Abstract
Primary cytomegalovirus (CMV) infection of the mother during pregnancy presents risk of CMV infection of the fetus with resulting permanent disability. CMV IgM antibody is generated following primary CMV infection but also can appear during nonprimary CMV infection and is thus of limited diagnostic use by itself. In contrast, the presence of low CMV IgG avidity has been shown to be a unique and reliable serologic indicator of primary CMV infection. We measured CMV IgG and IgM antibody levels and IgG avidity in sera from a population sample of 6,067 U.S. women aged 12 to 49 years from NHANES (National Health and Nutrition Examination Survey). The CMV IgG prevalence was 58% overall and increased strongly with age. The CMV IgM prevalence was 3.0% overall and remained relatively flat across age groups. The prevalence of low IgG avidity was 2.0% overall, decreased sharply with age, and was seen mainly among IgM-positive sera. Fourteen to 18% of the CMV IgM-positive sera were low IgG avidity, presumably representing primary CMV infection. High CMV IgM antibody titer was a strong predictor of low IgG avidity. The ability to reliably identify primary CMV infection during pregnancy is important for management of the pregnancy, including possible treatment options for the fetus. Both IgM and IgG avidity measurements provide useful clinical information for evaluating primary CMV infection, although commercial tests for CMV IgG avidity are not yet widely available in the United States.
INTRODUCTION
Human cytomegalovirus (CMV) is a ubiquitous virus that rarely causes disease in healthy individuals yet can cause serious disease in the fetus and in immunosuppressed individuals. Fetal infection is the greatest public health burden associated with CMV, occurring in 0.7% of overall live births worldwide with 15 to 20% of infected infants having permanent disability, including hearing loss, visual impairment, and cognitive deficit (8, 12). The most severe outcomes of congenital CMV infection tend to result from primary (i.e., first) CMV infection of the mother during pregnancy that leads to intrauterine transmission to the fetus (9, 24). Thus, accurate diagnosis of primary infection versus nonprimary infection (i.e., reactivation or reinfection with a different strain) during pregnancy provides important information for clinical management (1–3, 7) and would allow for the possibility of prenatal treatment (19).
Unequivocal diagnosis of primary CMV infection is achieved with documented CMV IgG seroconversion. However, since women are not routinely tested for CMV antibody, documentation of seroconversion is rare. When only one serum specimen is available, several parameters of the serologic response to viral infection can be helpful for diagnosis of primary infection, including the class (IgM versus IgG), concentration, and avidity of the antibodies produced. The transient presence of specific IgM antibody has long been used as a diagnostic marker for primary CMV infection, but IgM can also be present during viral reactivation or reinfection (18, 20) and so is not unique to primary infection. In contrast to IgM, low-avidity IgG is present only with primary infection, increasing over 3 to 5 months to high avidity (11, 14), a process that is referred to as maturation of the humoral immune response. IgG avidity has thus gained diagnostic importance in identifying primary CMV infection, mainly in Europe, where several commercial CMV avidity tests are available (2, 10, 15, 16, 20). Several groups have reported substantial improvements in the identification of at-risk pregnancies using diagnostic algorithms that incorporate both IgG avidity and IgM measurements (16, 17).
We measured CMV IgM antibody and IgG avidity in sera from 6,067 nationally representative U.S. women aged 12 to 49 years to determine the prevalence, demographic trends, and risk factors for these serologic measures. To our knowledge, there have been no previous studies of IgM and IgG avidity conducted in a large population-based sample of the United States. A secondary purpose of the study included testing of additional sera not from the NHANES (National Health and Nutrition Examination Survey) collection to examine whether parameters of IgM antibody measurements were associated with low IgG avidity, since CMV IgG avidity testing is not yet widely available in the United States.
MATERIALS AND METHODS
Study specimens and CMV IgG/IgM antibody testing.
This research has complied with all relevant federal guidelines and the CDC Institutional Review Board. CMV IgG was measured using the SeraQuest enzyme immunoassay (EIA; Miami, FL) together with the Vidas (bioMérieux Vitek Inc., Durham, NC) automated test as described by Staras et al. (25). We performed IgG determination on all available sera (n = 22,000) from the nationally representative National Health and Nutrition Examination Survey III (NHANES III). CMV IgM was measured using the Vidas test. We measured IgM in women of childbearing age by testing all available samples in NHANES from 12- to 49-year-old females (n = 6,067). The Vidas instrument performs an enzyme-linked fluorescent immunoassay that provides semiquantitative universal arbitrary units per milliliter (UA/ml) comparable to optical density (OD) of other automated serology tests. We performed endpoint serial dilutions on 6 CMV IgM-positive sera from the NHANES collection with various levels of antibody to confirm that the UA/ml approximates antibody titer for the range shown (see Fig. 1).
Fig. 1.
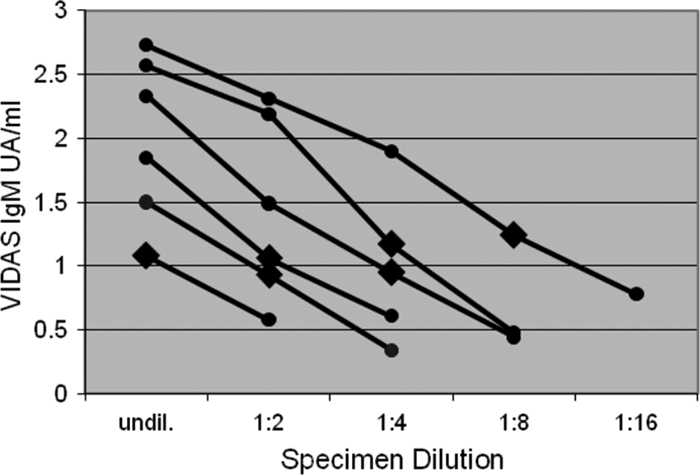
Relationship between CMV IgM antibody levels measured by UA/ml and endpoint antibody titer. Twofold serial dilutions were performed on six IgM-positive sera from the NHANES collection. UA/ml decreases approximately linearly with dilution. Sera with higher initial UA/ml values have higher endpoint titers, shown by enlarged black diamonds. The Vidas test cutoff for IgM positivity is 0.90 UA/ml.
For the comparison of IgM antibody levels and IgG avidities shown in Fig. 2 and Table 4, we used 270 NHANES sera plus an additional 54 deidentified CMV IgM-positive sera from other U.S. serological surveys because the NHANES serum collection lacked sufficient numbers of IgM-positive sera to perform the analysis. The 54 additional sera were chosen to span the positive range of the IgM test without prior knowledge of their IgG avidity status.
Fig. 2.
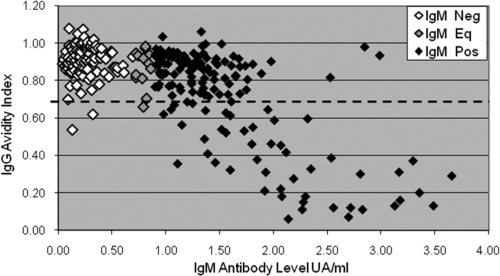
Association between CMV IgM antibody levels and CMV IgG avidity. CMV IgM-negative (white), IgM-equivocal (gray), and IgM-positive (black) serum specimens are plotted according to their IgM antibody level measured by UA/ml and their IgG avidity index. The assay cutoff for IgM positivity is 0.9 UA/ml. The avidity test cutoff used for this analysis was 0.7, marked by the dashed line.
Table 4.
Association between CMV IgM antibody level and low IgG aviditya
| IgM UA/ml | No. of sera (total = 324) | % low avidity |
|---|---|---|
| IgM negative or equivalent, <0.90 | 154 | 1.9 |
| 0.90–1.09 | 50 | 6.0 |
| 1.10–1.29 | 26 | 15.4 |
| 1.30–1.49 | 29 | 17.2 |
| 1.50–1.99 | 32 | 40.6 |
| ≥2.00–4.00 | 33 | 78.8 |
Sera are listed by increasing levels of IgM antibody, showing the proportion that were low IgG avidity. Chi-square test for trend, P < 0.0001. The 0.7 avidity test cutoff was used to define low IgG avidity.
CMV IgG avidity testing.
The Vidas test from bioMérieux was used for IgG avidity testing. All sera from the NHANES collection that were CMV IgG and IgM positive with sufficient volumes (n = 126) were tested, as were a similar number of IgG-positive/IgM-negative sera (n = 129) randomly selected from the total of 3,384 IgG-positive/IgM-negative sera. For measuring antibody avidity, two CMV-specific IgG tests were performed in parallel, a reference IgG determination and a second test in which 6 M urea was added to the wash buffer as a protein denaturing agent to weaken the antigen-antibody bond. The avidity index was calculated by dividing the optical density (OD) with urea by the OD of the reference sample. We analyzed the results using three different single-point cutoff values. The index value of 0.8 (≥0.8, high avidity suggestive of past infection; <0.8, low avidity suggestive of recent infection) in one study gave good separation of sera from past and recent infection (6) but is recommended by the manufacturer for separation of high avidity and intermediate avidity. Other avidity cutoff values analyzed were 0.7 (≥0.7, high avidity suggestive of past infection; <0.7, low avidity suggestive of recent infection) (1, 3); and 0.6 (≥0.6, high avidity suggestive of past infection; <0.6, low avidity suggestive of recent infection). Cutoff values recommended by the manufacturer of ≥0.8 for high avidity, <0.8 to 0.2 for intermediate (indeterminate) avidity, and <0.2 for low avidity were not analyzed because most of the test range assigns indeterminate avidity and because the <0.2 cutoff has been shown to be too low to identify many sera from acute CMV infection (1, 3, 6, 7, 23).
To test whether high IgG antibody titers can give false avidity values as reported by Dangel et al. (6), we performed 2-fold serial dilutions with 0.9% NaCl and repeated the avidity testing on 14 sera with high IgG antibody titers (7 with high avidity and 7 with low avidity) chosen from the NHANES collection based on antibody titer and sufficient volume remaining for additional testing.
Statistical methods.
Of the 7,235 NHANES III female participants aged 12 to 49 years, 6,067 (84%) had sera that were available for both CMV IgG and CMV IgM testing. Availabilities of sera were similar by race, age, and household income. We used SUDAAN software version 9.0 (RTI International) to account for the two-stage sample design and to incorporate sampling weights, allowing IgG and IgM estimates to represent the United States population. We estimated the IgG, IgM, and IgG avidity prevalences and 95% confidence intervals with direct standardization. All linear tests for trend were conducted with chi-square tests. Estimates for untested sera were not weighted, due to the assumptions that our tested sera represented our untested sera.
RESULTS
CMV IgG and IgM prevalence.
The overall CMV IgG seroprevalence in the study participants 12 to 49 years old was 58% and rose steadily by decade (Table 1). The CMV IgM seroprevalence was 3.0% and did not vary significantly by age (Table 1). All but 3 of the CMV IgM-positive samples were also CMV IgG positive. There was no evidence of an association between CMV IgM seropositivity and the age, race, or household income of study participants. Serial dilution of CMV IgM-positive sera confirmed that UA/ml values approximated antibody titer for the range shown. IgM UA/ml values decreased steadily with dilution, and sera with higher UA/ml values had higher endpoint antibody titers (Fig. 1).
Table 1.
CMV IgG and IgM prevalence estimates (weighted) by age among 5,992 women from the NHANES national surveya
| Age of women (yr) | No. tested | % positive for: |
|
|---|---|---|---|
| CMV IgG | CMV IgM | ||
| 12–19 | 1,525 | 47.3 | 2.6 |
| 20–29 | 1,643 | 54.4 | 4.5 |
| 30–39 | 1,616 | 59.7 | 2.3 |
| 40–49 | 1,208 | 69.8 | 2.4 |
| Total | 5,992 | 57.9 | 3.0 |
Three of the IgM+ sera were IgG−; the rest were IgG+.
Prevalence estimates for low-avidity IgG and trends with age.
All IgG avidity testing was conducted only with IgG-positive sera. In several previous studies, 90% or more of CMV low-IgG-avidity sera identified were CMV IgM positive (4, 14, 22); therefore, our avidity testing and analysis focused on the IgM-positive sera. Table 2 shows weighted prevalence estimates for low avidity by age among all available IgM-positive sera from the NHANES collection (n = 126) using three different avidity test cutoffs. The 0.80 test cutoff identified 31.1% of the IgM-positive sera as low avidity, showing a significant association between low avidity and young age (P = 0.004). The 0.70 test cutoff identified 13.5% of the IgM-positive sera as low avidity with a significant association with young age (P = 0.048). The 0.60 test cutoff identified 7.6% of the IgM-positive sera as low avidity and a similar association with young age though nonsignificant.
Table 2.
Prevalence estimates (weighted) for CMV IgG low avidity by age among the 126 IgM+ sera from the NHANES national survey
| Age of women (yr) | No. tested | Median % positive (95% CI) by test cutoff for low aviditya |
||
|---|---|---|---|---|
| 0.8 | 0.7 | 0.6 | ||
| 12–19 | 36 | 67.4 (35.3–88.6) | 33.0 (11.6–64.9) | 12.3 (2.9–40.1) |
| 20–29 | 52 | 30.7 (16.8–49.1) | 16.5 (7.1–33.8) | 10.4 (3.8–25.6) |
| 30–49 | 38 | 17.1 (7.1–35.7) | 3.1 (0.6–14.0) | 3.1 (0.6–14.0) |
| All ages | 126 | 31.1 (20.1–44.6) | 13.5 (6.1–27.5) | 7.6 (3.5–15.9) |
P values for trends with age were 0.004, 0.048, and 0.3 for cutoff values of 0.8, 0.7, and 0.6, respectively. 95% CI, 95% confidence intervals.
Table 3 shows unweighted prevalence estimates for low avidity including IgM-negative sera using three different avidity test cutoffs. To estimate low avidity for all of the NHANES sera, we made the assumption that the 129 tested and 3,384 untested IgM-negative sera had the same proportions of low-avidity sera. We did not use the survey weights in any of the groups (IgM positive, IgM negative tested, or IgM negative untested) because of the strong assumptions used in this estimate. Thus, our estimates of low IgG avidity in Table 3 differ from the weighted estimates in Table 2, and Table 3 estimates are not considered United States population estimates. The small number of low-avidity sera among IgM-negative sera did not allow stratification by age. Analysis of low-IgG-avidity prevalence among all sera by race or household income was not possible because of low prevalence.
Table 3.
Low-CMV-IgG-avidity prevalence estimates (unweighted) for both CMV IgM-positive and IgM-negative sera from the NHANES survey, according to avidity test cutoff
| CMV IgM status (no. tested) | % prevalence of low IgG avidity (no. of specimens) by avidity test cutoff |
||
|---|---|---|---|
| 0.80 | 0.70 | 0.60 | |
| IgM+ (126) | 34.9 (44) | 18.3 (23) | 11.1 (14) |
| IgM− (129) | 8.5 (11) | 2.3 (3) | 0.8 (1) |
| IgM+ and IgM− combined (255) | 6.6 (55) | 2.0 (26) | 0.8 (15) |
We also investigated whether high CMV IgG titer sera can give false low-avidity status as reported by Dangel et al. (6), using the index value of 0.7 as the cutoff for low avidity. Dilution of high-titer sera (100 to 285 UA/ml) to below 50 UA/ml was performed on 7 high-avidity and 7 low-avidity sera. For the high-avidity sera, dilution changed the avidity status to borderline low avidity (index, 0.65 to 0.69) for 2/7 sera and did not change the avidity status for 5/7 sera. Dilution did not change the avidity status for any of the low-avidity sera. Similar results were seen with 0.6 and 0.8 avidity test cutoffs (data not shown).
Relationship between IgM antibody levels and IgG avidity.
CMV IgM-negative or equivocal sera (n = 154) and IgM-positive sera (n = 170) of various antibody levels were examined for IgG avidity. Table 4 shows 1.9% prevalence for low IgG avidity among IgM-negative and equivocal sera and the steadily increasing proportion of sera that are low avidity as the IgM UA/ml increases, up to 78.8% for sera with IgM UA/ml of 2.0 to 4.0 (Table 4) (chi-square test for trend of decreasing IgG avidity with increasing IgM levels, P < 0.0001). Figure 2 shows actual IgM UA/ml and avidity values for the 324 sera presented in Table 4. Sera with the highest IgM levels had the lowest IgG avidity index values. The 0.7 avidity test cutoff was used to define low IgG avidity for this analysis, although similar strong trends were also seen with the 0.8 and 0.6 test cutoffs (data not shown).
DISCUSSION
Our results present the first prevalence estimates for CMV IgM and low IgG avidity in a nationally representative U.S. population. CMV IgM seroprevalence among women ranged from 2.3 to 4.5% across age groups and did not show significant trends with age. These results are consistent with the understanding that CMV IgM can be produced throughout life after primary CMV infection or as a result of reinfection or reactivation (18, 20) and suggest that older people may be as likely to have a recurrent episode as younger people are to have a primary infection. No risk factors based on race, ethnicity, age, or household income emerged for CMV IgM seroprevalence in contrast to risk factors such as race/ethnicity and household income for CMV IgG seroprevalence reported by Staras et al. (25). The lack of identifiable risk factors for CMV IgM may be due to the relatively low number of observations (3.0% prevalence for IgM), and because over 80% of the IgM reactivity in our population sample was high avidity and thus presumably from nonprimary CMV infection, which may be less associated with identifiable risk than primary infection. In addition, a portion of the IgM-positive sera may have been false-positive determinations known to occur with CMV IgM testing (13).
Testing all available IgM-positive sera and a sample of IgM-negative sera from the NHANES repository for IgG avidity allowed us to provide prevalence estimates for low IgG avidity (Tables 2 and 3) not previously reported. We applied 3 different cutoffs in the Vidas test for determination of low IgG avidity: an index value of 0.80, shown to reliably identify sera from acute CMV infection in one study (7); an index value of 0.7, recommended as a cutoff for low avidity by some studies (1, 3); and an index value of 0.6. The cutoff value of 0.80 showed a strong association between low avidity and young age (Table 2) but gave an unfeasibly high prevalence for low avidity of 6.6% compared to U.S. incident infection estimates for CMV of 1 to 2% (5), indicating low specificity for identification of primary CMV infection as shown in other studies (1, 3, 5). The cutoff value of 0.70 gave a low-avidity prevalence estimate of 2.0%, disproportionately excluding IgM-negative sera compared to the 0.8 test cutoff, and showed a significant association between young age and low avidity (Table 2). The index value of 0.60 resulted in a low-IgG-avidity prevalence of 0.8% with no association between low avidity and young age (Table 2). The 0.6 test cutoff likely decreased test sensitivity because 3 of the 11 specimens excluded from low avidity compared to the 0.7 cutoff were from young females 12 to 19 years old with strong CMV IgM reactivity suggestive of recent primary infection. Our results and other studies (1, 3, 5) indicate that the optimal single-point cutoff for the Vidas avidity test is likely 0.7 or possibly midway between 0.6 and 0.7, which was not analyzed. Thus, 0.7 was used as the cutoff for the main results of this study. Use of a two-point cutoff with an indeterminate zone benefits many serology tests but was not applied to the three cutoffs in our study since it would have created too many result categories for analysis. The optimal test cutoff for any serology test depends on whether the test is used primarily for screening, where sensitivity is more important, or confirmation, where specificity is more important. As with nearly all serology tests, adjusting the cutoff involves a tradeoff between sensitivity and specificity of the test.
IgG avidity testing of 170 IgM-positive sera showed that high IgM antibody titers (>2.0 UA/ml) were strongly associated with low avidity and presumably primary CMV infection. This extended work by Prince and Leber (22), who measured IgG avidity in 64 CMV IgM-positive sera that were primarily low titer or high titer and showed that the high-titer group was 93% low IgG avidity. Our study analyzed about 3 times more IgM-positive sera with titers spanning the test range and showed a steadily increasing proportion of low IgG avidity with increasing IgM antibody level, and the high-antibody group was 79% low IgG avidity.
Strengths of the study include the large national sample size and first population-based estimates for CMV IgM and IgG avidity. Limitations of the study include the cross-sectional design of NHANES, which did not allow documentation of IgG seroconversion to support low-avidity determination. The sample size of 6,000 lacked sufficient power to analyze risk factors for low avidity and primary CMV infection. The avidity analysis would have benefited from testing more of the IgM-negative sera (129/3,384 were tested), but this became evident during analysis after the serum collection had been returned to NHANES. Commercial CMV IgG avidity testing has the general limitation of being relatively new and requiring further standardization among commercial avidity tests recently highlighted by Revello et al. (23). Borderline avidity values should be interpreted with caution and considered together with CMV IgM determination.
In summary, our findings support the understanding that CMV IgM reactivity can occur throughout life as the result of primary and nonprimary CMV infection. Our prevalence estimates for low IgG avidity suggest that at a given point in time, 14 to 18% of CMV IgM reactivity represents primary CMV infection, consistent with results from a large study in France where 10 to 16% of the IgM-positive sera identified from pregnant women were low avidity (21). Because about 90% of the low-avidity sera were IgM positive, screening for IgM can greatly enrich the testing pool for low-IgG-avidity sera and should remain part of a testing algorithm. High IgM antibody titer was a strong predictor for low IgG avidity, which may aid the identification of primary infection and risk for congenital CMV infection, especially with the current limited availability of avidity testing. To the best of our knowledge, CMV IgG avidity testing in the United States is available at only two reference laboratories (Focus Diagnostics, Cypress, CA, and ARUP Laboratory, Salt Lake City, UT), and kits are sold through Abbott Laboratories (Abbott Park, IL) but only for use on the Abbott instrument.
ACKNOWLEDGMENTS
This work was supported by the Centers for Disease Control and Prevention.
We thank the following individuals for their excellent assistance: Kay Radford and William Hendley (laboratory testing), Elizabeth Henderson (graphics), and Toni Roberson (manuscript preparation).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Baccard-Longere M., Freymuth F., Cointe D., Seigneurin J. M., Grangeot-Keros L. 2001. Multicenter evaluation of a rapid and convenient method for determination of cytomegalovirus immunoglobulin G avidity. Clin. Diagn. Lab. Immunol. 8:429–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blackburn N. K., Besselaar T. G., Schoub B. D., O'Connell K. F. 1991. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J. Med. Virol. 33:6–9 [DOI] [PubMed] [Google Scholar]
- 3. Bodeus M., Beulne D., Goubau P. 2001. Ability of three IgG-avidity assays to exclude recent cytomegalovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 20:248–252 [DOI] [PubMed] [Google Scholar]
- 4. Bodeus M., Feyder S., Goubau P. 1998. Avidity of IgG antibodies distinguishes primary from non-primary cytomegalovirus infection in pregnant women. Clin. Diagn. Virol. 9:9–16 [DOI] [PubMed] [Google Scholar]
- 5. Colugnati F. A., Staras S. A., Dollard S. C., Cannon M. J. 2007. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect. Dis. 7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dangel V., Bader U., Enders G. 2006. Improvement of cytomegalovirus avidity testing by adjusting the concentration of CMV-specific IgG in test samples. J. Clin. Virol. 35:303–309 [DOI] [PubMed] [Google Scholar]
- 7. de Souza S., Bonon S. H., Costa S. C., Rossi C. L. 2003. Evaluation of an in-house specific immunoglobulin G (IgG) avidity ELISA for distinguishing recent primary from long-term human cytomegalovirus (HCMV) infection. Rev. Inst. Med. Trop. Sao Paulo 45:323–326 [DOI] [PubMed] [Google Scholar]
- 8. Dollard S. C., Grosse S. D., Ross D. S. 2007. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 17:355–363 [DOI] [PubMed] [Google Scholar]
- 9. Fowler K. B., et al. 1992. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 326:663–667 [DOI] [PubMed] [Google Scholar]
- 10. Grangeot-Keros L., et al. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J. Infect. Dis. 175:944–946 [DOI] [PubMed] [Google Scholar]
- 11. Hedman K., Seppala I. 1988. Recent rubella virus infection indicated by a low avidity of specific IgG. J. Clin. Immunol. 8:214–221 [DOI] [PubMed] [Google Scholar]
- 12. Kenneson A., Cannon M. J. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 17:253–276 [DOI] [PubMed] [Google Scholar]
- 13. Lazzarotto T., Dalla Casa B., Campisi B., Landini M. P. 1992. Enzyme-linked immunoadsorbent assay for the detection of cytomegalovirus-IgM: comparison between eight commercial kits, immunofluorescence and immunoblotting. J. Clin. Lab. Anal. 6:216–218 [DOI] [PubMed] [Google Scholar]
- 14. Lazzarotto T., Guerra B., Lanari M., Gabrielli L., Landini M. P. 2008. New advances in the diagnosis of congenital cytomegalovirus infection. J. Clin. Virol. 41:192–197 [DOI] [PubMed] [Google Scholar]
- 15. Lazzarotto T., et al. 1997. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin. Diagn. Lab. Immunol. 4:469–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lazzarotto T., et al. 2000. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol. 13:137–141 [DOI] [PubMed] [Google Scholar]
- 17. Munro S. C., et al. 2005. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J. Clin. Microbiol. 43:4713–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nielsen S. L., Sorensen I., Andersen H. K. 1988. Kinetics of specific immunoglobulins M, E, A, and G in congenital, primary, and secondary cytomegalovirus infection studied by antibody-capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 26:654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nigro G., Adler S. P., La T. R., Best A. M. 2005. Passive immunization during pregnancy for congenital cytomegalovirus infection. N. Engl. J. Med. 353:1350–1362 [DOI] [PubMed] [Google Scholar]
- 20. Pass R. F., Griffiths P. D., August A. M. 1983. Antibody response to cytomegalovirus after renal transplantation: comparison of patients with primary and recurrent infections. J. Infect. Dis. 147:40–46 [DOI] [PubMed] [Google Scholar]
- 21. Picone O., et al. 2009. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG 116:818–823 [DOI] [PubMed] [Google Scholar]
- 22. Prince H. E., Leber A. L. 2002. Validation of an in-house assay for cytomegalovirus immunoglobulin G (CMV IgG) avidity and relationship of avidity to CMV IgM levels. Clin. Diagn. Lab. Immunol. 9:824–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Revello M. G., et al. 2010. Comparative evaluation of eight commercial human cytomegalovirus IgG avidity assays. J. Clin. Virol. 48:255–259 [DOI] [PubMed] [Google Scholar]
- 24. Stagno S., et al. 1982. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N. Engl. J. Med. 306:945–949 [DOI] [PubMed] [Google Scholar]
- 25. Staras S. A., et al. 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 43:1143–1151 [DOI] [PubMed] [Google Scholar]