the vaccinated mice still remained free of bacteria (data not shown), indicating the generation of sterilizing immunity.
Fig. 4.
Vaccination i.n. with iYp plus IL-12 prevents colonization of Y. pestis in lungs, livers, and spleens of BALB/c mice (4/group) after challenge with 103 CFU of Y. pestis CO92. ‘×' at 72 h indicates death of an infected mouse. *, P < 0.05, iYp-plus-IL-12 group compared to the PBS group. Hatched horizontal lines indicate the lower limits of CFU detection. Error bars represent means ± SD. These results are representative of two independent experiments.
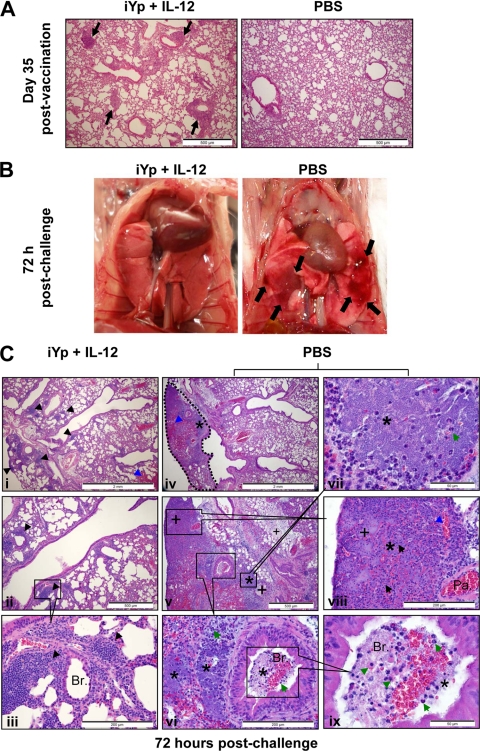
Vaccination with iYp plus IL-12 induces pulmonary cellular aggregations and reduced pulmonary pathology after Y. pestis challenge.
Tissue damage was assessed by pulmonary histopathology. On day 35 postvaccination, the lungs of iYp-plus-IL-12–vaccinated mice had developed peribronchial and perivascular cellular aggregates in the lung parenchyma (Fig. 5A), reminiscent of previously described inducible bronchus-associated lymphoid tissue (22). These cellular aggregates were absent from PBS-treated mice. At 72 h after Y. pestis CO92 challenge, the lungs of iYp-plus-IL-12–vaccinated mice were normal in gross appearance, while the lungs of PBS-treated control mice contained hemorrhagic areas with surrounding erythema (Fig. 5B). Microscopically at 72 h after challenge, the lungs of iYp-plus-IL-12–vaccinated mice had intact pulmonary architecture except for the presence of small hemorrhages and perivascular and peribronchiolar cellular aggregates in the lung parenchyma (Fig. 5C). These cellular aggregates appeared to consist predominantly of mononuclear and polymorphonuclear cells (Fig. 5Ci to Ciii). No bacteria were observed in the pulmonary parenchyma of iYp-plus-IL-12–vaccinated mice, and alveoli were clear of cellular infiltrates and exudates (Fig. 5Ciii). In contrast, the lungs of PBS-treated and challenged mice developed severe lesions (Fig. 5Civ). Within these lesions, there was a complete loss of normal pulmonary architecture due to extensive bacterial multiplication, massive hemorrhages, edema, neutrophilic infiltrations in parenchyma and bronchioles, and necrosis (Fig. 5Civ to Cix). In addition, the alveoli were damaged and filled with edematous fluid and neutrophilic infiltration (Fig. 5Cix). Dead red blood cells and “nuclear dust” from dead neutrophils were also evident in the pulmonary parenchyma, which showed evidence of necrosis (Fig. 5Cvi to Cix). Overall, it appeared that PBS-treated control mice developed overwhelming bacterial replication that resulted in severe bronchopneumonia and destruction of normal lung architecture. These features were absent from mice vaccinated with iYp plus IL-12.
Fig. 5.
Pulmonary cellular aggregates after i.n. vaccination with iYp plus IL-12 and decreased pulmonary pathology after i.n. challenge with 103 CFU of Y. pestis CO92 in BALB/c mice. (A) H&E-stained lung sections showing pulmonary cellular aggregates in mice 35 days after vaccination (black arrows). (B) Development of erythematous pulmonary lesions (black arrows) in PBS control mice but not vaccinated mice 72 h after challenge with i.n. Y. pestis CO92. (C) H&E-stained lung sections showing histopathological changes in vaccinated and control mice after challenge. Black arrowhead, cellular aggregates; blue arrowhead, hemorrhages; asterisk, Y. pestis; plus sign, necrosis; green arrow, neutrophils; green arrowhead, alveolar macrophages; and black arrow, dead neutrophils. Br., bronchiole; Pa., pulmonary artery. These results are representative of two independent experiments.
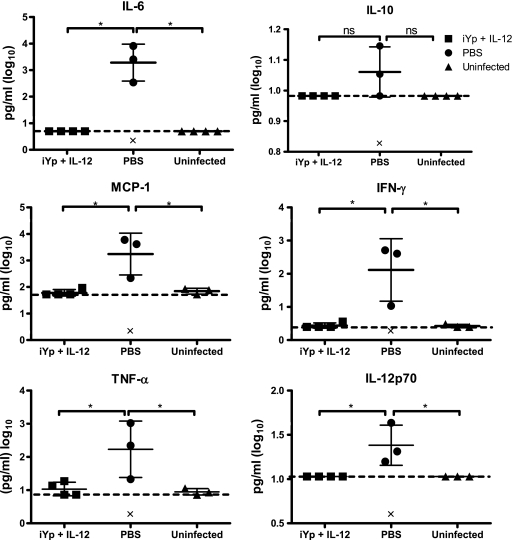
Reduced pulmonary pathology is correlated with reduced levels of pulmonary proinflammatory cytokines.
The levels of proinflammatory cytokines in the lungs of iYp-plus-IL-12 mice were also determined and found to be significantly lower at 72 h postinfection than those in the lungs of control mice (Fig. 6). While PBS-treated mice had high levels of lung IL-6, monocyte chemoattractant protein 1 (MCP-1), gamma interferon (IFN-γ), TNF-α, and IL-12p70, iYp-plus-IL-12–vaccinated mice were indistinguishable from uninfected, naïve mice. The levels of IL-10 were not statistically different in any group.
Fig. 6.
Reduction in the levels of pulmonary proinflammatory cytokines in iYp-plus-IL-12–vaccinated BALB/c mice (4/group) 72 h after i.n. challenge with 103 CFU of Y. pestis CO92. ‘×' indicates death of a mouse due to infection. *, P < 0.05; all compared to the PBS or uninfected control groups. Hatched lines represent the limits of cytokine detection by bead-based immunoassay. Error bars represent means ± SD. These results are representative of two independent experiments.
FcR-mediated humoral immunity for protection against pneumonic plague.
To determine if antibodies are sufficient in providing protection against pneumonic plague, IMS obtained from iYp-plus-IL-12–vaccinated mice or normal mouse serum (NMS) was passively transferred into naïve mice, and these recipients were then infected i.n. with Y. pestis CO92 followed by further passive transfer of serum. All mice receiving NMS failed to survive challenge, with a median survival time of only 4 days. However, 80% of mice receiving IMS survived the infection. Even a single dose of IMS at 18 h following infection resulted in 40% survival (Fig. 7A). Thus, serum antibodies are sufficient to provide protection against pneumonic plague. Passive transfer of IMS to FcRγ−/− mice (Jackson Laboratory) resulted in only 20% protection, while again, IMS protected 80% of FcRγ+/+ mice (Charles River Laboratories) (Fig. 7B). To determine the requirement of FcRs for active immunity, C57BL/6 FcRγ−/− and FcRγ+/+ mice were i.n. vaccinated and then challenged with Y. pestis CO92. Whereas all FcRγ+/+ mice survived, 60% of FcRγ−/− mice succumbed to infection (Fig. 7C). The prechallenge IgG titers in FcRγ+/+ and FcRγ−/− mice were not significantly different (data not shown). These results suggest that Fc receptors play a pivotal role of mediating antibody-based immunity against pneumonic plague.
Fig. 7.
FcRs are required for specific antibody-mediated passive and active immunity against pneumonic plague. (A) Adoptive transfer of NMS or Y. pestis-specific IMS into wild-type BALB/c mice (5 mice/group). *, P < 0.05, compared to the NMS group. These results are representative of two independent experiments. (B) Adoptive transfer of NMS or Y. pestis-specific IMS into FcRγ+/+ and FcRγ−/− mice (5 mice/group). *, P < 0.05, compared to the IMS transferred into the FcRγ−/− group. These results are representative of two independent experiments. (C) Vaccination and Y. pestis CO92 challenge 4 weeks later for FcRγ+/+ and FcRγ−/− mice (4 or 5 mice/group). *, P < 0.05, compared to the FcγR−/− group. All survival curves were evaluated by log rank (Mantel-Cox) tests.
Complement deficiency does not impair antibody-mediated protection.
To determine the role of complement in the effector phase of antibody-mediated immunity, IMS and NMS were transferred into C3−/− (Jackson Laboratory) and C3+/+ mice (Charles River Laboratories), which were then infected i.n. with Y. pestis CO92. While all C3+/+ and C3−/− mice receiving NMS died, 60% of C3−/− mice inoculated with IMS survived, which was not significantly different from the survival rate of C3+/+ mice (Fig. 8). Thus, C3 appears to have a minimal role in antibody-mediated protection against pneumonic plague.
Fig. 8.
Adoptive transfer of NMS or Y. pestis specific IMS to C3−/− mice (4 or 5 mice/group). Survival curves were evaluated by log rank (Mantel-Cox) test. These results represent a single experiment.
CD4 and CD8 T cells are not required during the effector phase of vaccine-induced protective immunity against Y. pestis.
We next investigated the potential roles of CD4 and CD8 T cells in mediating protection after i.n. vaccination with iYp-plus-IL-12–vaccinated mice. To assess the role of these cells in the effector phase of protection (rather than the inductive phase), mice were vaccinated and then depleted of T cells by using anti-CD4 and anti-CD8 MAbs (Fig. 9A). Depletion of CD4 and CD8 T cells was confirmed by flow cytometry analysis (see Fig. S1 in the supplemental material). After i.n. infection, all vaccinated mice treated with isotype control IgG survived the infection, while unvaccinated mice all succumbed by day 4 postchallenge. Of interest is that all of the vaccinated mice that were depleted of either CD4 or CD8 T cells, or depleted of both T-cell subsets, survived (Fig. 9B). Thus, Y. pestis-specific antibodies can fully protect mice against pneumonic plague in the absence of T cells.
Fig. 9.
CD4 and CD8 T cells are not required for protective immunity at the time of i.n. challenge with Y. pestis CO92. (A) Experimental protocol. (B) Survival of i.n. iYp-plus-IL-12–vaccinated mice that were depleted of CD4 and/or CD8 T cells after vaccination but before i.n. infection with 103 CFU of Y. pestis CO92 (5 mice/group). **, P < 0.01, compared to the PBS group. These results represent a single experiment.
DISCUSSION
In this study, the safety, immunogenicity and efficacy of an i.n. administered iYp-plus-IL-12 vaccine platform was evaluated in a mouse model. Furthermore, the mechanisms of vaccine-mediated protective immunity against pneumonic plague were also established. It was found that 107 CFU of formalin-killed Y. pestis CO92 administered i.n. was safe, yet effective in protecting mice against lethal pneumonic plague. The use of IL-12 as an adjuvant helped in increasing immunogenicity while lowering the bacterial mass required to generate protective immunity. Protection induced by iYp plus IL-12 was correlated with higher serum IgG and lung IgA titers, alleviation of lung pathology, and reduced levels of pulmonary proinflammatory cytokines. In addition, vaccination prevented bacterial colonization following challenge and provided sterilizing immunity. Passive transfer of serum antibodies conferred protection against lethal pneumonic plague, and this protection was partially dependent upon recipient FcR expression. On the other hand, depletion of CD4 and CD8 T cells in the vaccinated mice had minimal impact on protection. Together, these data indicate that iYp plus IL-12 is a safe vaccine that provides sterilizing immunity against pneumonic plague by an antibody-dependent mechanism.
Several studies have suggested that iYp vaccines do not protect against pneumonic plague (18, 33, 34, 37–39). In fact, owing to reports of the reactogenicity and inefficacy of whole killed-cell vaccines against pneumonic plague, the previously licensed plague vaccine USP containing iYp was discontinued in the United States. In contrast, other studies have provided evidence that iYp can protect against pneumonic plague (3, 9, 38, 39). These latter studies were performed using parenteral administration of iYp, and only one study reported protection by administering iYp in s.c. prime–i.n. boost fashion (3). In this case, protection was only partial. Interestingly, a DNA vaccine coexpressing IL-12 and Y. pestis antigens administered by the i.m. route was recently shown to enhance protective immunity against pneumonic plague (42).
Vaccine safety experiments revealed that a dose of 107 CFU of iYp plus IL-12 was safe as it generated only a mild, transient weight loss with no detectable systemic effects like ruffled coat, malaise, weight loss, and behavioral changes or death, yet provided 100% protection against pneumonic plague. Furthermore, this dose of iYp caused only transient elevation of TNF-α, the primary mediator of fever, anorexia, and weight loss in mice (5, 36). Previous studies utilizing s.c. iYp vaccination approaches for plague likewise resulted in mild adverse reactions such as fever and headache, although in some cases, there were severe reactions, including generalized urticaria, wheezing, and hypotension (15, 21). The studies by Meyer et al. (19, 21) found that reactogenicity was correlated with the dose of vaccine used. Additionally, a large study over a period of 21 years found that adverse reactions in humans due to iYp were related to the amount of bacterial mass in the vaccine dose and reducing the booster vaccine dose 2 to 4 times resulted in a 70% reduction in local reactions and a 65% reduction in systemic reactions (15). The absence of adverse reactions in our study might be due to the bacterial mass of iYp that was used, which was approximately 20-fold lower than that used by Russell et al. (29), together with the i.n. route of vaccination and the lack of preservatives.
Vaccination by the i.n. route is an attractive strategy for protection against respiratory pathogens as it is an easy, rapidly deployable, effective, and needle-free method of mass-scale antigen delivery (25). A variety of bacteria and viruses, as well as their subunits, have been successfully used to i.n. vaccinate mice, large animals, and humans (8). The i.n. route of vaccination has also been found to be superior to other vaccination routes for inducing immune responses against bacterial polysaccharides (30). However, the pulmonary innate immune system is typically quiescent under homeostatic conditions (40). In order to overcome this immune quiescence, adjuvants are therefore typically required, particularly for nonviable vaccines. In our study, IL-12 was used as an adjuvant since it has been reported to elicit robust cellular and humoral immune responses against a variety of antigens (16, 17). While it was found that administering 107 CFU of iYp alone provided only partial immunity, the addition of IL-12 resulted in 100% protection against pneumonic plague. These results are consistent with previous reports that demonstrated the effectiveness of IL-12 for vaccination against respiratory pathogens such as influenza virus (2), Streptococcus pneumoniae (1), and Francisella tularensis (4). It should be noted that IL-12 is safe when administered by the i.n. route (11). The use of IL-12 also likely allowed a lower amount of iYp to be used to induce protection and hence resulted in decreased reactogenicity. In fact, 107 CFU of formalin-killed Y. pestis CO92 coadministered i.n. with IL-12 also protects C57BL/6 mice (data not shown). Finally, we noted that IL-12 treatment caused greater recruitment of immune cells to the lungs. Studies to establish the nature and the role of these cells in providing protection are under way.
Passive transfer of decomplemented IMS from iYp-plus-IL-12–vaccinated mice fully protected naïve mice against pneumonic plague. However, IMS-mediated protection was partially lost in FcRγ−/− mice, suggesting that antibodies mediate protection via FcR-dependent opsonophagocytosis. Consistent with these results, only partial protection was observed in iYp-plus-IL-12–vaccinated FcRγ−/− mice. Early work of Yersin, Calmette, and Borrel (described in reference 20) established that sera from rabbits vaccinated with inactivated cultures of Y. pestis could protect naïve animals against plague, although the mechanism by which immune sera mediated protection against pneumonic plague was not known (31). In a more recent study, it was also found that i.n. administration of anti-F1 and anti-LcrV antibodies could protect mice from pneumonic plague (10).
Perhaps surprisingly, depletion of CD4 and/or CD8 T cells before challenge did not affect the survival of iYp-plus-IL-12–vaccinated mice. Since passive transfer of IMS was sufficient to protect mice against pneumonic plague, it is conceivable that iYp plus IL-12 induced an antibody response that was qualitatively and quantitatively superior to those seen for other vaccination strategies. Indeed, vaccination with iYp plus IL-12 generated a robust humoral immune response against a diverse range of Y. pestis antigens, i.e., at least 31 different polypeptides that by Western blot analysis ranged in size from 6 to 185 kDa (unpublished observations). In contrast, subunit vaccines, such as F1- and LcrV-based vaccines, generate immune responses that are directed against a highly limited number of B- and T-cell epitopes. It should be noted that we tested the requirement for T cells during the effector phase, subsequent to the induction of specific antibodies. To confirm the contribution of T cells in protection, we also transferred immune serum to Rag1−/− mice before i.n. challenge with Y. pestis CO92 (see Fig. S2 in the supplemental material). However, it was found that IMS alone could not protect Rag1−/− mice, a result that was surprising considering that depletion of T cells from vaccinated mice did not result in the loss of protection. One possible explanation is that absolute depletion of T cells, which is very difficult to achieve by antibody treatment, may be required to ablate protective immunity. Although we measured percentages of T cells in the spleens and lungs after depletion, it is difficult to deplete the entire mouse, including the bone marrow, where memory T cells reside. Therefore, the survival of T-cell-depleted vaccinated mice may have been due to the incomplete depletion of effector and memory T cells that aided antibody-mediated protection. Indeed, it has been previously shown that TNF-α and IFN-γ contribute to protection against pneumonic plague (14). It is possible that while WT mice may have sufficiently activated phagocytic cells that aid in killing antibody-coated Y. pestis, Rag1−/− mice may lack sufficient expression of essential soluble and cellular factors required for this process. Thus, it is likely that stimulation of both humoral and cellular immune responses will ultimately be required for the generation of optimal protection.
During the inductive phase, as Jones et al. (13) have shown, CD4 T cells are absolutely required for the generation of vaccine-induced protective immunity against pneumonic plague, whereas CD8 T cells play only a minor role in inducing protection. Further, Chattopadhyay et al. (6) reported the importance of CD4 cells, but not CD8 T cells, in mice vaccinated i.m. with vesicular stomatitis virus (VSV)-vectored vaccine expressing Y. pestis LcrV antigen. Nevertheless, in their studies approximately 70% of mice still survived infection in the absence of CD4 cells, which further suggests the predominant protective role of humoral immunity. Multiple reports have shown the protective role of CD4 and CD8 T cells with attenuated live vaccines (24, 28, 32), at the time of challenge or after adoptive transfer of primed T cells. However, all of these studies were conducted in B-cell-deficient μMT mice, which omitted the contribution of B cells in the development of vaccine-primed immunity. Based on the results of previous studies (24, 28, 32) and our work, it is likely that there exist redundant humoral and cellular immune mechanisms that can prevent pneumonic plague.
In summary, the present work has demonstrated that iYp combined with the mucosal adjuvant IL-12 confers protection against pneumonic plague. In addition to increasing immunogenicity, the inclusion of IL-12 likely reduced the amount of bacterial mass required to generate protective immunity and consequently increased safety. Our results indicate that other iYp-based vaccines might be improved by (i) optimizing the vaccine dose for safety and efficacy, (ii) using an optimal route for vaccination such as the i.n. route, and (iii) using a potent mucosal adjuvant such as IL-12. The strategy of using iYp plus IL-12 administered in an i.n. prime-boost fashion warrants further study in nonhuman primates such as cynomolgus macaques and African green monkeys, animals that have been difficult in other studies to protect with iYp vaccines.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge financial support from the U.S. Department of Defense—Office of Naval Research (ONR award no. N00014-06-1-1176) and NIH grant RO1 AI41715. Disclosure: We have no financial conflicts of interest.
We also thank Michelle Wyland, Sharon Salmon, and Shannan Zilles from the Center for Immunology and Microbial Disease, who helped in maintaining the mouse colonies and performing ABSL-3 challenge studies, and Yili Lin of the CIMD Immunology Core. Yersinia pestis strain CO92 was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH.
Footnotes
Supplemental material for this article may be found at http://cvi.asm.org/.
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Arulanandam B. P., Lynch J. M., Briles D. E., Hollingshead S., Metzger D. W. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718–6724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arulanandam B. P., O'Toole M., Metzger D. W. 1999. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. . J. Infect. Dis. 180:940–949 [DOI] [PubMed] [Google Scholar]
- 3. Baca-Estrada M., et al. 2000. Intranasal immunization with liposome-formulated Yersinia pestis vaccine enhances mucosal immune responses. Vaccine 18:2203–2211 [DOI] [PubMed] [Google Scholar]
- 4. Baron S. D., Singh R., Metzger D. W. 2007. Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect. Immun. 75:2152–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cerami A., Ikeda Y., Le Trang N., Hotez P. J., Beutler B. 1985. Weight loss associated with an endotoxin-induced mediator from peritoneal macrophages: the role of cachectin (tumor necrosis factor). Immunol. Lett. 11:173–177 [DOI] [PubMed] [Google Scholar]
- 6. Chattopadhyay A., et al. 2008. Single-dose, virus-vectored vaccine protection against Yersinia pestis challenge: CD4+ cells are required at the time of challenge for optimal protection. Vaccine 26:6329–6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darling R., Woods J. (ed.) 2004. USAMRIID's medical management of biological casualties handbook, 4th ed. U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD. [Google Scholar]
- 8. Davis S. S. 2001. Nasal vaccines. Adv. Drug Deliv. Rev. 51:21–42 [DOI] [PubMed] [Google Scholar]
- 9. Ehrenkranz N. J., Meyer K. F. 1955. Studies on immunization against plague. VIII. Study of three immunizing preparations in protecting primates against pneumonic plague. . J. Infect. Dis. 96:138–144 [DOI] [PubMed] [Google Scholar]
- 10. Hill J., et al. 2006. Administration of antibody to the lung protects mice against pneumonic plague. Infect. Immun. 74:3068–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huber V., et al. 2003. Delivery of IL-12 intranasally leads to reduced IL-12-mediated toxicity. Int. Immunopharmacol. 3:801–809 [DOI] [PubMed] [Google Scholar]
- 12. Inglesby T. V., et al. 2000. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA 283:2281–2290 [DOI] [PubMed] [Google Scholar]
- 13. Jones A., et al. 2010. Protection against pneumonic plague following oral immunization with a non-replicating vaccine. Vaccine 28:5924–5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin J. S., et al. 2010. TNFalpha and IFNgamma contribute to F1/LcrV-targeted immune defense in mouse models of fully virulent pneumonic plague. Vaccine 29:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marshall J. D., Jr., Bartelloni P. J., Cavanaugh D. C., Kadull P. J., Meyer K. F. 1974. Plague immunization. II. Relation of adverse clinical reactions to multiple immunizations with killed vaccine. J. Infect. Dis. 129(Suppl.):S19–S25 [DOI] [PubMed] [Google Scholar]
- 16. Metzger D. 2009. IL-12 as an adjuvant for the enhancement of protective humoral immunity. Expert Rev. Vaccines 8:515–518 [DOI] [PubMed] [Google Scholar]
- 17. Metzger D. 2010. Interleukin-12 as an adjuvant for induction of protective antibody responses. Cytokine 52:102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyer K. 1970. Effectiveness of live or killed plague vaccines in man. Bull. World Health Organ. 42:653–666 [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer K., Cavanaugh D., Bartelloni P., Marshall J. J. 1974. Plague immunization. I. Past and present trends. . J. Infect. Dis. 129(Suppl.):S13–S18 [DOI] [PubMed] [Google Scholar]
- 20. Meyer K. F. 1947. The prevention of plague in the light of newer knowledge. Ann. N. Y. Acad. Sci. 48:429–467 [Google Scholar]
- 21. Meyer K. F., Smith G., Foster L. E., Marshall J. D., Cavanaugh D. C. 1974. Plague immunization. IV. Clinical reactions and serologic response to inoculations of Haffkine and freeze-dried plague vaccine. . J. Infect. Dis. 129(Suppl.):S30–S36 [DOI] [PubMed] [Google Scholar]
- 22. Moyron-Quiroz J. E., et al. 2004. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10:927–934 [DOI] [PubMed] [Google Scholar]
- 23. Ogra P. L., Faden H., Welliver R. C. 2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14:430–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parent M. A., et al. 2005. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect. Immun. 73:7304–7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Partidos C. D. 2000. Intranasal vaccines: forthcoming challenges. Pharm. Sci. Technol. Today 3:273–281 [DOI] [PubMed] [Google Scholar]
- 26. Partidos C. D., Beignon A. S., Semetey V., Briand J. P., Muller S. 2001. The bare skin and the nose as non-invasive routes for administering peptide vaccines. Vaccine 19:2708–2715 [DOI] [PubMed] [Google Scholar]
- 27. Perry R., Fetherston J. 1997. Yersinia pestis-etiologic agent of plague. Clin. Microbiol. Rev. 10:35–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Philipovskiy A. V., Smiley S. T. 2007. Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect. Immun. 75:878–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Russell P., et al. 1995. A comparison of plague vaccine, USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine 13:1551–1556 [DOI] [PubMed] [Google Scholar]
- 30. Shen X., et al. 2001. Effect of pre-existing immunity for systemic and mucosal immune responses to intranasal immunization with group B Streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugate. Vaccine 19:3360–3368 [DOI] [PubMed] [Google Scholar]
- 31. Smiley S. T. 2008. Immune defense against pneumonic plague. Immunol. Rev. 225:256–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Szaba F. M., et al. 2009. D27-pLpxL, an avirulent strain of Yersinia pestis, primes T cells that protect against pneumonic plague. Infect. Immun. 77:4295–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Titball R. W., Williamson E. D. 2001. Vaccination against bubonic and pneumonic plague. Vaccine 19:4175–4184 [DOI] [PubMed] [Google Scholar]
- 34. Titball R. W., Williamson E. D. 2004. Yersinia pestis (plague) vaccines. Expert Opin. Biol. Ther. 4:965–973 [DOI] [PubMed] [Google Scholar]
- 35. Torrieri-Dramard L., et al. 2011. Intranasal DNA vaccination induces potent mucosal and systemic immune responses and cross-protective immunity against influenza viruses. Mol. Ther. 19:602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tracey K. J., et al. 1988. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J. Exp. Med. 167:1211–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williamson E. D. 2009. Plague. Vaccine 27(Suppl. 4):D56–D60 [DOI] [PubMed] [Google Scholar]
- 38. Williamson E. D., et al. 1995. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol. Med. Microbiol. 12:223–230 [DOI] [PubMed] [Google Scholar]
- 39. Williamson E. D., et al. 1997. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against pneumonic plague. Vaccine 15:1079–1084 [DOI] [PubMed] [Google Scholar]
- 40. Wissinger E., Goulding J., Hussell T. 2009. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin. Immunol. 21:147–155 [DOI] [PubMed] [Google Scholar]
- 41. Wu H.-Y., Nahm M. H., Guo Y., Russell M. W., Briles D. E. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. . J. Infect. Dis. 175:839–846 [DOI] [PubMed] [Google Scholar]
- 42. Yamanaka H., et al. 2009. An IL-12 DNA vaccine co-expressing Yersinia pestis antigens protects against pneumonic plague. Vaccine 27:80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamanaka H., et al. 2008. A nasal interleukin-12 DNA vaccine coexpressing Yersinia pestis F1-V fusion protein confers protection against pneumonic plague. Infect. Immun. 76:4564–4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.