Abstract
Chagas' disease is caused by the protozoan parasite Trypanosoma cruzi and is one of the most important endemic problems in Latin America. Lately, it has also become a health concern in the United States and Europe. Currently, a diagnosis of Chagas' disease and the screening of blood supplies for antiparasite antibodies are achieved by conventional serological tests that show substantial variation in the reproducibility and reliability of their results. In addition, the specificity of these assays is curtailed by antigenic cross-reactivity with sera from patients affected by other endemic diseases, such as leishmaniasis. Here we used a highly sensitive chemiluminescent enzyme-linked immunosorbent assay (CL-ELISA) to evaluate a recombinant protein core of a mucin-like molecule (termed trypomastigote small surface antigen [TSSA]) for the detection of specific serum antibodies in a broad panel of human sera. The same samples were evaluated by CL-ELISA using as the antigen either a mixture of native T. cruzi trypomastigote mucins or an epimastigote extract and, for further comparison, by conventional serologic tests, such as an indirect hemagglutination assay and indirect immunofluorescence assay. TSSA showed ∼87% sensitivity among the seropositive Chagasic panel, a value which was increased up to >98% when only parasitologically positive samples were considered. More importantly, TSSA showed a significant increase in specificity (97.4%) compared to those of currently used assays, which averaged 80 to 90%. Overall, our data demonstrate that recombinant TSSA may be a useful antigen for the immunodiagnosis of Chagas' disease.
INTRODUCTION
American trypanosomiasis, or Chagas' disease, is a major health and economic problem in Latin America caused by the protozoan parasite Trypanosoma cruzi. It is estimated that 8 million people are currently infected and that 90 million individuals living in areas of endemicity are at risk of infection (27, 32). The parasite is transmitted to humans through the feces of infected, blood-sucking triatomine bugs, blood transfusion, organ transplantation, or congenital contamination or by the ingestion of tainted food and fluids (35). In recent years, several efforts have been successfully undertaken to control vectorial transmission in Latin American countries, with a concomitant decrease in the actual numbers of acute infections (28). However, since T. cruzi infection is asymptomatic in most cases, chronically infected individuals can serve as parasite reservoirs throughout their lifetimes. Thus, there is a consensus that congenital T. cruzi infection will be a pressing public health problem for at least the next 20 years (30). In addition, the risk of acquiring Chagas' disease through infected blood transfusion is becoming a problem even in areas of nonendemicity, such as the United States and Europe, and some cases have already been reported (18, 23, 32). Owing to the risk of transmission by blood transfusion and organ transplantation, most blood donations in the United States have routinely been screened in recent years (1). Nevertheless, in many developed countries, the blood supply is not yet regularly tested for anti-T. cruzi antibodies (18, 32).
Due to the low parasite levels present in the chronic phase of the disease, its detection in blood samples by direct examination, hemoculture, or xenodiagnosis is difficult and time-consuming (19). Several PCR- and real-time-PCR-based procedures have been reported that, though highly specific and sensitive, might not be appropriate for routine use in blood supplies or health centers (16, 31). Detection of anti-T. cruzi antibodies is still the most effective method for demonstrating direct exposure to the parasite. At present, the most widely used serologic methods are indirect hemagglutination assays (IHAs), indirect immunofluorescence (IIF), and enzyme-linked immunosorbent assays (ELISAs) using total parasite homogenates or semipurified antigenic fractions from epimastigotes, the noninfective parasite form present in the digestive tract of the insect vector (19). However, these tests show variations in the reproducibility and reliability of their results that can be attributed to the poor standardization of the reagents (12). The advent of recombinant DNA technology allowed the production and one-step purification of large amounts of highly pure T. cruzi immunodominant antigens, some of which were evaluated by way of multicenter trials (12, 19, 25). The use of linear and/or branched synthetic peptides spanning B-cell epitopes has also been successfully applied (20, 22, 34). Both recombinant antigens and synthetic peptides minimize the extent of specificity problems, one of the major drawbacks of immunodiagnosis of Chagas' disease (19). As previously shown, sera from individuals with leishmaniasis, mycoses, and/or certain autoimmune disorders cross-react with crude preparations of T. cruzi antigens (6, 33).
The mucin coat that covers the surfaces of bloodstream trypomastigotes (trypomastigote glycosylphosphatidylinositol [tGPI] mucins) is decorated with highly immunogenic α-galactosyl (α-Gal) epitopes (3, 4). Chagasic anti-α-Gal antibodies have a complement-independent lytic effect on bloodstream trypomastigote forms (26), and more importantly, their titer is considerably reduced in benznidazole-treated patients in the early stage of chronicity (5, 13), suggesting that they might also be useful for monitoring patients after drug treatment. The trypomastigote small surface antigen (TSSA) is a mucin-like glycoprotein displayed on the surface of infective trypomastigote forms (15) and potentially involved in host cell recognition (G. E. Cánepa and C. A. Buscaglia, unpublished data). Two main isoforms of TSSA were originally recognized in the two major lineages or subgroups into which the T. cruzi species has been divided (36): one isoform present in lineage I (currently known as T. cruzi discrete typing unit I [DTU TcI]) parasite stocks and one isoform present in TcIIe (now DTU TcVI) isolates. Sequence variations between the isoforms were shown to have a major impact on TSSA antigenicity, leading to negligible cross-reactivity between them (15). This property was proposed to have great epidemiological value, as it allowed for identification of the lineage of the infecting strain by simple serologic methods (9). Recent studies challenged this idea by showing that TSSA is significantly more diverse in amino acid structure than previously described (7), although the antigenic impact of these changes, if any, remains uncertain.
Here we report a thorough study of the sensitivity and specificity of TSSA which demonstrates that this recombinant antigen is a useful molecule for the immunodiagnosis of Chagas' disease. In order to improve the sensitivity of the assay, a high-throughput chemiluminescent ELISA (CL-ELISA) was employed (3).
MATERIALS AND METHODS
Study populations.
Human serum samples (n = 617) were obtained from the Hospital das Clínicas da Universidade de São Paulo (HC-USP), the Laboratório de Investigaçao Médica-Parasitológica, Instituto de Medicina Tropical de São Paulo (IMT), Departamento de Moléstias Infecciosas, HC-USP, and the Fundação Hemocentro de Ribeirão Preto, São Paulo, Brazil. The use of these human serum samples was approved by the institutional review boards of the IMT and the Instituto de Ciências Biomédicas, USP (ICM-USP). Some of the samples were part of a serum panel stocked by the ICM-USP. These sera had been codified upon collection, and therefore no information (e.g., name, age, sex, etc.) regarding the patient was available. They were approved for use in the current study by the institutional review boards of both the ICM-USP and the IMT. Sera were collected from clotted blood obtained by venipuncture, diluted 1:2 with high-grade glycerol for conservation, and stored at −80°C until use. Three panels of human sera were used in this work. The first panel was composed of 237 samples collected from individuals that were grouped into seropositive Chagasic samples (those rendering positive results only for conventional serology tests [n = 185]) or parasitologically positive Chagasic samples (those rendering positive results for both conventional serology tests and hemoculture [n = 52]). The second panel was composed of 200 samples from healthy, noninfected individuals (NHS) rendering negative results for T. cruzi by three independent assays (ELISA using total parasite homogenate, IHA, and IIF) and was thus defined as the seronegative panel. The third panel, termed the specificity control panel, was composed of 180 samples collected from non-Chagasic individuals affected by unrelated diseases, as defined by the clinical and serologic diagnoses of their respective pathologies. Twenty-nine of these 180 samples were from patients infected with cutaneous leishmaniasis, 31 were from patients with visceral leishmaniasis, 4 were from histoplasmosis patients, 9 were from patients infected with Mycobacterium leprae, and 28 were from patients affected by different helminth and protozoan infections. Specifically, 4 of the last samples were from individuals infected with Schistosoma spp., 5 were from individuals infected with Giardia lamblia, 1 was from an individual infected with Hymenolepis nana, 4 were from individuals infected with Tricuris trichiura, 2 were from individuals infected with Strongyloides stercoralis, 2 were from individuals infected with Ancylostoma spp., 3 were from individuals infected with Ascaris lumbricoides, 1 was from an individual infected with Enterobius vermicularis, 3 were from individuals infected with Toxocara canis, and 3 were from individuals infected with Cryptosporidium spp. In addition, this panel contained 79 samples from individuals afflicted by autoimmune disorders: 53 with rheumatoid arthritis and 26 with systemic lupus erythematosus.
Parasites.
Cell-derived trypomastigotes from the Y strain (DTU TcII) were collected from the supernatant of Mycoplasma-free LLC-MK2 cells (American Type Culture Collection, Manassas, VA) grown in Dulbecco's modified Eagle medium (DMEM) containing 4.5% glucose, 10% fetal bovine serum (FBS), and antibiotics (8). After three washings in phosphate-buffered saline (PBS), parasites were lyophilized and stored at −70°C until use. Epimastigotes from the same strain were cultured in liver infusion tryptose (LIT) medium containing 10% FBS and 5% glucose (10).
Purification of parasite tGPI mucins and preparation of total epimastigote extracts.
tGPI mucins from 1010 parasites were purified from delipidated butan-1-ol-water extracts by hydrophobic interaction chromatography as described previously (4). A total epimastigote extract (EpEx) was obtained as described previously (3).
Expression and purification of recombinant GST-TSSA proteins.
Genes coding for the Sylvio X-10/1 TSSA (TSSA I; GenBank accession number ACY02865.1) and the CL Brener TSSA (TSSA VI, formerly TSSA II; GenBank accession number ACY54510) have been described (7, 15). To reduce the risk of false-positive sampling, the recombinant TSSA proteins used in this work consisted only of the predicted, full-length, mature products, i.e., without most of the endoplasmic reticulum and GPI anchor signals (Fig. 1 A). Briefly, previously described glutathione S-transferase (GST)-TSSA clones were reamplified by PCR using the oligonucleotides EMT5/s and EMT5/a (15), digested with BamHI/EcoRI, and cloned into the pGEX-2T vector (GE). Supernatants of Escherichia coli cultures induced for 3 h at 28°C with 0.1 mM isopropyl-β-d-thiogalactopyranoside were purified by glutathione-Sepharose chromatography (15) and dialyzed against PBS. The purity of GST-TSSA samples was assessed with silver-stained SDS-PAGE gels (Fig. 1B). Some of the faint bands that can be seen in the gel correspond to multimeric forms and/or degradation products of TSSA molecules, as judged by anti-GST Western blot analysis (not shown).
Fig. 1.
Features of evaluated TSSA molecules. (A) Schematic illustration of TSSA products showing the predicted signal peptide (SP) and GPI-anchoring signals. The sequences of the TSSA I and TSSA VI regions expressed as GST fusion proteins are indicated. Variable positions between both proteins are shaded. The predicted SP cleavage site is indicated with an inverted triangle, and amino acids (aa) predicted to be O-glycosylated in the TSSA VI protein are indicated with asterisks. B-cell epitopes recognized by human Chagasic sera in the TSSA VI protein are underlined. (B) The purity of the recombinant GST fusion proteins used for the CL-ELISAs was ascertained by silver staining of an SDS-PAGE gel containing 1 μg of the indicated protein. Molecular size markers (in kDa) are indicated.
CL-ELISA.
Polystyrene ELISA microplates (FluoroNunc; Nunc, Roskilde, Denmark) were coated with the corresponding antigen (50 μl containing 1.2 ng tGPI mucins, 2.2 ng EpEx, or 10 ng TSSA per well), diluted in 50 mM carbonate-bicarbonate buffer, pH 9.6 (CBB). After 18 h at 4°C, plates were blocked with CBB containing 0.1% bovine serum albumin (CBB-BSA) and washed 3 times with 300 μl of PBS, pH 7.2, containing 0.05% Tween 20 (PBS-T). Sera were diluted in PBS-T as follows: (i) 1:2,000 for a chemiluminescent ELISA (CL-ELISA) with tGPI mucins or EpEx and (ii) 1:200 for a CL-ELISA with TSSA I and TSSA VI (15). In all cases, sera were incubated for 1 h at 37°C, and plates were then washed as before and developed by the addition of biotin-labeled anti-human IgG antibody (50 μl, 1:1,000 dilution; Amersham, GE Healthcare, Piscataway, NJ), followed by streptavidin coupled to horseradish peroxidase (HRP) (50 μl, 1:2,000 dilution; Amersham, GE Healthcare). Plates were washed as before, and 50 μl of ECL reagent (Amersham, GE Healthcare) diluted 1:20 in CBB was added. Reading of the plates was carried out with a Fluoroskan Ascent FL apparatus (Thermo Labsystems, Helsinki, Finland), and values were expressed in relative luminescence units (RLU). The cutoff value for each antigen was calculated with the equation, cutoff = k(mn − mb), where k is the number of standard deviations (SD) obtained for the negative-control sera separating the maximum RLU value for the negative-control sera and the minimum RLU value for the positive-control sera, and mn and mb are the mean values of results from the negative-control sera and the background (without the addition of serum), respectively. The k values were estimated as 3 for the tGPI mucins and 2 for EpEx and the GST-TSSA recombinant proteins. In order to compare the results of different CL-ELISAs using distinct antigens, serological titers were calculated by dividing the RLU values of individual serum samples by the cutoff value for each antigen.
Conventional serologic tests (IHA, IIF, and ELISA).
Serum samples were tested by IHA and IIF as described previously (21), using commercial reagents (Cecon, São Paulo, Brazil). For the latter, fluorescein isothiocyanate (FITC)-coupled anti-human IgG antibodies were used (New England BioLabs, Beverly, MA). The cutoff value determined for both techniques was 1:40, and in both cases, a 1:20 titer was considered inconclusive. A commercial ELISA kit composed of a crude extract of epimastigote forms was purchased from Embrabio (São Paulo, Brazil) and used according to the manufacturer's guidelines. For ELISA and CL-ELISA, the corresponding cutoff value ± 10% was considered inconclusive.
RESULTS
Development of the T. cruzi mucin-based CL-ELISA.
The quality of the purified antigenic GST-TSSA I and GST-TSSA VI (formerly GST-TSSA II [15]) recombinant proteins was assessed with silver-stained SDS-PAGE gels (Fig. 1A). These proteins rendered a single band of the expected molecular mass (∼35 kDa) (Fig. 1B), whereas purified tGPI mucins migrated as a broad smear ranging from 40 to 250 kDa, which is barely visible upon silver staining (4, 8; not shown). This heterogeneity is the result of the coexpression of multiple and heterogeneous apomucin polypeptides subjected to different extents of posttranslational modifications, mainly the addition of O-glycans (2, 8). The optimal concentration of protein per assay and the dilution of the serum samples were determined for each antigen by antigen-serum cross-titration, and they are defined as the antigen concentration and serum dilution that gave the highest ratio between a blindly selected pool of parasitologically positive sera (n = 10) and a blindly selected pool of NHS from our seronegative panel (n = 10). For tGPI mucins and EpEx, the optimal conditions were achieved at concentrations of 1.2 and 2.2 ng/well, respectively, and in testing the samples at a 1:2,000 dilution. In the case of the GST-TSSA VI molecule, optimal results were achieved by using 10 ng/well and diluting the serum samples 1:200. Accordingly, the same conditions were used for the evaluation of the GST-TSSA I recombinant protein. The cutoff and k values for each antigen were calculated as indicated in Materials and Methods by the use of (i) 52 parasitologically positive sera and (ii) 100 NHS samples which were blindly selected from our seronegative panel.
Sensitivity of the CL-ELISA using seropositive and parasitologically positive human sera.
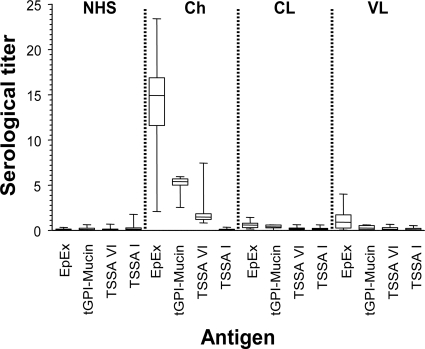
A panel of 237 samples was analyzed by CL-ELISA using EpEx, purified tGPI mucins, and recombinant GST-TSSA VI and GST-TSSA I proteins as antigens. For comparison, all the samples were analyzed by three widely used serodiagnostic techniques: IIF, IHA, and ELISA. Two groups can be defined among the tested samples: those showing positive results in conventional serology tests and hemoculture (52 parasitologically positive samples) and those showing positive results only in conventional serology tests (185 seropositive samples), which were collected either in Brazil (n = 160) or Chile (n = 25). Box-and-whisker plots expressing the results obtained in each case are shown in Fig. 2, and the raw data are summarized in Table 1. When tGPI mucins were used as antigens, 100% sensitivity was obtained for both groups of samples, in accordance with previous data (3). This value is even higher than that obtained by IHA or IIF and also higher than that obtained with EpEx, with which 1 out of 237 samples rendered negative results. On the other hand, the overall sensitivity of GST-TSSA VI is 86.9% and thus in the range of values for other recombinant parasite antigens proposed for Chagas' disease immunodiagnosis (12, 25). However, and according to previous results, sensitivities clearly differed between hemoculture-tested and -untested sera (15). The value for the first group was >98% (only 1 out of 52 samples produced inconclusive results), whereas a significant reduction to 83.7% was verified for the second group. No significant differences in the sensitivity indexes were observed when sera collected from Brazil and Chile were compared (83.7 and 84.0%, respectively), although the mean values recorded for the Brazilian samples were consistently higher (not shown). In line with previous results, none of the tested sera rendered positive values when evaluated by the control GST-TSSA I protein (Table 1), which could be attributed to (i) low levels of expression/antigenicity of the TSSA isoform present in DTU TcI parasite stocks and/or (ii) the predominance of non-DTU TcI infections in the domestic and peridomestic cycles of Chagas' disease in the Southern Cone countries of Latin America (11, 15).
Fig. 2.
Recognition of evaluated antigens by sera from T. cruzi- and Leishmania-infected individuals. Reactivities of sera collected from individuals infected with T. cruzi (Chagasic [Ch]) (n = 237) or Leishmania spp. (n = 60) and from healthy individuals (NHS) (n = 200) to the indicated T. cruzi-derived antigens were determined by CL-ELISA. Results are presented by box-and-whisker plots, indicating the median serum titer (horizontal line inside box), SD (box's upper and lower lines), and maximal and minimal serum titers (whiskers). The serological titer for each sample was determined as described in Materials and Methods. Sera from Leishmania-infected individuals were grouped according to the manifestation of the disease. VL, visceral leishmaniasis (n = 31); CL, cutaneous leishmaniasis (n = 29).
Table 1.
Sensitivities and specificities of CL-ELISA with distinct T. cruzi antigens and of conventional serologic methods
| Index | No. of samples | Result (%) using CL-ELISA with: |
Result (%) using: |
|||||
|---|---|---|---|---|---|---|---|---|
| EpEx | tGPI mucin | TSSA VI | TSSA Id | IHA | IIF | ELISA | ||
| Sensitivity | 237 | 99.6 | 100 | 86.9 | 0 | 97.5 | 98.7 | 100 |
| Specificitya | 200 | 100 | 100 | 99 | NA | 100 | 100 | 100 |
| Specificityb | 180 | 69.4 | 100 | 94.4 | NA | 77.8 | 58.3 | 57.8 |
| Specificityc | 380 | 85.5 | 100 | 97.4 | NA | 89.5 | 80.3 | 80 |
Specificity determined with seronegative human sera.
Specificity determined with specificity control human sera.
Specificity determined with seronegative and specificity control human sera.
NA, not applicable (did not render a positive value).
Specificity of the CL-ELISA.
The specificity of each antigen in our CL-ELISA was analyzed by using 380 serum samples collected from non-Chagasic individuals (Table 1). Among these samples, 200 were from healthy, noninfected blood donors (NHS; seronegative panel) and 180 from individuals affected by other infectious or autoimmune diseases which might elicit cross-reactive humoral responses toward T. cruzi constituents (specificity panel). No reactivity against tGPI mucins was observed among NHS samples, leading to a 100% specificity (3). The same results were obtained when these samples were evaluated either by conventional serology techniques (IIF, IHA, and a commercial ELISA kit) or by CL-ELISA using EpEx as the antigen. In the case of GST-TSSA VI, one inconclusive and one positive value were recorded. It is noteworthy that the latter sample also rendered positive results when tested with GST-TSSA I (not shown), suggesting that the reacting antibodies recognize a common determinant on both proteins, thus showing a specificity different from that of antibodies present in samples from patients with chronic Chagas' disease (15). Whether this recognition is directed toward the GST molecule or toward a TSSA epitope lying outside the central and variable regions between both isoforms was not tested.
The most striking differences between conventional serologic assays and our proposed TSSA VI antigen were obtained when the specificity panel of serum samples was evaluated (Table 2). IIF, IHA, and ELISA techniques and the EpEx-based CL-ELISA displayed high levels of recognition of cross-reacting antibodies, particularly those from Leishmania-infected patients and individuals with autoimmune disorders (Fig. 1 and Table 2, respectively). In addition, EpEx-based and nonchemiluminescent ELISAs using a commercial kit yielded positive results for some samples from individuals infected with protozoan or multicellular parasites. In sharp contrast, the specificity recorded for tGPI mucins was 100%. GST-TSSA VI showed a slightly reduced specificity (94.4%) when evaluated with the specificity panel of serum samples, which is still significantly better than that reported for conventional serodiagnostic tests, such as IIF, IHA, and ELISA (ranging from 58 to 78%). Overall, these results highlight that the major advantage for the use of TSSA is at the specificity level.
Table 2.
Specificities of CL-ELISA with distinct T. cruzi antigens and of conventional serologic methods to heterologous human sera
| Disease or infection (n)a | Test resultb | No. of samples with each result with CL-ELISA and: |
No. of samples with each result with: |
||||
|---|---|---|---|---|---|---|---|
| EpEx | tGPI mucin | TSSA VI | IHA | IIF | ELISA | ||
| Leishmaniasis (60) | Positive | 36 | 0 | 0 | 35 | 46 | 40 |
| Inconclusive | 6 | 0 | 1 | 3 | 5 | 0 | |
| Negative | 18 | 60 | 59 | 22 | 9 | 20 | |
| Specificity | 30 | 100 | 98.3 | 36.7 | 15 | 33.3 | |
| Histoplasmosis (4) | Positive | 0 | 0 | 0 | 0 | 0 | 0 |
| Inconclusive | 0 | 0 | 0 | 0 | 0 | 0 | |
| Negative | 4 | 4 | 4 | 4 | 4 | 4 | |
| Specificity | 100 | 100 | 100 | 100 | 100 | 100 | |
| Mycobacterium leprae (9) | Positive | 0 | 0 | 0 | 0 | 3 | 0 |
| Inconclusive | 0 | 0 | 0 | 0 | 2 | 0 | |
| Negative | 9 | 9 | 9 | 9 | 4 | 9 | |
| Specificity | 100 | 100 | 100 | 100 | 44.5 | 100 | |
| Helminth and protozoan infections (28) | Positive | 2 | 0 | 0 | 0 | 0 | 5 |
| Inconclusive | 2 | 0 | 1 | 0 | 0 | 2 | |
| Negative | 24 | 28 | 27 | 28 | 28 | 21 | |
| Specificity | 85.7 | 100 | 96.4 | 100 | 100 | 75 | |
| Rheumatoid arthritis (53) | Positive | 4 | 0 | 3 | 0 | 4 | 20 |
| Inconclusive | 1 | 0 | 4 | 0 | 5 | 0 | |
| Negative | 48 | 53 | 46 | 53 | 44 | 33 | |
| Specificity | 90.6 | 100 | 86.6 | 100 | 83.1 | 62.3 | |
| Systemic lupus erythematosus (26) | Positive | 4 | 0 | 1 | 2 | 4 | 9 |
| Inconclusive | 0 | 0 | 0 | 0 | 6 | 0 | |
| Negative | 22 | 26 | 25 | 24 | 16 | 17 | |
| Specificity | 84.6 | 100 | 96.2 | 92.3 | 61.5 | 65.4 | |
n, number of samples from affected individuals.
Specificity is expressed as a percentage.
DISCUSSION
Serologic methods are widely employed for the diagnosis of Chagas' disease, particularly in the indeterminate and chronic phases, when parasitemia is extremely low and very hard to detect. The World Health Organization has long emphasized the need to employ defined antigens as a way of improving the serodiagnosis of Chagas' disease. In order to be useful, these antigens must meet several criteria: (i) they should be present in T. cruzi isolates from different areas of endemicity, (ii) they should be absent from other infectious disease agents, (iii) they should be highly immunogenic in populations with different genetic backgrounds, and (iv) they should be stable and easily amenable to quality control tests to guarantee reproducibility (19). Considering these guidelines, we herein evaluated the recombinant protein core of the TSSA antigen, derived from a mucin molecule expressed by the bloodstream forms of T. cruzi, in the development of a sensitive and specific CL-ELISA system for the diagnosis of Chagas' disease. The choice was based on previous results in which the TSSA isoform from DTU TcVI parasite stocks displayed a high reactivity with human infection sera from Southern Cone countries (15). Although the spectra of circulating parasite stocks, and therefore of their expressed TSSA isoforms, seem to vary in different areas of endemicity, a high frequency of TSSA VI recognition, as assessed by Western blotting, was also recently observed in patients with chronic Chagas' disease from Colombia, Venezuela, and Mexico (29).
By using a much larger and more comprehensive serum panel, we estimated the overall sensitivity of GST-TSSA VI as 86.9% (Table 1). Even though the sensitivity of this antigen is in the range of other recombinant proteins proposed for Chagas' disease immunodiagnosis (12, 25), its sensitivity among parasitologically positive samples was >98%. More importantly, TSSA VI showed minimal cross-reactivity with blood samples from Leishmania-infected individuals, a common source of false-positive results in conventional serodiagnosis tests for Chagas' disease (Tables 1 and 2). In accordance, TSSA seems to be restricted to T. cruzi, since no homologous gene has been detected in the genome databases of other trypanosomatids (reference 17 and our unpublished results).
For comparison purposes, the same samples were evaluated in parallel using tGPI mucins, a putative gold standard for the diagnosis and follow-up of the treatment of Chagas' disease (3, 5, 13), which resulted in 100% sensitivity and 100% specificity with the extensive panel of serum samples tested here. The basis for this sensitivity can be attributed in part to the abundance of tGPI mucins on the surface of a parasite (26) and to the high antigenicity of their nonreducing, terminal α-Gal epitopes (4). Accordingly, treatment of tGPI mucins with α-galactosidase abolished most (80 to 100%) of the reactivity of individual human Chagasic sera against these GPI-anchored glycoproteins (I. C. Almeida, unpublished data). In spite of its optimal specificity and sensitivity, some drawbacks could be envisaged for the routine implementation of tGPI mucins in serodiagnosis. First, the material is purified from cultured infective forms of the parasite, and this process is expensive and time-consuming. In addition, purification is a multistep process, requiring lyophilization of the parasites, extraction in organic solvents, and hydrophobic-interaction chromatography. These disadvantages are eased by the fact that very small amounts of tGPI mucins are required (∼1 ng/well). Therefore, a single-batch preparation from 109 trypomastigotes should render enough material to perform 20,000 assays. Different protocols to speed up and simplify the tGPI mucin purification processes should be evaluated.
It becomes clear that in order to improve the sensitivity of TSSA VI, it should be supplemented with other antigens, either by including it in existing serologic diagnostic kits or by developing a new mixture of antigens that might well include a synthetic terminal α-Gal-containing epitope(s) recognized by Chagasic anti-Gal antibodies in tGPI mucins. In this regard, it is worth noting that chemical synthesis of complex oligosaccharides attached to T. cruzi mucins has been recently achieved (14, 24). We speculate that the presence of both peptide and glycan epitopes in a single serodiagnostic reagent may render optimal results in terms of sensitivity and specificity. In summary, our results show that TSSA VI is a better alternative to the epimastigote extracts currently employed in T. cruzi serodiagnosis. It could be combined with other recombinant antigens to improve the sensitivities of current kits or used alone or in combination with other antigens as a confirmatory diagnostic test, as recommended by the World Health Organization (19). The CL-ELISA developed here also has a highly increased specificity, in particular with respect to Leishmania infections, compared to other, more cumbersome diagnostic techniques for T. cruzi detection, such as IHA and IIF.
ACKNOWLEDGMENTS
We are grateful to Dimas Covas and Lea Soussumi (Fundação Hemocentro de Ribeirão Preto, São Paulo, Brazil) and Maria A. Shikanai-Yasuda (Hospital das Clinicas da Faculdade de Medicina, USP) for kindly providing several serum samples.
This work was supported by a grant from the Fundação de Amparo à Pesquisa do Estado do São Paulo (FAPESP), Brazil, to I.C.A. and by grants from the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR), World Health Organization, the Fundación Florencio Fiorini, and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina, to C.A.B. I.C.A. was also partially supported by grants 2G12RR008124-16A1 and 2G12RR008124-16A1S1 from the National Institutes of Health.
A.C.C.F. and C.A.B. are researchers from the CONICET.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Agapova M., Busch M. P., Custer B. 2010. Cost-effectiveness of screening the US blood supply for Trypanosoma cruzi. Transfusion 50:2220–2232 [DOI] [PubMed] [Google Scholar]
- 2. Almeida I. C., et al. 2000. Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 19:1476–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Almeida I. C., Covas D. T., Soussumi L. M., Travassos L. R. 1997. A highly sensitive and specific chemiluminescent enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion 37:850–857 [DOI] [PubMed] [Google Scholar]
- 4. Almeida I. C., Ferguson M. A., Schenkman S., Travassos L. R. 1994. Lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem. J. 304(Part 3):793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrade A. L., et al. 2004. Short report: benznidazole efficacy among Trypanosoma cruzi-infected adolescents after a six-year follow-up. Am. J. Trop. Med. Hyg. 71:594–597 [PubMed] [Google Scholar]
- 6. Araujo F. G. 1986. Analysis of Trypanosoma cruzi antigens bound by specific antibodies and by antibodies to related trypanosomatids. Infect. Immun. 53:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhattacharyya T., et al. 2010. Analysis of molecular diversity of the Trypanosoma cruzi trypomastigote small surface antigen reveals novel epitopes, evidence of positive selection and potential implications for lineage-specific serology. Int. J. Parasitol. 40:921–928 [DOI] [PubMed] [Google Scholar]
- 8. Buscaglia C. A., et al. 2004. The surface coat of the mammal-dwelling infective trypomastigote stage of Trypanosoma cruzi is formed by highly diverse immunogenic mucins. J. Biol. Chem. 279:15860–15869 [DOI] [PubMed] [Google Scholar]
- 9. Buscaglia C. A., Di Noia J. M. 2003. Trypanosoma cruzi clonal diversity and the epidemiology of Chagas' disease. Microbes Infect. 5:419–427 [DOI] [PubMed] [Google Scholar]
- 10. Camargo M. E. 1966. Fluorescent antibody test for the serodiagnosis of American trypanosomiasis. Technical modification employing preserved culture forms of Trypanosoma cruzi in a slide test. Rev. Inst. Med. Trop. Sao Paulo 8:227–235 [PubMed] [Google Scholar]
- 11. Coura J. R., Junqueira A. C., Fernandes O., Valente S. A., Miles M. A. 2002. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 18:171–176 [DOI] [PubMed] [Google Scholar]
- 12. da Silveira J. F., Umezawa E. S., Luquetti A. O. 2001. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol. 17:286–291 [DOI] [PubMed] [Google Scholar]
- 13. de Andrade A. L., et al. 1996. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 348:1407–1413 [DOI] [PubMed] [Google Scholar]
- 14. de Lederkremer R. M., Agusti R. 2009. Glycobiology of Trypanosoma cruzi. Adv. Carbohydr. Chem. Biochem. 62:311–366 [DOI] [PubMed] [Google Scholar]
- 15. Di Noia J. M., Buscaglia C. A., De Marchi C. R., Almeida I. C., Frasch A. C. 2002. A Trypanosoma cruzi small surface molecule provides the first immunological evidence that Chagas' disease is due to a single parasite lineage. J. Exp. Med. 195:401–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duffy T., et al. 2009. Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in Chagas disease patients. PLoS Negl. Trop. Dis. 3:e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El-Sayed N. M., et al. 2005. Comparative genomics of trypanosomatid parasitic protozoa. Science 309:404–409 [DOI] [PubMed] [Google Scholar]
- 18. Gascon J., Bern C., Pinazo M. J. 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 115:22–27 [DOI] [PubMed] [Google Scholar]
- 19. Gomes Y. M., Lorena V. M., Luquetti A. O. 2009. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem. Inst. Oswaldo Cruz 104(Suppl. 1):115–121 [DOI] [PubMed] [Google Scholar]
- 20. Hernandez Marin M., Hernandez Spengler I., Ramos Martinez G., Pozo Pena L. 2006. Chimeric synthetic peptides as antigens for detection of antibodies to Trypanosoma cruzi. Biochem. Biophys. Res. Commun. 339:89–92 [DOI] [PubMed] [Google Scholar]
- 21. Hoshino-Shimizu S., Camargo M. E., Umezawa E. S. 1975. A rapid slide flocculation test for the diagnosis of American trypanosomiasis using Trypanosoma cruzi fragments preserved by lyophilization. Comparison with hemagglutination, immunofluorescence, and complement fixation tests. Am. J. Trop. Med. Hyg. 24:586–589 [DOI] [PubMed] [Google Scholar]
- 22. Houghton R. L., et al. 2000. Multiepitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in patients with treated or untreated Chagas' disease. J. Infect. Dis. 181:325–330 [DOI] [PubMed] [Google Scholar]
- 23. Leiby D. A. 2004. Threats to blood safety posed by emerging protozoan pathogens. Vox Sang. 87(Suppl. 2):120–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mendoza V. M., Kashiwagi G. A., de Lederkremer R. M., Gallo-Rodriguez C. 2010. Synthesis of trisaccharides containing internal galactofuranose O-linked in Trypanosoma cruzi mucins. Carbohydr. Res. 345:385–396 [DOI] [PubMed] [Google Scholar]
- 25. Pastini A. C., et al. 1994. Immunoassay with recombinant Trypanosoma cruzi antigens potentially useful for screening donated blood and diagnosing Chagas disease. Clin. Chem. 40:1893–1897 [PubMed] [Google Scholar]
- 26. Pereira-Chioccola V. L., et al. 2000. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J. Cell Sci. 113(Part 7):1299–1307 [DOI] [PubMed] [Google Scholar]
- 27. Rassi A., Jr., Rassi A., Marin-Neto J. A. 2010. Chagas disease. Lancet 375:1388–1402 [DOI] [PubMed] [Google Scholar]
- 28. Reithinger R., Tarleton R. L., Urbina J. A., Kitron U., Gurtler R. E. 2009. Eliminating Chagas disease: challenges and a roadmap. BMJ 338:b1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Risso M. G., et al. 2011. Immunological identification of Trypanosoma cruzi lineages in human infection along the endemic area. Am. J. Trop. Med. Hyg. 84:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Russomando G., Sanchez Z., Meza G., de Guillen Y. 2010. Shed acute-phase antigen protein in an ELISA system for unequivocal diagnosis of congenital Chagas disease. Expert Rev. Mol. Diagn. 10:705–707 [DOI] [PubMed] [Google Scholar]
- 31. Schijman A. G., et al. 2011. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl. Trop. Dis. 5:e931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmunis G. A., Yadon Z. E. 2010. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 115:14–21 [DOI] [PubMed] [Google Scholar]
- 33. Schnaidman B. B., Yoshida N., Gorin P. A., Travassos L. R. 1986. Cross-reactive polysaccharides from Trypanosoma cruzi and fungi (especially Dactylium dendroides). J. Protozool. 33:186–191 [DOI] [PubMed] [Google Scholar]
- 34. Vergara U., et al. 1991. Assay for detection of Trypanosoma cruzi antibodies in human sera based on reaction with synthetic peptides. J. Clin. Microbiol. 29:2034–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoshida N. 2009. Molecular mechanisms of Trypanosoma cruzi infection by oral route. Mem. Inst. Oswaldo Cruz 104(Suppl. 1):101–107 [DOI] [PubMed] [Google Scholar]
- 36. Zingales B., et al. 2009. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz 104:1051–1054 [DOI] [PubMed] [Google Scholar]