Abstract
Type 1 cytokine responses are known to play an important role in immunity to tuberculosis (TB) in children, although little is known about other factors that might be important. In addition, children are more prone to developing extrapulmonary manifestations of TB than adults. To identify the immune responses important both in control of infection and in extrapulmonary dissemination, we examined mycobacterium-specific cytokine responses of children with pulmonary TB (PTB) and extrapulmonary TB (ETB) and compared them with those of healthy control children (HC). No significant differences were found in the cytokine responses either with no stimulation or following mycobacterial-antigen (Ag) stimulation between children with PTB and ETB. On the other hand, children with active TB compared with HC showed markedly diminished production of type 1 (gamma interferon [IFN-γ] and tumor necrosis factor alpha [TNF-α]), 2 (interleukin 4 [IL-4] and IL-13), and 17 (IL-17A, IL-21, and IL-23)-associated cytokines with no stimulation and in response to mycobacterial antigens. This was not associated with significantly altered production of IL-10 or transforming growth factor β (TGF-β). Among children with ETB, those with neurologic involvement exhibited more significantly diminished Ag-driven IFN-γ and IL-17 production. Pediatric TB is characterized by diminished type 1, 2, and 17 cytokine responses, with the most profound diminution favoring development of neurologic TB, suggesting a crucial role for these cytokines in protection against pediatric tuberculosis.
INTRODUCTION
One million children are estimated to develop tuberculosis (TB) globally every year, and TB is among the top 10 causes of death among children worldwide (28, 38). Children belong to the category of relatively susceptible individuals, and young children are highly likely to develop active disease after infection (25, 28, 38). Indeed, the risk of developing active TB following exposure ranges from 20 to 40% in children below 5 years old (42). In addition, a unique aspect of TB in children is the rapid progression from infection with Mycobacterium tuberculosis to disease. The mechanism by which children become more susceptible to developing active pulmonary disease, as well as extrapulmonary dissemination following exposure, is not fully understood. The risk for developing disease after infection is determined by various factors, including age at exposure, nutritional and immune status, genetic factors, virulence of the organism, and magnitude of the initial infection (38). Young age (28, 42) and HIV coinfection (8, 22) are the most important risk factors for severe or disseminated disease, and lymphadenopathy (TB lymphadenitis) and central nervous system [CNS] involvement (neuro-TB) are the most common extrapulmonary manifestations of TB infection in children (38). The relative immaturity of the immune system in terms of both antigen (Ag)-presenting cell function and T cell function also contributes to the development of disease. The only licensed vaccine against TB, Mycobacterium bovis Bacille Calmette-Guérin (BCG), affords variable and mostly poor protection against pulmonary TB in children while having a more significant protective effect against the severe forms of childhood TB, such as miliary TB and TB meningitis (40). Moreover, BCG has very little protective effect in HIV-infected children; rather, BCG may cause disease in this population (15, 16).
Based on murine models, immunity to M. tuberculosis requires Th1 and (to a lesser extent) Th17 responses (4, 6). Thus, interleukin 12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) (along with IL-17 and IL-23) all play important roles in induction and maintenance of protective immune responses against tuberculous disease (5, 7, 20). Although CD4+ T lymphocytes of the Th1 type are critical for protective immunity (30), evidence exists that CD8+ T cells, as well as unconventional T cells (γδ T cells and CD-1-restricted T cells), contribute to optimum protection in susceptible-animal models (11, 18, 21). IL-12, IFN-γ, and TNF-α are also known to play pivotal roles in immunity to infections in humans. In addition, in the guinea pig model of TB infection, which is a model for susceptibility to infection and development of granulomas, it has been shown that CD4+ T cells and the production of cytokines, TNF-α, IL-12, IFN-γ, and IL-2, all play an important role in the response to infection (43). Children with deficiencies in the IL-12 or IFN-γ pathways are known to be susceptible to disseminated disease (17, 29, 32). Further, polyfunctional T cells have been associated with resistance to infection in animal models, although human data are still scarce (9, 12, 27). In contrast, a number of regulatory factors, including regulatory T cells (Tregs), IL-10, transforming growth factor β (TGF-β), cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4), and programmed death 1 (PD-1) have been implicated in establishment of chronic M. tuberculosis infection by downmodulating protective immune responses (2, 14, 19, 33, 39).
To study the role of type 1, type 2, type 17, and immunoregulatory cytokines implicated in susceptibility to TB infection and to extrapulmonary dissemination, we examined Ag-specific induction of these cytokines in children with pulmonary TB (PTB), children with extrapulmonary TB (ETB), and healthy control (HC) children. We showed that children with active TB fail to mount effective type 1, type 2, and type 17 cytokine responses and that the neurologic forms of ETB in children are characterized by even more profound suppression of IFN-γ and IL-17 production than is seen with other forms of ETB.
MATERIALS AND METHODS
Study population.
We studied a group of 30 Indian children with TB—13 with PTB and 17 with ETB—as well as 18 HC children in South India, where the childhood TB case rate is currently 6% of all newly reported cases (35). TB diagnosis was made on the basis of sputum microscopy and culture, and TB-infected children were studied before the commencement of treatment. Quantiferon TB-Gold enzyme-linked immunosorbent assay (ELISA) was performed to determine latent infection (26). All the children were HIV negative. The demographics of the children in the study are shown in Table 1. Children with ETB (n = 17) comprised those with TB meningitis and spinal TB (n = 9), abdominal TB (including peritonitis or tuberculomas [n = 2]), or TB lymphadenitis (n = 6). Among the 30 subjects, only 11 of the PTB, 3 of the ETB, and 5 of the HC children had received BCG vaccination. All the HC children were negative by Quantiferon ELISA. All individuals were examined as part of a clinical protocol approved by the Institutional Review Board of the Tuberculosis Research Center, and informed written consent was obtained from the parents of all participants.
Table 1.
Demographics of the study population
| Characteristic | Value for group | ||
|---|---|---|---|
| Pulmonary TB | Extrapulmonary TB | Control | |
| No. of patients | 13 | 17 | 18 |
| Median age (range) | 8 (1–15) | 6 (2–15) | 7 (3–12) |
| Gender (M/Fa) | 6/7 | 7/10 | 13/5 |
| BCG status (+/−) | 11/2 | 5/12 | 5/13 |
| Quantiferon assay (+/−) | 6/7 | 6/11 | Negative |
M, male; F, female.
Isolation of PBMCs.
Heparinized blood was collected, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll diatrizoate gradient centrifugation (LSM; ICN Biomedicals, Aurora, OH). Erythrocytes were lysed using ACK lysis buffer (8,024 mg/liter NH4Cl, 1,001 mg/liter KHCo2, and 3,722 mg/liter EDTA-Na2-2H2o; Biosource International, Camarillo, CA). Cells were then washed and cultured in RPMI-1640 (BioWhittaker, Walkersville, MD) supplemented with 20 mM glutamine (BioWhittaker), 10% heat-inactivated fetal calf serum (FCS) (Harlan Bioproducts for Science, Madison, WI), and 50 μg/ml of gentamicin (Mediatech, Herndon, VA). PBMCs from different groups of children did not differ significantly in baseline hematological and immunological parameters, including total, differential white blood cell counts and CD4+ and CD8+ T cell counts.
Ags.
Mycobacterial purified protein derivative of tuberculin (PPD) (Statens Serum Institute, Copenhagen, Denmark) and culture filtrate Ag from M. tuberculosis H37 Rv (here referred to as CFP; kind gift of P. Selvaraj, Tuberculosis Research Center, India) were used as the antigenic stimuli, and anti-CD3 antibody (Ab) was used as the positive control. Final concentrations were 10 μg/ml for PPD and CFP and 5 μg/ml for anti-CD3.
In vitro culture.
PBMCs were cultured with PPD, M. tuberculosis CFP, or anti-CD3 in 24-well tissue culture plates (Corning, Corning, NY) at concentrations of 5 × 106/well. After 24 h, culture supernatants were collected and analyzed for cytokines. PBMCs were also cultured for 48 and 72 h, but optimal cytokine production was detected at 24 h. The percent cell viability at the end of 24 h was between 85 and 90% for the all the conditions and did not differ significantly between the groups.
ELISA.
The levels of cytokines in the culture supernatants were measured using the Bioplex multiplex cytokine assay system (Bio-Rad, Hercules, CA). Net cytokine levels indicate cytokine values following subtraction of baseline values. The cytokines analyzed were IL-2, IFN-γ, TNF-α, IL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, and IL-1β. TGF-β, IL-21, and IL-23 were measured by ELISA using the kit from R&D Systems (Minneapolis, MN).
Statistical analysis.
Comparisons were done using the nonparametric Mann-Whitney test, and P values were determined using the Holm correction method for multiple comparisons. All statistics were performed using the GraphPad Prism software program, version 5 for Windows (GraphPad Software, Inc., San Diego, CA).
RESULTS
Children with TB have diminished spontaneous type 1, type 2, and type 17 cytokine responses in vitro.
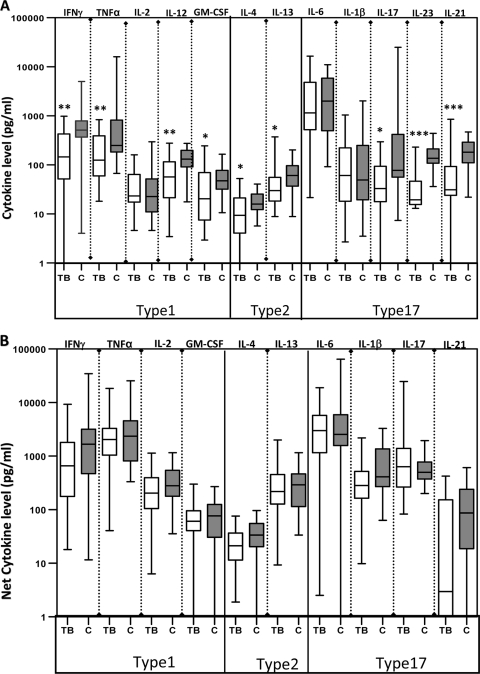
To determine the impact of pediatric TB on cytokine responses, we assessed their production by PBMCs from TB-infected or HC children with no stimulation or following stimulation with anti-CD3. First, when those with PTB and those with ETB were considered separately, there were no intrinsic differences in the levels of type 1 (IFN-γ, TNF-α, IL-2, IL-12, or GM-CSF), type 2 (IL-4 or IL-13), and type 17 (IL-17, IL-21, IL-23, IL-6, or IL-1β) cytokines produced spontaneously or in response to anti-CD3 (data not shown). Compared with HC children, however, TB-infected children exhibited significantly diminished production of type 1 (IFN-γ, TNF-α, and GM-CSF), type 2 (IL-4 and IL-13), and type 17 (IL-17, IL-21, and IL-23) cytokines spontaneously (Fig. 1A). No significant differences were observed following anti-CD3 stimulation for any of the above cytokines (Fig. 1B), suggesting that the potential to express these cytokines is not affected in TB-infected children. Group data (geometric means for the individual cytokines examined and the P values for the statistical differences between TB-infected and HC children) for cytokine values either without stimulation or following anti-CD3-stimulation are shown in Table S1 in the supplemental material.
Fig. 1.
Children with TB have diminished spontaneous type 1, type 2, and type 17 cytokine responses in vitro. PBMC from TB (n = 30) and HC (C) (n = 18) children were stimulated with medium alone (A) or anti-CD3 (5 μg/ml) (B) for 24 h. Levels of type 1-associated cytokines IL-2, IFN-γ, TNF-α, IL-12, and GM-CSF, type 2 cytokines IL-4 and IL-13, and type 17-associated cytokines IL-17, IL-23, IL-6, IL-1β, and IL-21 were measured by ELISA. Results are shown as raw values for nonstimulated and net cytokine production (with medium control values subtracted) for anti-CD3 using box-and-whisker plots, with whiskers showing minimum and maximum values. P values were calculated using the Mann-Whitney test (“*” denotes P < 0.05; “**” denotes P < 0.005; “***” denotes P < 0.001).
Children with TB have diminished type 1, type 2, and type 17 cytokine responses following stimulation with mycobacterial antigens.
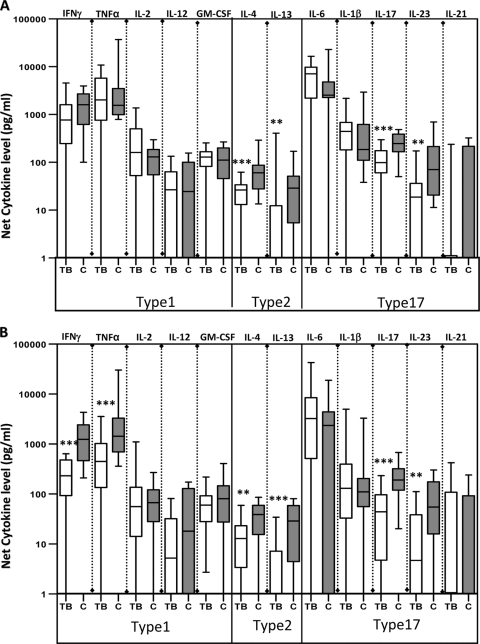
To determine the impact of pediatric TB on antigen-specific cytokine responses, we assessed their production by PBMCs from TB-infected or HC children following stimulation with two different mycobacterial Ags, PPD and CFP. First, when those with PTB and those with ETB were considered separately, there were no intrinsic differences in the levels of type 1, type 2, and type 17 cytokines following PPD or CFP stimulation (data not shown). However, upon stimulation with PPD (Fig. 2A) or CFP (Fig. 2B), those with active TB showed a marked inhibition of mycobacterium-specific type 1 (IFN-γ and TNF-α), type 2 (IL-4 and IL-13), and type 17 (IL-17 and IL-23) cytokines, indicating that antigen-specific cytokine responses are compromised in TB-infected children. Group data (geometric means for the individual cytokines examined and the P values for the statistical differences between TB-infected and HC children) for cytokine values following stimulation with PPD and CFP are shown in Table S1 in the supplemental material.
Fig. 2.
Children with TB have diminished mycobacterial antigen-specific type 1, type 2, and type 17 cytokine responses. PBMC from TB (n = 30) and HC (n = 18) children were stimulated with PPD (10 μg/ml) (A) or CFP (10 μg/ml) (B) for 24 h. Levels of type 1-associated cytokines IL-2, IFN-γ, TNF-α, IL-12, and GM-CSF, type 2 cytokines IL-4 and IL-13, and type 17-associated cytokines IL-17, IL-23, IL-6, IL-1β, and IL-21 were measured by ELISA. Results are shown as net cytokine production (with medium control values subtracted) using box-and-whisker plots with whiskers showing minimum and maximum values. P values were calculated using the Mann-Whitney test (“*” denotes P < 0.05; “**” denotes P < 0.005; “***” denotes P < 0.001).
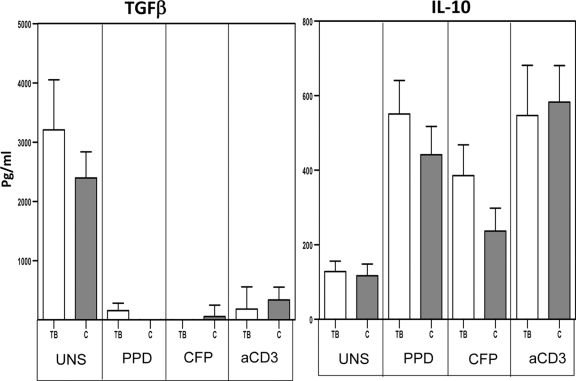
Children with TB infection do not have altered production of TGF-β and IL-10.
Because TGF-β and IL-10 are associated with downregulation of immune responses in adult TB (2, 39), we postulated that either or both of these cytokines could be involved in the diminished cytokine responses observed in TB-infected children. Therefore, we examined production of TGF-β and IL-10 in TB-infected and HC children. As shown in Fig. 3, we observed no significant differences in the levels of both cytokines produced spontaneously in vitro or following mycobacterial Ag or anti-CD3 stimulation.
Fig. 3.
Children with TB infection do not have altered production of TGF-β and IL-10. PBMCs from TB (n = 30) and HC (n = 18) children were stimulated with PPD (10 μg/ml), M. tuberculosis CFP (10 μg/ml), or anti-CD3 (5 μg/ml) for 24 h, and levels of the immunoregulatory cytokines TGF-β and IL-10 were measured by ELISA. Results are shown as raw values for nonstimulated and net cytokine production (with medium control values subtracted) for PPD, CFP, and anti-CD3 using bar graphs showing geometric means and 95% confidence intervals. P values were calculated using the Mann-Whitney test.
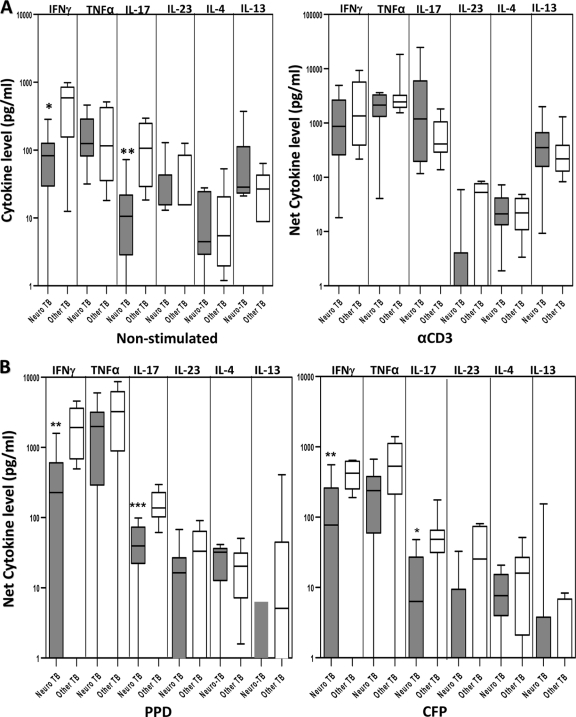
Children with neuro-TB infection have significantly diminished spontaneous and Ag-induced IFN-γ and IL-17 production.
Since neuro-TB was the most common manifestation (and often the most serious) of extrapulmonary TB in our study, we wanted to determine the Th1, Th2, and Th17 responses in children with neuro-TB compared with those in children with other forms of ETB. As shown in Fig. 4A, we observed significantly lower levels of IFN-γ (geometric mean, 39.8 pg/ml in neuro-TB versus 310.2 pg/ml in other forms of ETB; P = 0.0111) and IL-17 (geometric mean, 8.2 pg/ml in neuro-TB versus 82.5 pg/ml in other forms of ETB; P = 0.0016) production with no stimulation but not after anti-CD3 stimulation. In addition, IFN-γ (geometric mean, −152.5 pg/ml versus 1,875 pg/ml [P = 0.0016] for PPD; geometric mean, −139.5 pg/ml versus 426 pg/ml [P = 0.0079] for CFP) and IL-17 (geometric mean, 33.2 pg/ml versus 145.6 pg/ml [P = 0.0006] for PPD; geometric mean, −130.3 pg/ml versus 42.5 pg/ml [P = 0.0152] for CFP) levels following mycobacterial Ag stimulation were also significantly lower in children with neurologic involvement than in those with other forms of ETB (Fig. 4B). Examination of the other cytokines revealed no significant differences either with no stimulation or following Ag or anti-CD3 stimulation. Thus, both IFN-γ and IL-17 production are significantly compromised in children with neurologic forms of TB, although the potential to produce both cytokines is still intact as demonstrated by the anti-CD3-stimulated response.
Fig. 4.
Children with neurologic TB infection have significantly diminished nonstimulated and Ag-induced IFN-γ and IL-17 production. PBMCs from children with neurologic TB (n = 9) and other forms of ETB (n = 8) were stimulated with medium alone or anti-CD3 (5 μg/ml) (A) or PPD (10 μg/ml) or M. tuberculosis CFP (10 μg/ml) (B) for 24 h, and levels of type 1 cytokines IFN-γ and TNF-α, type 2 cytokines IL-4 and IL-13, and type 17 cytokines IL-17 and IL-23 were measured by ELISA. Results are shown as raw values for nonstimulated and net cytokine production (with medium control values subtracted) for PPD, CFP, and anti-CD3 using box-and-whisker plots, with whiskers showing minimum and maximum values. P values were calculated using the Mann-Whitney test (“*” denotes P < 0.05; “**” denotes P < 0.005; “***” denotes P < 0.001).
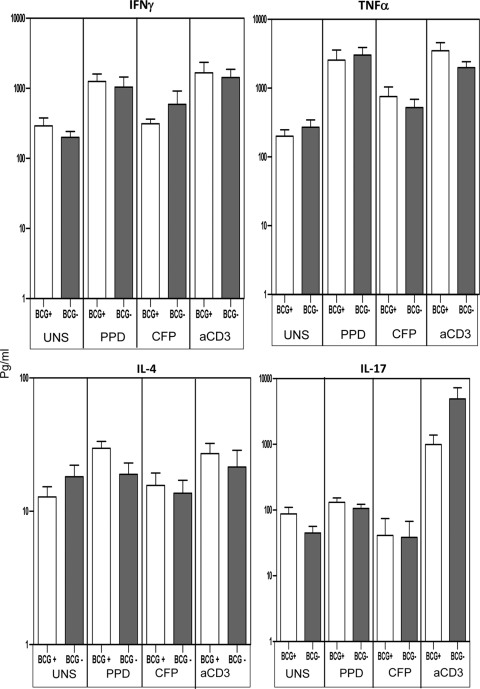
BCG vaccination status does not impact production of type 1, type 2, or type 17 cytokines.
Because BCG vaccination could potentially be a confounding factor in analyzing type 1, type 2, or type 17 cytokine responses in TB-infected children, we analyzed production of IFN-γ, TNF-α, IL-4, and IL-17 in BCG-vaccinated and nonvaccinated TB-infected children. As shown in Fig. 5, we observed no significant differences in production of IFN-γ, TNF-α, IL-4, and IL-17 between BCG-vaccinated and nonvaccinated children with no stimulation or following mycobacterial Ag or anti-CD3 stimulation. Thus, the defective type 1, type 2, or type 17 cytokine responses seen in TB-infected children cannot be attributed to their BCG vaccination status. In addition, BCG vaccination status also did not affect the cytokine responses in HC (data not shown), indicating that cytokine responses observed in HC children are probably due to priming by environmental mycobacteria.
Fig. 5.
Bacillus Calmette-Guérin vaccination status does not impact the production of type 1, type 2 or type 17 cytokines. PBMC production of IFN-γ, TNF-α, IL-4, and IL-17 following 24-h stimulation with PPD (10 μg/ml) or M. tuberculosis CFP (10 μg/ml) in Bacillus Calmette-Guérin-vaccinated (n = 14) (BCG+) or nonvaccinated (n = 16) (BCG-) TB-infected children is analyzed. Results are shown as raw values for nonstimulated and net cytokine production (with medium control values subtracted) for PPD, CFP, and anti-CD3 using bar graphs showing geometric means and 95% confidence intervals. P values were calculated using the Mann-Whitney test.
DISCUSSION
TB infection in children is a devastating problem globally, and children have a higher risk of disseminated TB disease and death (28, 38). Successful vaccination requires persistent immunologic memory that confers protection against infection or disease, and CD4+ T cells are critical, since depletion of CD4+ T cells, as seen in HIV infection, leads to increased susceptibility to TB (4). CD8+ T cells are felt to be equally important for protection (4). T cell differentiation into Th1 and Th2 lineages based on their cytokine profile and transcription factor expression has served as the basis for our understanding of the pathogenesis of a variety of infectious and allergic diseases (1), although with the advent of newer approaches, CD4+ T cell differentiation has expanded into many more subsets, including Tregs, Th17 cells, and polyfunctional T cells, among others (31).
We wanted to examine the role of type 1-, type 2-, and type 17-associated cytokines both in susceptibility to disease, which would manifest as differences in responses between TB-infected and noninfected children, and in dissemination or extrapulmonary manifestations, which would manifest as differences in responses between PTB- and ETB-infected children. Interestingly, we did not find any significant difference in either spontaneous in vitro or Ag-driven type 1, 2, or 17 or immunoregulatory cytokine responses between PTB and ETB children. These data suggest that conventional cytokine responses may not be solely responsible for susceptibility to TB dissemination and that other factors may need to be examined to understand this process. In addition, this cannot be attributed solely to differences in T cell numbers since the CD4+ and CD8+ T cell numbers in the two groups were not significantly different. Despite the lack of differences in cytokine production between these two groups with active TB, our study identified several important features of cytokine responses that distinguish TB disease/infection in children.
The major finding from our study is that diminished type 1, type 2, and type 17 cytokine responses with no stimulation and following mycobacterial Ag stimulation are a characteristic and distinctive feature of TB-infected children. Since Th1 cells are absolutely essential for resistance to TB in mice (4) and might also play an important role in the immune response to TB infection in guinea pigs (43), we first examined type 1 cytokine responses in TB-infected and HC children and found striking differences in nonstimulated and M. tuberculosis culture filtrate protein-induced production of IFN-γ, TNF-α, and IL-12. In addition, the differential cytokine response was not seen upon stimulation with a polyclonal stimulus, anti-CD3, suggesting that this is a defect in mycobacterial Ag-specific responses. Increased Th2 responses have also been postulated to play a role in susceptibility to TB (34), with IL-4 and IL-13 having been shown to modulate Th1-mediated immunity and to drive inappropriate alternative activation of macrophages (10, 23, 34). In contrast to studies using animal models whose findings have suggested that increased IL-4 and/or IL-13 would be detrimental to host resistance (10, 34) to M. tuberculosis, our study suggests that active TB infection in children is associated with both diminished Th1 and Th2 responses. In addition, the ability of HC to respond to TB antigens is perhaps a reflection of the priming of the immune system by environmental mycobacteria. Also, the production of IFN-γ to PPD and CFP but the absence of a response in the Quantiferon TB Gold test (in both TB and HC children) is due to the fact that the Quantiferon test uses antigens—ESAT-6, CFP-10, and TB7.7—which are present in M. tuberculosis but absent in almost all environmental mycobacteria (13).
We also examined induction of type 17 cytokine responses in children with TB, because Th17 cells appear to be critical in induction of the M. tuberculosis-specific memory response and mediation of protection against challenge infections and vaccinations (20), and the IL-23/IL-17 axis has been found to be important in the human immune response to TB (3, 36). Our finding that TB-infected children have significantly impaired IL-17 and IL-23 production spontaneously in vitro and following Ag stimulation and IL-21 production with no stimulation, compared with results for HC children, suggests that a lack of Th17 upregulation is one of the main characteristics of susceptibility to infection/disease. This is in agreement with recent reports of diminished IL-17 production in adults with active TB (3) and, to our knowledge, is the first study highlighting the importance of type 17 cytokine responses in childhood TB.
Various factors have recently been found to play important roles in dampening immune responses in infectious diseases, autoimmunity, and tumor biology. The most important of these are the inhibitory cytokines IL-10 and TGF-β, both of which have been shown to mediate suppression of immune responses in anergic TB patients (2, 33, 39). We found no significant differences in production of IL-10 or TGF-β between TB-infected or HC children either nonstimulated or following Ag stimulation. Thus, our preliminary findings indicate that IL-10 or TGF-β might not be primarily responsible for differential production of type 1, 2, or 17 cytokines in TB-infected and uninfected children. We are examining the role of adaptive Tregs (TR1) and natural Tregs (nTregs) in regulation of immune responses in pediatric TB.
A unique aspect of TB in children is the rapid progression from exposure to infection to disease, with pulmonary parenchymal disease and intrathoracic adenopathy being the most common clinical manifestations of pediatric TB, accounting for 60 to 80% of all cases (24). Among extrapulmonary manifestations, lymphadenopathy is the most common (67%), followed by CNS involvement (13%) (24). In our study population, we sought to investigate the differential response observed in neurologic forms of TB compared with other forms of ETB. Indeed, we found that children with neuro-TB had the most profound defect in spontaneous as well as Ag-driven production of both IFN-γ and IL-17. These data suggest that impaired type 1 and type 17 cytokine responses may underlie the development of neuro-TB.
Finally, because BCG vaccination of children has been demonstrated to induce complex cytokine profiles in both CD4+ and CD8+ T cells (37), we wanted to exclude a role for BCG vaccination in the observed immune responses in TB-infected children. When we analyzed production of the typical type 1, 2, and 17 cytokines on the basis of BCG vaccination status in TB-infected children, we found no significant difference in any of the cytokines, indicating that BCG had very little, if any, role in inducing a differential immune response in pediatric TB.
Very little is known about the adaptive immune response to M. tuberculosis in children in the setting of very high TB incidence. As biomarkers of protection against TB are gaining importance as possible indicators for efficient immune responses leading to protection (41), this study highlights some of these that characterize susceptibility to TB in children. While we have not examined children with latent infection alone in this study, our data nevertheless suggest that a complex network of cytokine responses involving type 1-, 2-, and 17-associated cytokines might play a vital role in mediating resistance or susceptibility to TB infection. It also implicates a role for Ag-specific type 1 and 17 cytokine responses in protection against one of the most common forms of ETB in children: neuro-TB. These data significantly improve our understanding of the fundamental nature of pediatric TB and may offer promising leads to design better therapeutics and vaccines for this highly prevalent infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of Kanchi Kamakoti CHILDS Trust Hospital, Chennai, India, for valuable assistance in recruiting the patients for this study, Sajid Q. Bhat and Jovvian George of the NIH-ICER for technical assistance, and NIAID intramural editor Brenda Rae Marshall for editorial assistance.
Because T.B.N. and S.B. are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMed Central for display and use by the public, and PubMed Central may tag or modify the work consistent with its customary practices. You can establish rights outside the U.S. subject to a government use license.
We have no potential conflicts of interest to report.
This study received financial support from the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://cvi.asm.org/.
Published ahead of print on 28 September 2011.
REFERENCES
- 1. Abbas A. K., Murphy K. M., Sher A. 1996. Functional diversity of helper T lymphocytes. Nature 383:787–793 [DOI] [PubMed] [Google Scholar]
- 2. Boussiotis V. A., et al. 2000. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Invest. 105:1317–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen X., et al. 2010. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am. J. Respir. Crit. Care Med. 181:734–742 [DOI] [PubMed] [Google Scholar]
- 4. Cooper A. M. 2009. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27:393–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper A. M., et al. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper A. M., Khader S. A. 2008. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol. Rev. 226:191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooper A. M., Magram J., Ferrante J., Orme I. M. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cotton M. F., et al. 2008. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int. J. Tuberc. Lung Dis. 12:225–227 [PubMed] [Google Scholar]
- 9. Day C. L., et al. 2008. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. J. Infect. Dis. 197:990–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deretic V., Levine B. 2009. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5:527–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flynn J. L., Goldstein M. M., Triebold K. J., Koller B., Bloom B. R. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U. S. A. 89:12013–12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forbes E. K., et al. 2008. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J. Immunol. 181:4955–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goletti D., et al. 2005. Selected RD1 peptides for active tuberculosis diagnosis: comparison of a gamma interferon whole-blood enzyme-linked immunosorbent assay and an enzyme-linked immunospot assay. Clin. Diagn. Lab. Immunol. 12:1311–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guyot-Revol V., Innes J. A., Hackforth S., Hinks T., Lalvani A. 2006. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 173:803–810 [DOI] [PubMed] [Google Scholar]
- 15. Hesseling A. C., et al. 2009. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin. Infect. Dis. 48:108–114 [DOI] [PubMed] [Google Scholar]
- 16. Hesseling A. C., et al. 2007. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine 25:14–18 [DOI] [PubMed] [Google Scholar]
- 17. Holland S. M. 2007. Interferon gamma, IL-12, IL-12R and STAT-1 immunodeficiency diseases: disorders of the interface of innate and adaptive immunity. Immunol. Res. 38:342–346 [DOI] [PubMed] [Google Scholar]
- 18. Jullien D., Stenger S., Ernst W. A., Modlin R. L. 1997. CD1 presentation of microbial nonpeptide antigens to T cells. J. Clin. Invest. 99:2071–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jurado J. O., et al. 2008. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J. Immunol. 181:116–125 [DOI] [PubMed] [Google Scholar]
- 20. Keane J., et al. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098–1104 [DOI] [PubMed] [Google Scholar]
- 21. Koga T., et al. 1989. T cells against a bacterial heat shock protein recognize stressed macrophages. Science 245:1112–1115 [DOI] [PubMed] [Google Scholar]
- 22. Lawn S. D., Bekker L. G., Middelkoop K., Myer L., Wood R. 2006. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin. Infect. Dis. 42:1040–1047 [DOI] [PubMed] [Google Scholar]
- 23. Liu P. T., Modlin R. L. 2008. Human macrophage host defense against Mycobacterium tuberculosis. Curr. Opin. Immunol. 20:371–376 [DOI] [PubMed] [Google Scholar]
- 24. Marais B. J., et al. 2006. The spectrum of disease in children treated for tuberculosis in a highly endemic area. Int. J. Tuberc. Lung Dis. 10:732–738 [PubMed] [Google Scholar]
- 25. Marais B. J., Schaaf H. S. 2010. Childhood tuberculosis: an emerging and previously neglected problem. Infect. Dis. Clin. North Am. 24:727–749 [DOI] [PubMed] [Google Scholar]
- 26. Mazurek G. H., et al. 2005. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm. Rep. 54:49–55 [PubMed] [Google Scholar]
- 27. Mueller H., et al. 2008. Mycobacterium tuberculosis-specific CD4+, IFNgamma+, and TNFalpha+ multifunctional memory T cells coexpress GM-CSF. Cytokine 43:143–148 [DOI] [PubMed] [Google Scholar]
- 28. Nelson L. J., Wells C. D. 2004. Global epidemiology of childhood tuberculosis. Int. J. Tuberc. Lung Dis. 8:636–647 [PubMed] [Google Scholar]
- 29. Newport M. J., et al. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941–1949 [DOI] [PubMed] [Google Scholar]
- 30. North R. J., Jung Y. J. 2004. Immunity to tuberculosis. Annu. Rev. Immunol. 22:599–623 [DOI] [PubMed] [Google Scholar]
- 31. O'Shea J. J., Paul W. E. 2010. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327:1098–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ottenhoff T. H., Kumararatne D., Casanova J. L. 1998. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol. Today 19:491–494 [DOI] [PubMed] [Google Scholar]
- 33. Roberts T., Beyers N., Aguirre A., Walzl G. 2007. Immunosuppression during active tuberculosis is characterized by decreased interferon-gamma production and CD25 expression with elevated forkhead box P3, transforming growth factor-beta, and interleukin-4 mRNA levels. J. Infect. Dis. 195:870–878 [DOI] [PubMed] [Google Scholar]
- 34. Rook G. A. 2007. Th2 cytokines in susceptibility to tuberculosis. Curr. Mol. Med. 7:327–337 [DOI] [PubMed] [Google Scholar]
- 35. Satyanarayana S., et al. 2010. Characteristics and programme-defined treatment outcomes among childhood tuberculosis (TB) patients under the national TB programme in Delhi. PLoS One 5:e13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scriba T. J., et al. 2008. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 180:1962–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soares A. P., et al. 2008. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J. Immunol. 180:3569–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swaminathan S., Rekha B. 2010. Pediatric tuberculosis: global overview and challenges. Clin. Infect. Dis. 50(Suppl 3):S184–S194 [DOI] [PubMed] [Google Scholar]
- 39. Toossi Z., Gogate P., Shiratsuchi H., Young T., Ellner J. J. 1995. Enhanced production of TGF-beta by blood monocytes from patients with active tuberculosis and presence of TGF-beta in tuberculous granulomatous lung lesions. J. Immunol. 154:465–473 [PubMed] [Google Scholar]
- 40. Trunz B. B., Fine P., Dye C. 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367:1173–1180 [DOI] [PubMed] [Google Scholar]
- 41. Wallis R. S., et al. 2010. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect. Dis. 10:68–69 [DOI] [PubMed] [Google Scholar]
- 42. Walls T., Shingadia D. 2004. Global epidemiology of paediatric tuberculosis. J. Infect. 48:13–22 [DOI] [PubMed] [Google Scholar]
- 43. Williams A., Hall Y., Orme I. M. 2009. Evaluation of new vaccines for tuberculosis in the guinea pig model. Tuberculosis (Edinb.) 89:389–397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.