Abstract
Anaplasma phagocytophilum is the zoonotic cause of granulocytic anaplasmosis. We hypothesized that immune response, specifically gamma interferon (IFN-γ), plays a role in disease severity. To test this, horses were infected and IFNG expression was pharmacologically downregulated using corticosteroids. Eight horses were infected with A. phagocytophilum; 4 received dexamethasone on days 4 to 8 of infection. Clinical signs, hematologic parameters, and transcription of cytokine/chemokine genes were compared among treated and untreated horses. Infection was quantitated by msp2 real-time PCR and microscopy. As anticipated, there was significantly greater leukopenia, thrombocytopenia, and anemia in infected versus uninfected horses. The A. phagocytophilum load was higher for dexamethasone-treated horses. Dexamethasone reduced IFNG transcription by day 12 and IL-8 and IL-18 by days 7 to 9 and increased IL-4 on day 7. The ratio of IL-10 to IFNG was increased by dexamethasone on day 9. There were no hematologic differences between the infected horses. Dexamethasone suppression of proinflammatory response resulted in delayed infection-induced limb edema and decreased icterus, anorexia, and reluctance to move between days 6 and 9 and lower fever on day 7. These results underscore the utility of the equine model of granulocytic anaplasmosis and suggest that Th1 proinflammatory response plays a role in worsening disease severity and that disease severity can be decreased by modulating proinflammatory response. A role for Th1 response and macrophage activation in hematologic derangements elicited by A. phagocytophilum is not supported by these data and remains unproven.
INTRODUCTION
Anaplasma phagocytophilum is an obligate intracellular alpha- proteobacterium identified as the causative agent of granulocytic anaplasmosis (GA) in humans (HGA) and equines (EGA) (11, 37). A great diversity in clinical disease presentations exists among humans and animals infected by A. phagocytophilum, ranging from asymptomatic or subclinical infection to severe inflammatory syndromes, opportunistic infections, and death (10, 11). Disease in humans and some animals presents acutely with sudden onset of fever, accompanied by headache, myalgia, and malaise in most human infections and by fever, anorexia, depression, limb edema, icterus, and ataxia in horses (10, 20, 23). Both humans and horses develop similar hematologic abnormalities, including thrombocytopenia, leukopenia, and evidence of mild or moderate hepatic injury (20). In fact, histopathologic findings among humans, horses, and other infected animals bear remarkable similarities, underscoring the concept of conserved pathogenetic mechanisms among infected mammals (13, 14, 20). However, the pathogenetic mechanisms underlying disease are incompletely understood.
Early studies of humans linked disease severity with pathogen burden and preexisting complicating diseases (3, 6). How-ever, murine models and recent human studies implicate host immune responses, chiefly gamma interferon (IFN-γ), interleukin-12 (IL-12), and IL-10 as critical factors in disease and both clinical and histopathologic severity (5, 10, 25, 26). Based upon these studies, we hypothesized that host immune response to infection, chiefly the balance of pro- and anti-inflammatory responses, is a critical factor in both clinical disease manifestations and hematologic abnormalities observed with GA. In this study we used the GA horse model to evaluate the effect of pharmacological suppression of proinflammatory pathways, including IFNG, IL12B, and IL-10 transcription, by dexamethasone on the severity of clinical signs, leukopenia, and thrombocytopenia.
MATERIALS AND METHODS
Cultivation of A. phagocytophilum in vitro.
A. phagocytophilum was cultivated in vitro as previously described (8). Briefly, low-passage (<10 in vitro passages) A. phagocytophilum strain Webster was propagated in HL-60 cells in RMPI 1640 medium supplemented with 5% fetal bovine serum (FBS) and 2 mM l-glutamine until heavily infected (>95% of cells in culture). When heavily infected, cells were harvested, and a total of 106 infected cells were used for initial equine infections.
Horses.
Thirteen healthy adult horses confirmed seronegative for exposure to A. phagocytophilum and lacking A. phagocytophilum DNA in peripheral blood by real-time quantitative PCR were obtained from the Center for Equine Heath, UC Davis. All studies were approved by the Institutional Animal Care and Use Committees of The Johns Hopkins University School of Medicine and the University of California, Davis.
In vivo adaptation of in vitro-propagated A. phagocytophilum.
Our pilot studies confirmed that inoculation of horses with culture-derived A. phagocytophilum does not reproducibly cause virulent disease as observed with some natural infections, but that brief passage in vivo by intravenous administration to horses reproducibly restores virulence (37). Thus, we inoculated one horse with 106 A. phagocytophilum-infected HL-60 cells propagated in vitro by intravenous injection and observed for onset of fever, morulae in neutrophils, and A. phagocytophilum DNA in peripheral blood. Upon detection of infection on day 13 postinoculation, 120 ml of fresh acid citrose dextran (ACD)-anticoagulated peripheral blood was transfused into a second horse. Upon evidence of fever and PCR detection of A. phagocytophilum, this animal served as the source of inoculum for the remaining study horses.
Experimental infection.
Eight horses were infected intravenously with 18 ml of whole ACD-anticoagulated peripheral blood from the second experimentally infected horse. Four of the 8 horses (group 1) received 40 mg of dexamethasone on days 4 to 8 after infection, and the remaining 4 (group 2) did not. The inoculum was calculated to contain approximately 106 infected cells (1.14 × 106 infected neutrophils), in accordance with prior experimental infections in horses (29, 31, 37). Clinical assessments were conducted on days 0, 2, 4, 5, 6, 7, 9, and 12, and limb edema was also evaluated on days 14, 16, and 18. Blood samples for A. phagocytophilum quantitative bacteremia by real-time PCR and cytokine/chemokine gene transcription in peripheral blood cells were obtained on days 0, 4, 7, 9, and 12; routine hematologic evaluation was conducted as well on these days, including day 6. The study was terminated on day 18, when infection and clinical signs had resolved in all animals.
Control horses.
Four additional horses were mock infected with 18 ml of whole equine blood from a healthy A. phagocytophilum PCR-negative donor horse. Two mock-infected horses received 40 mg of dexamethasone on days 4 to 8 (group 3), and the remaining 2 (group 4) did not.
Quantitative A. phagocytophilum bacteremia.
In order to assess the overall bacteremia with A. phagocytophilum, two methods were employed. First, routine Wright's stained blood smears were assessed for at least 100 neutrophils to determine the proportion containing A. phagocytophilum morulae, and this value was used to determine the absolute infected neutrophil count. Likewise, direct bacterial burden in blood was determined by quantitative PCR targeting the 5′ conserved region of A. phagocytophilum msp2, a gene with >100 paralogous copies in the A. phagocytophilum HZ strain genome (30, 31, 37), with all results normalized to those for total-blood DNA. For this, total bacteremia was calculated based on a comparison of threshold cycle (CT) values from a standard curve of DNA obtained from known bacterial quantities (37). The values were examined by comparing treated and untreated horses on the same days, and total bacteremia over the course of the infection was calculated by determining the area under the curve for each group. Differences at individual days postinfection were assessed using one-sided, two-sample, unequal-variance Student t tests, with a P value of <0.05 considered significant. For overall quantitative bacteremia, the area under the curve was calculated for each condition and compared using the χ2 test, with a P value of <0.05 considered significant.
Clinical and hematologic assessment.
Blood was obtained from horses on days 0, 4, 6, 7, 9, and 12 by jugular vein venipuncture. Complete blood counts were determined using an automated hematology analyzer (Advia 120; Bayer Corporation, Norwood, MA) to characterize laboratory features of infection (Veterinary Medical Teaching Hospital, UC Davis). Clinical measurements of severity included the following: lethargy, ranging from slightly quiet and less responsive to obtunded (grade 1 to 4); reluctance to move, ranging from slow walking with leading to refusal to walk even with leading (grade 1 to 4); anorexia, ranging from slight to severe (grade 1 to 4); petechiae and icterus, ranging from slight to severe (grade 1 to 4); limb edema, ranging from slight congestion above the fetlock to edema above and below the carpus and hock (grade 1 to 4); and ataxia, graded as present or absent. These features were evaluated on days 0, 2, 4, 5, 6, 7, 9, and 12. Limb edema was observed additionally on days 14, 16, and 18. Severity values were ranked and assessed using repeated measures of analysis of variance (ANOVA) with a Bonferroni correction, with a P value of <0.05 considered significant. Observations were made by a single equine veterinarian (J.E.M.) with more than 40 years of experience, who was not blinded to treatment with dexamethasone.
Cytokine gene transcription measurements.
Transcription of cytokine genes among peripheral blood cells was assessed by real-time reverse transcriptase qPCR on days 0, 4, 7, 9, and 12 of infection. Total cellular RNA was isolated using the QIAamp RNA blood minikit (Qiagen, Inc., Valencia, CA) from the whole peripheral blood of A. phagocytophilum- and mock-infected horses as described previously (15); ex vivo antigenic stimulation was not used. Equine cytokine transcripts analyzed included IL-4, IL-8, IL-10, IL-12B (IL-12 p40 subunit gene), IL-18, IFNG, and CCL5 (RANTES) (32, 33). Quantitation was performed using the ΔΔCT method and reported as relative transcription or the n-fold difference relative to the cDNA calibrator (22). For comparative purposes, transcription was normalized to that observed on day 0 for each horse; for infected horses, transcription was further normalized to account for changes in uninfected controls. Differences in transcription were assessed using repeated-measure ANOVA with a Bonferroni correction, with a P value of <0.05 considered significant.
RESULTS
A. phagocytophilum PCR and microscopic quantitation.
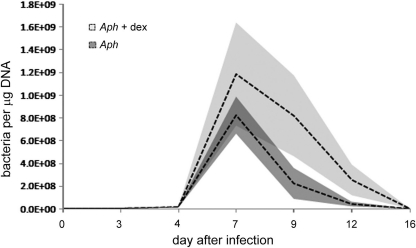
All horses inoculated with A. phagocytophilum were confirmed infected by real-time qPCR on days 4 through 12, with peak pathogen burden on days 7 and 9; infection was also confirmed by microscopic examination of peripheral blood smears. Although the absolute number of infected neutrophils was higher on days 7 through 12 for the dexamethasone-treated horses, significant differences in the A. phagocytophilum load did not exist when individual days were compared. However, the bacterial burden over the entire course of infection was higher for dexamethasone-treated than for untreated horses (P = 0.022, χ2 test) (Fig. 1). Peak bacteremia coincided with nadirs of both leukocyte and platelet counts and coincided with or preceded peak severity of limb edema, anorexia, icterus, and reluctance to move.
Fig. 1.
Anaplasma phagocytophilum peripheral blood load, as determined by quantitative PCR and normalized to total-blood DNA content in untreated and dexamethasone-treated horses. The dashed lines represent the mean values at each time point; the shaded areas delineate the standard error of the mean (SEM) for the means for each group. Although bacterial loads did not differ between the groups at individual days postinfection, the overall bacterial load (area under the curve) was significantly higher for dexamethasone-treated than for untreated A. phagocytophilum-infected horses (P = 0.022, χ2 test).
Clinical findings.
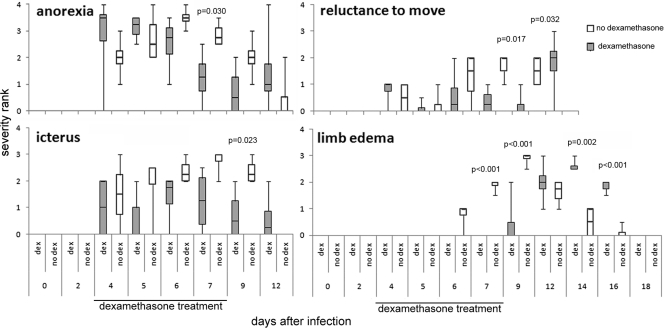
All infected horses manifested signs of disease regardless of treatment. By day 4, signs, including moderate anorexia, lethargy, reluctance to move, and icterus, appeared. Limb edema and ataxia appeared by day 6. The most dramatic difference between treated and untreated infected horses was a delay in limb edema with dexamethasone, as evident with lower edema scores for treated horses on days 6 to 9, reversing on days 14 to 16 (Fig. 2) (P < 0.001). In addition, other clinical conditions were worse for untreated horses on days 6 through 9, including anorexia (P ≤ 0.029), icterus (P ≤ 0.029), and reluctance to move (P ≤ 0.029); fever was worse on day 7 (P < 0.001; Fig. 2). Ataxia did not differ between the treatment groups.
Fig. 2.
Clinical severity of infection in untreated horses was worse or accelerated compared with these features in dexamethasone-treated horses after experimental infection with A. phagocytophilum. The median value is shown by the central bar within each box; the 1st and 3rd quartiles are delineated by the boxes, and the error bars show the maximum and minimum values after ranking. Significant differences between the treatment groups are illustrated by P values above comparisons for each graph.
Hematologic parameters.
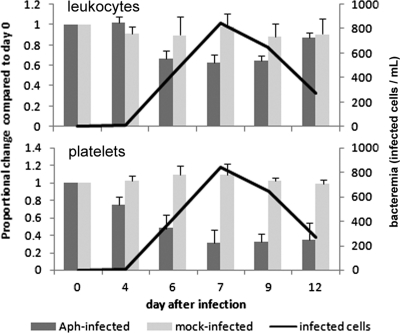
As anticipated from a comparison with equine reference intervals, all infected horses developed thrombocytopenia, leukopenia, and anemia by day 6 of infection; uninfected horses did not develop any hematologic abnormalities. Infected horses developed marked thrombocytopenia on days 6 through 12 (P < 0.001) (mean, 58,770 μl−1; reference interval, 100,000 to 225,000 μl−1), anemia on days 4 through 7 (P = 0.038) (mean, 25%; reference interval, 30 to 46%), leukopenia on days 6 through 9 (P = 0.009) (mean, 4,490 μl−1; reference interval, 5,000 to 11,600 μl−1), and lymphopenia on days 4 through 9 (P < 0.014) (mean, 789 μl−1; reference interval, 1,600 to 5,800 μl−1). There were no significant differences between dexamethasone-treated and untreated horses, with the exception of suppressed peripheral blood eosinophil counts in dexamethasone-treated horses on day 9 only (P = 0.018). The nadirs of thrombocytopenia and leukopenia corresponded closely with times of peak bacteremia (Fig. 3).
Fig. 3.
A. phagocytophilum infection induced significant leukopenia and thrombocytopenia for which nadirs corresponded to peak bacteremia regardless of dexamethasone treatment. The bars (left axis) depict leukocyte or platelet count changes normalized to day 0 values; the line (right axis) shows the level of bacteremia determined by microscopic counts of morulae-containing neutrophils in peripheral blood of infected (Aph-infected) horses only.
Cytokine gene transcriptional changes. (i) Mock-infected horses.
Compared to untreated horses on the same day, dexamethasone did not alter transcription of IFNG, IL-10, IL-12B, IL-18, IL-4, or CCL5. Only IL-12B transcription was decreased more on day 4 in untreated horses than in treated horses post-mock infection (P = 0.024). To determine the stability of cytokine gene transcription, we compared responses on days 4, 7, 9, and 12 to those on day 0. Measurements did not significantly differ over time except for changes in CCL5 and IL-4 on day 9, immediately after termination of dexamethasone treatment, and for IL-10 and the ratio of IL-10 to IFNG on day 12.
(ii) A. phagocytophilum-infected horses.
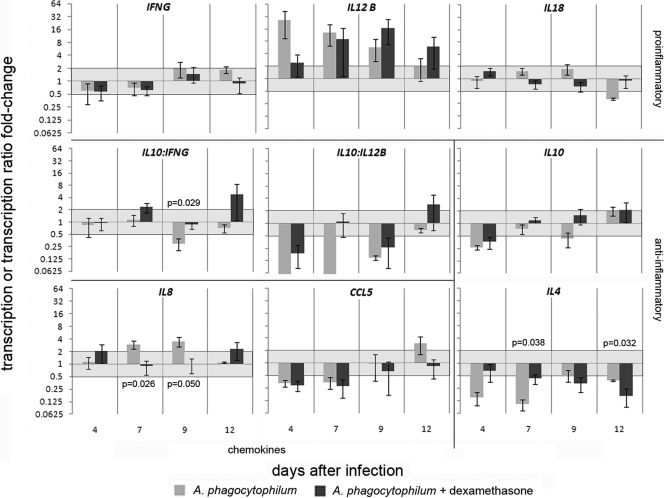
Because transcription levels in mock-infected animals varied and were not directly related to dexamethasone treatment, cytokine gene transcription in infected horses was normalized to that in mock-infected horses to more precisely identify dexamethasone effects on infection-related cytokine gene expression. Infection had several broad effects on cytokine gene transcription in peripheral blood (Fig. 4), including a generalized increase in IL-12B, IL-18, and IL-8 and reduction in IL-4 and CCL5. After infection, regardless of dexamethasone treatment, transcription was upregulated 2- to 27-fold for IL-12B and 2-fold for IL-8 and downregulated 2- to 4-fold for IL-4 (P < 0.008), 3-fold for CCL5 (P < 0.001), and 3-fold for IL-10 (P < 0.001). Dexamethasone treatment abrogated the initial 9.5-fold downregulation of IL-4 transcription in infected horses on days 7 and 12 to 2.3-fold (P = 0.038); this was followed by suppressed IL-4 transcription at day 12 (6.0- versus 2.6-fold; P = 0.032), after dexamethasone treatment was terminated. Similarly, 2.9- to 3.5-fold increases in IL-8 transcription on days 7 to 9 were suppressed by dexamethasone (P = 0.026 and 0.050, respectively) (Fig. 4). Dexamethasone treatment resulted in increasingly higher transcription ratios of IL-10 to IFNG (IL-10 transcript excess) over the course of infection, although this was significant only on day 9 (P = 0.029). Although the ratio of IL-10 to IL-12B was higher in dexamethasone-treated horses at every interval, the differences were not statistically significant in this small group.
Fig. 4.
Cytokine and chemokine transcription in peripheral blood with experimental A. phagocytophilum infection compared to mock-infected controls differs modestly between untreated and dexamethasone-treated horses. A. phagocytophilum infection in untreated horses (gray bars) induced a proinflammatory response with reduced IL-4 and IL-10 transcription and abundant IL-12 and modest IFNG transcription. Dexamethasone treatment during infection (black bars) dampened or delayed IL-12B and IL-18 transcription and abrogated the suppressed IL-4 transcription, resulting in altered ratios of IL-10 to IFNG and IL-10 to IL-12B throughout most of the infection. Significant differences (repeated-measure ANOVA; P < 0.05) in transcription between untreated and dexamethasone-treated horses are denoted by P values. The lines between panels delineate groups of proinflammatory or anti-inflammatory cytokine or chemokine gene transcriptional responses or ratios of selected responses. The light-gray zones delineate the 2-fold up- and downregulation limits compared to those of mock-infected controls.
Taken as a group, the changes in proinflammatory cytokine gene (IFNG, IL-12B, and IL-18) transcription were not significantly altered by the 4-day course of dexamethasone, and anti-inflammatory cytokine gene (IL-4 and IL-10) transcription was significantly lower only in the absence of dexamethasone at day 7 (ANOVA). However, when the ratios of IL-10 transcriptional change to IFNG and IL-12B transcriptional change were considered together, higher ratios that correspond to a lower overall proinflammatory response on both days 7 and 9 (P = 0.029, ANOVA), most likely impacted by the dexamethasone treatment on days 4 to 8, were observed.
DISCUSSION
The mechanisms by which A. phagocytophilum causes tissue injury and disease are unclear. Only limited data demonstrate any link between pathogen load and disease severity, and recent evaluations of infection in mice and in humans clearly establish links between immune response and histopathology or disease manifestations and severity (5, 7, 8, 10, 26). The chief immunological marker is IFN-γ, which when depleted results in a marked increase in pathogen load but complete loss of tissue lesions in mice (1, 26). In contrast, infection in mice that lack IL-10, a cytokine that suppresses IFN-γ expression, results in markedly worsened tissue lesions but no alteration in pathogen load (26). These findings indicate that in mice, A. phagocytophilum triggers a polarized response that favors inflammatory tissue injury and activation of macrophage effector functions (5, 8, 10, 35, 36, 38). In humans with A. phagocytophilum infection, moderate or high serum IFN-γ and IL-10 levels are observed, and a high ratio of IL-10 to IFN-γ is associated with increased disease severity, a likely reflection of the extent to which pro- and anti-inflammatory signaling occurs (10). In addition, serum IL-12, ferritin, and triglyceride levels correlate with increased disease severity, further reflecting the downstream activation of macrophages by the proinflammatory response and the association of this feature with a disease phenotype.
Unfortunately, the murine model suffers from the lack of clinical disease signs. To circumvent this shortfall in an experimental setting, horses offer the advantage that they develop a disease very similar to that in humans and share underlying histopathology after A. phagocytophilum infection with both experimentally infected mice and naturally infected humans (4, 21, 24). Among several disadvantages of the horse model, the inability to experimentally manipulate discrete immunological responses is potentially problematic. Here, we took advantage of the anecdotal observation that treatment of horses with dexamethasone results in a phenotype similar to that observed with IFN-γ knockout mice, in which blood pathogen loads increase substantially and disease manifestations seem curtailed. Dexamethasone has broad immunological effects, but major targets of its action include transrepression of NF-κB and AP-1 transcriptional activation by ligated glucocorticoid receptors and regulation of STAT-1-modulated IFNG transcription and IFN-γ expression and signaling (16, 19, 28, 34).
Here we confirm some observations with murine models of GA which show that disease severity is lessened with attenuated host inflammatory and immune responses to A. phagocytophilum infection (26, 35, 38). Moreover, these data extend the observations with murine models by documenting the association of worsened clinical disease manifestations, such as anorexia, limb edema, icterus, reluctance to move, and fever with unmodified inflammatory response despite an overall lower bacterial burden. A feature of this analysis shows that IFNG transcription in peripheral blood is only marginally altered by dexamethasone treatment after established infection and that the only time point at which significant differences in IFNG transcription are detected is after the key differences in clinical features are observed, raising questions about the association of these features. However, assessment of cytokine responses among circulating leukocytes is not an absolute surrogate of responses or their magnitude in mononuclear phagocyte organs or at sites of inflammation. Perhaps subsequent experiments could better assess this possibility with ex vivo stimulation of peripheral blood elements by A. phagocytophilum, a situation that would mimic contact of immune cells with the pathogen in tissues. Moreover, proinflammatory responses attributable to IFN-γ signaling are amplified after priming or by feed-forward loops even when IFN-γ concentrations are low and are counterbalanced by the effects of anti-inflammatory signaling of IL-10 and similar cytokines (17). Thus, these data could be underestimations, may not be reflective of local immunological responses, or could indicate more important roles for IL-12, IL-8, or other cytokines in horses. In contrast, the relationship between cytokine profiles that conform to Th1 proinflammatory or anti-inflammatory/regulatory responses, as reflected by transcription ratios of IL-10 to IFNG and IL-10 to IL-12B (9), is associated with the temporal occurrence of histopathologic lesions or clinical signs, as previously shown with mice and with humans with increased disease severity (10, 26). Proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, are known to synergistically activate macrophages; however, the inability to detect significant quantities of these factors in humans and animal models of A. phagocytophilum infection argued against their further study (10, 12, 25). In sum, A. phagocytophilum infection toggles the host response to a proinflammatory, macrophage-activating environment associated with histopathologic injury and clinical disease. In contrast, partial treatment with dexamethasone interferes with events, perhaps transcriptional, interwoven into the proinflammatory host response and tip its bias toward that often observed with the regulation of inflammation, dominated by suppressed proinflammatory cytokines and the presence of anti-inflammatory IL-10 (9, 27).
Interestingly, modulation of cytokine and inflammatory/innate immune responses did not substantially impact any of the hematologic changes associated with A. phagocytophilum, including leukopenia, thrombocytopenia, or anemia. This finding seems to contradict our prior hypothesis that such hematologic alterations in HGA are the result of IFN-γ-mediated macrophage activation, at least to the degree that such was modified by dexamethasone treatment after infection. This hypothesis posited that the predominant mechanism of pancytopenia involves macrophage activation by IFN-γ, leading to nonspecific extramedullary destruction of platelets and leukocytes after excessive or unregulated nonspecific phagocytosis or induction of inflammatory mediators within mononuclear phagocyte organs (2). However, here it must be noted that despite the inability to discern a linkage between the hematologic alterations and suppressed cytokine production, the dexamethasone treatment regimen used was limited to 5 days and was started only after onset of detectable bacteremia at a time when initiation of cytokine cascades that determine macrophage activation likely already occurred. Thus, further study will be needed to accurately determine the relationship between macrophage activation after cytokine stimulation and the development of pancytopenia in A. phagocytophilum infections. In contrast, Klein et al. proposed that the induced production of myelosuppressing cytokines and chemokines detected in vitro with A. phagocytophilum could account for the typical hematologic changes (18). The lack of an association between the alterations in IL-8 or CCL5 transcription and leukopenia, anemia, or thrombocytopenia provides evidence to refute this hypothesis.
The ramifications of these findings are potentially important for management of the severe consequences of A. phagocytophilum infection, since control of bacterial load appears to be only one consideration for minimizing disease, especially among the 7% or more with severe infections (10, 11). Rather, adjunctive measures that diminish inflammatory cascade induction, especially those that lead to macrophage activation, should be considered when disease becomes more severe, as with multiorgan involvement or acute respiratory distress syndrome. Additionally, as consideration is given to vaccines for animals or humans, it is imperative that the constructs used be carefully evaluated to avoid the triggering of such inflammatory sequelae (9). To better understand how this could be accomplished, studies will be needed to determine which components of A. phagocytophilum infection trigger the response (7), which cells in the host are predominantly responsible (8), and why some but not all individuals develop more profound inflammatory or innate immune responses that accompany or lead to severe inflammatory disease manifestations (10). Given the involvement of macrophage activation and the Th1 cytokine cascade, much attention should be given to the stimulation of this process by cytokines, such as IFN-γ, by innate immune subsets, such as NK and NK T lymphocytes, and control of the process by cytolytic or regulatory lymphocytes whose function is also in part down-modulation of macrophage activation.
ACKNOWLEDGMENTS
This study was funded by R01 AI41213 and R56 AI41213 from The National Institutes of Allergy and Infectious Diseases to J.S.D.
We acknowledge D. Grab for his careful comments and reading of the manuscript.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Akkoyunlu M., Fikrig E. 2000. Gamma interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect. Immun. 68:1827–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arceci R. J. 2008. When T cells and macrophages do not talk: the hemophagocytic syndromes. Curr. Opin. Hematol. 15:359–367 [DOI] [PubMed] [Google Scholar]
- 3. Bakken J. S., et al. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272:212–218 [PubMed] [Google Scholar]
- 4. Barlough J. E., Madigan J. E., DeRock E., Dumler J. S., Bakken J. S. 1995. Protection against Ehrlichia equi is conferred by prior infection with the human granulocytotropic Ehrlichia (HGE agent). J. Clin. Microbiol. 33:3333–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Browning M. D., Garyu J. W., Dumler J. S., Scorpio D. G. 2006. Role of reactive nitrogen species in development of hepatic injury in a C57bl/6 mouse model of human granulocytic anaplasmosis. Comp. Med. 56:55–62 [PubMed] [Google Scholar]
- 6. Chen S. M., Dumler J. S., Bakken J. S., Walker D. H. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi K. S., Dumler J. S. 2007. Mitogenic component in polar lipid-enriched A. phagocytophilum membranes. Clin. Vaccine Immunol. 14:1260–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi K. S., Scorpio D. G., Barat N. C., Dumler J. S. 2007. Msp2 variation in Anaplasma phagocytophilum in vivo does not stimulate T cell immune responses or interferon-gamma production. FEMS Immunol. Med. Microbiol. 49:374–386 [DOI] [PubMed] [Google Scholar]
- 9. Couper K. N., Blount D. G., Riley E. M. 2008. IL-10: the master regulator of immunity to infection. J. Immunol. 180:5771–5777 [DOI] [PubMed] [Google Scholar]
- 10. Dumler J. S., Barat N. C., Barat C. E., Bakken J. S. 2007. Human granulocytic anaplasmosis and macrophage activation. Clin. Infect. Dis. 45:199–204 [DOI] [PubMed] [Google Scholar]
- 11. Dumler J. S., et al. 2005. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 11:1828–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dumler J. S., Trigiani E. R., Bakken J. S., Aguero-Rosenfeld M. E., Wormser G. P. 2000. Serum cytokine responses during acute human granulocytic ehrlichiosis. Clin. Diagn. Lab. Immunol. 7:6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foley J. E., Lerche N. W., Dumler J. S., Madigan J. E. 1999. A simian model of human granulocytic ehrlichiosis. Am. J. Trop. Med. Hyg. 60:987–993 [DOI] [PubMed] [Google Scholar]
- 14. Foley J. E., Leutenegger C. M., Dumler J. S., Pedersen N. C., Madigan J. E. 2003. Evidence for modulated immune response to Anaplasma phagocytophila sensu lato in cats with FIV-induced immunosuppression. Comp. Immunol. Microbiol. Infect. Dis. 26:103–113 [DOI] [PubMed] [Google Scholar]
- 15. Hodzic E., et al. 2001. Infection of mice with the agent of human granulocytic ehrlichiosis after different routes of inoculation. J. Infect. Dis. 183:1781–1786 [DOI] [PubMed] [Google Scholar]
- 16. Honda K., Taniguchi T. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6:644–658 [DOI] [PubMed] [Google Scholar]
- 17. Hu X., Chakravarty S. D., Ivashkiv L. B. 2008. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol. Rev. 226:41–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klein M. B., Hu S., Chao C. C., Goodman J. L. 2000. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 182:200–205 [DOI] [PubMed] [Google Scholar]
- 19. Kracht M., Saklatvala J. 2002. Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine 20:91–106 [DOI] [PubMed] [Google Scholar]
- 20. Lepidi H., et al. 2000. Comparative pathology and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 62:29–37 [DOI] [PubMed] [Google Scholar]
- 21. Lepidi H., et al. 2004. Cardiac valves in patients with Whipple endocarditis: microbiological, molecular, quantitative histologic, and immunohistochemical studies of 5 patients. J. Infect. Dis. 190:935–945 [DOI] [PubMed] [Google Scholar]
- 22. Leutenegger C. M., et al. 1999. Quantitative real-time PCR for the measurement of feline cytokine mRNA. Vet. Immunol. Immunopathol. 71:291–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madigan J. E., Gribble D. 1987. Equine ehrlichiosis in northern California: 49 cases (1968-1981). J. Am. Vet. Med. Assoc. 190:445–448 [PubMed] [Google Scholar]
- 24. Madigan J. E., et al. 1995. Transmission and passage in horses of the agent of human granulocytic ehrlichiosis. J. Infect. Dis. 172:1141–1144 [DOI] [PubMed] [Google Scholar]
- 25. Martin M. E., Bunnell J. E., Dumler J. S. 2000. Pathology, immunohistology, and cytokine responses in early phases of human granulocytic ehrlichiosis in a murine model. J. Infect. Dis. 181:374–378 [DOI] [PubMed] [Google Scholar]
- 26. Martin M. E., Caspersen K., Dumler J. S. 2001. Immunopathology and ehrlichial propagation are regulated by interferon-gamma and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am. J. Pathol. 158:1881–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller J. C., Ma Y., Crandall H., Wang X., Weis J. J. 2008. Gene expression profiling provides insights into the pathways involved in inflammatory arthritis development: murine model of Lyme disease. Exp. Mol. Pathol. 85:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newton R., Holden N. S. 2007. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol. Pharmacol. 72:799–809 [DOI] [PubMed] [Google Scholar]
- 29. Pusterla N., et al. 2002. Transmission of Anaplasma phagocytophila (human granulocytic ehrlichiosis agent) in horses using experimentally infected ticks (Ixodes scapularis). J. Vet. Med. B Infect. Dis. Vet. Public Health 49:484–488 [DOI] [PubMed] [Google Scholar]
- 30. Pusterla N., et al. 1999. Quantitative real-time PCR for detection of members of the Ehrlichia phagocytophila genogroup in host animals and Ixodes ricinus ticks. J. Clin. Microbiol. 37:1329–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pusterla N., et al. 1999. Quantitative evaluation of ehrlichial burden in horses after experimental transmission of human granulocytic Ehrlichia agent by intravenous inoculation with infected leukocytes and by infected ticks. J. Clin. Microbiol. 37:4042–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pusterla N., et al. 2006. Cytokine gene signatures in neural tissue of horses with equine protozoal myeloencephalitis or equine herpes type 1 myeloencephalopathy. Vet. Rec. 159:341–345 [DOI] [PubMed] [Google Scholar]
- 33. Pusterla N., Wilson W. D., Conrad P. A., Mapes S., Leutenegger C. M. 2006. Comparative analysis of cytokine gene expression in cerebrospinal fluid of horses without neurologic signs or with selected neurologic disorders. Am. J. Vet. Res. 67:1433–1437 [DOI] [PubMed] [Google Scholar]
- 34. Rogatsky I., Ivashkiv L. B. 2006. Glucocorticoid modulation of cytokine signaling. Tissue Antigens 68:1–12 [DOI] [PubMed] [Google Scholar]
- 35. Scorpio D. G., Akkoyunlu M., Fikrig E., Dumler J. S. 2004. CXCR2 blockade influences Anaplasma phagocytophilum propagation but not histopathology in the mouse model of human granulocytic anaplasmosis. Clin. Diagn. Lab. Immunol. 11:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scorpio D. G., Von Loewenich F. D., Bogdan C., Dumler J. S. 2005. Innate immune tissue injury and murine HGA: tissue injury in the murine model of granulocytic anaplasmosis relates to host innate immune response and not pathogen load. Ann. N. Y. Acad. Sci. 1063:425–428 [DOI] [PubMed] [Google Scholar]
- 37. Scorpio D. G., et al. 2008. Sequential analysis of Anaplasma phagocytophilum msp2 transcription in murine and equine models of human granulocytic anaplasmosis. Clin. Vaccine Immunol. 15:418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scorpio D. G., von Loewenich F. D., Gobel H., Bogdan C., Dumler J. S. 2006. Innate immune response to Anaplasma phagocytophilum contributes to hepatic injury. Clin. Vaccine Immunol. 13:806–809 [DOI] [PMC free article] [PubMed] [Google Scholar]