Abstract
As a consequence of continued spillover of Mycobacterium bovis into cattle from wildlife reservoirs and increased globalization of cattle trade with associated transmission risks, new approaches such as vaccination and novel testing algorithms are seriously being considered by regulatory agencies for the control of bovine tuberculosis. Serologic tests offer opportunities for identification of M. bovis-infected animals not afforded by current diagnostic techniques. The present study describes assay development and field assessment of a new commercial enzyme-linked immunosorbent assay (ELISA) that detects antibody to M. bovis antigens MPB83 and MPB70 in infected cattle. Pertinent findings include the following: specific antibody responses were detected at ∼90 to 100 days after experimental M. bovis challenge, minimal cross-reactive responses were elicited by infection/sensitization with nontuberculous Mycobacterium spp., and the apparent sensitivity and specificity of the ELISA with naturally infected cattle were 63% and 98%, respectively, with sensitivity improving as disease severity increased. The ELISA also detected infected animals missed by the routine tuberculin skin test, and antibody was detectable in bulk tank milk samples from M. bovis-infected dairy herds. A high-throughput ELISA could be adapted as a movement, border, or slaughter surveillance test, as well as a supplemental test to tuberculin skin testing.
INTRODUCTION
Tuberculosis (TB) in humans may result from exposure to any one of the tubercle bacilli included within the Mycobacterium tuberculosis complex, including Mycobacterium bovis. Unlike M. tuberculosis, M. bovis has a wide host range and is the species most often isolated from tuberculous cattle. Wildlife reservoir hosts for M. bovis infection of cattle include the Eurasian badger (Meles meles), brushtail possums (Trichosurus vulpecula), Eurasian wild boar (Sus scrofa), white-tailed deer (Odocoileus virginianus), and several other mammalian species. Wildlife reservoirs have made M. bovis eradication from national herds particularly difficult in several developed countries, including the United Kingdom, Ireland, New Zealand, Spain, and the United States (4, 5, 7, 9, 13). Additionally, movement of infected cattle between regions and countries accounts for several outbreaks in regions previously considered tuberculosis free; thus, trade agreements increasingly include regionalization principles for bovine tuberculosis control (12). Given the continued spillover of tuberculosis from wildlife reservoirs into cattle and increased risks associated with globalization of cattle trade, the development of new diagnostic strategies for effective control of bovine tuberculosis is urgently needed. Indeed, within the United States, a notable change in the tuberculosis eradication program has recently been proposed, reviewed, and implemented (1). A major component of this modification is to assist biologics companies in the development of diagnostic tests for tuberculosis control and to expedite evaluation of emerging diagnostic tests (1).
Current tests most widely used for the detection of tuberculosis in cattle and humans include measurement of delayed-type hypersensitivity (i.e., skin testing) to purified protein derivatives (PPD) and/or an in vitro assay for gamma interferon (IFN-γ) produced in response to mycobacterial antigen stimulation (i.e., Bovigam, from Prionics AG, Schlieren, Switzerland, and Quantiferon Gold from Cellestis Inc., Carnegie, Victoria, Australia). These tests rely on early cell-mediated responses, a hallmark of tuberculosis immunopathogenesis. Until recently, the poor sensitivity of antibody-based tests has prevented widespread use of these assays for early detection of tuberculous cattle (11). However, several serologic tests with promising accuracy have recently emerged (6, 12, 19, 22, 23). Additionally, antibody responses to M. bovis positively correlate with M. bovis-elicited pathology and M. bovis antigen burden (8, 17); therefore, data from these assays are supportive for immunopathogenesis and vaccine efficacy studies. Considering the ease of sample collection and test procedure, serologic (antibody-based) tests may be used in a wide range of applications and provide additional testing opportunities not afforded with cell-mediated response-based tests.
The present study describes a new commercial enzyme-linked immunosorbent assay (ELISA) for the detection of tuberculous cattle. Development of the assay consisted of proof-of-concept studies and test optimization using samples from experimentally infected cattle (including M. bovis and nontuberculous Mycobacterium spp.). Experimental infection trials were followed by field assessment of test utility and accuracy through evaluation of samples from multiple countries.
MATERIALS AND METHODS
Experimental infection trials: Mycobacterium spp., skin test procedures, and necropsy.
For studies performed at the National Animal Disease Center (NADC), calves were infected via an aerosol or intratonsillar route as previously described (10, 20, 17, 18). Briefly, 6-month-old castrated Holstein-Friesian bull calves received ∼104 CFU of M. bovis (strain 95-1315, MI deer isolate) by aerosol (n = 7; one dose) or ∼108 CFU of Mycobacterium avium subsp. paratuberculosis (strain 167, clinical bovine isolate), ∼109 CFU of M. avium subsp. avium (strain TMC 702, chicken isolate; here, M. avium), or ∼108 CFU of Mycobacterium kansasii (strain 03-6931, bovine isolate) by direct intratonsillar instillation of sedated calves (n = 8 per group for nontuberculous Mycobacterium spp.; inocula were delivered in two equal doses 2 weeks apart). The decision to use a dose of nontuberculous Mycobacterium spp. ∼104- to 105-fold larger than that of M. bovis was based upon prior studies (17, 18, 20) and the relatively low virulence of these species. A group of eight age-, breed-, and gender-matched calves was also included as noninfected calves. All calves were housed in a biosafety level 3 (BSL-3) facility according to institutional guidelines and approved animal care and use protocols. BSL-3 procedures were followed for the M. bovis-inoculated group of calves, whereas BSL-2 procedures were followed for all other groups. For studies performed at AgResearch/Hopkirk Research Institute in New Zealand, 8-month-old Holstein-Friesian calves were infected via intratracheal inoculation with 5,000 CFU of M. bovis strain WAg202 as described previously (2). The animals were kept on pasture in a containment area. All cattle experiments were cleared by local ethical review, and animal procedures were performed in accordance with institutional guidelines and approved animal care and use protocols.
For both NADC and AgResearch studies, mycobacterial culture and enumeration of challenge inoculum, postmortem procedures, culture of Mycobacterium spp. from tissues, and histopathology were performed as previously described (2, 10, 17) and using standard techniques. For measurement of delayed-type hypersensitivity (15), skin thickness was measured with calipers immediately prior to PPD administration and 72 h after injection. M. bovis and M. avium PPD were each applied at separate sites in the mid-cervical region. Time points for tuberculin skin test were ∼3 and 6 months after M. bovis inoculation (NADC studies), ∼3.25 months after M. bovis inoculation (AgResearch studies), and ∼4.5 months for noninoculated calves and after inoculation with M. avium, M. avium subsp. paratuberculosis, or M. kansasii (NADC studies). Necropsy was performed at ∼10 months (NADC) or 4 months (AgResearch) after M. bovis challenge or ∼ 5 months after M. avium, M. avium subsp. paratuberculosis, or M. kansasii challenge. For M. bovis infection, gross lesions typical of M. bovis infection were observed in the lungs and pulmonary lymph nodes, and M. bovis was isolated from all calves. As previously described (17, 18, 20), gross lesions were not detected in noninoculated or in M. avium-, M. avium subsp. paratuberculosis- or M. kansasii-inoculated calves.
Field samples.
The source and description of serum samples for determination of diagnostic accuracy of the IDEXX M. bovis ELISA (IDEXX Laboratories, Westbrook, ME) are provided in Table 1 (sensitivity) and Table 2 (specificity). Samples included serum obtained from cattle within the United States (including various states), Great Britain, New Zealand, Ireland, and Austria.
Table 1.
Sensitivity of IDEXX M. bovis antibody ELISA with sera collected from naturally infected cattle
|
M. bovis-infected serum type |
nd | No. of herds | Sensitivity (%) |
|||
|---|---|---|---|---|---|---|
| Source | ID or characterizationc | Lot 1 | Lot 2 | Lot 3 | ||
| Great Britain | AHVLA-2a | 134 | 31 | 74.6 | 72.4 | 73.1 |
| AHVLA-1b | 50 | >5 | 86 | 88 | 86 | |
| Ireland | No visible lesionsb | 50 | >5 | 48 | 44 | 46 |
| With visible lesionsb | 50 | >5 | 72 | 70 | 70 | |
| Skin test positive, Bovigam positive, with visible lesionsb | 30 | 22 | 96.7 | 86.7 | 90.0 | |
| New Zealand | AgResearchb | 42 | 7 | 42.9 | 40.5 | 35.7 |
| USA | Coloradoa | 81 | 1 | 44.4 | 45.7 | 49.4 |
| NVSL serum banka | 31 | 12 | 48.4 | 48.4 | 48.4 | |
| Michigana | 10 | 1 | 30 | 30 | 30 | |
| Overall value | 478 | >89 | 63.6 | 61.9 | 62.6 | |
Infection status determined by histopathology with IS6110 PCR and/or culture.
Infection status determined by culture or presence of gross lesions and/or from a tuberculosis-affected herd.
ID, identification. NVSL, National Veterinary Service Laboratory.
n, number of animals.
Table 2.
Specificity of IDEXX M. bovis antibody ELISA with sera collected from noninfected cattle from various geographic regions
| Source of noninfected seraa | nb | No. of herds | Specificity (%) |
||
|---|---|---|---|---|---|
| Lot 1 | Lot 2 | Lot 3 | |||
| Maine | 126 | 2 | 99.2 | 98.4 | 99.2 |
| Maine | 126 | 2 | 99 | 98.4 | 99.2 |
| Pennsylvania | 79 | 1 | 88.6 | 92.4 | 98.7 |
| Arkansas | 39 | 1 | 100 | 100 | 97.4 |
| New York | 84 | 1 | 98.8 | 100 | 98.8 |
| North Dakota | 110 | 1 | 96.3 | 97.2 | 99.1 |
| Washington | 84 | 2 | 98.8 | 98.8 | 98.8 |
| South Dakota | 84 | 1 | 98.8 | 100 | 100 |
| Missouri | 92 | >2 | 94.5 | 95.7 | 98.9 |
| Texas | 96 | >2 | 93.7 | 93.8 | 95.8 |
| Michigan | 92 | 2 | 100 | 100 | 100 |
| Iowa | 8 | 1 | 100 | 100 | 100 |
| Colorado | 121 | 11 | 99.2 | 100 | 99.2 |
| Great Britain (AHVLA) | 50 | >5 | 94 | 98 | 96 |
| Ireland (UCD/DAFF) | 92 | 16 | 100 | 100 | 95.7 |
| Austria | 316 | >10 | 97.5 | 99.1 | 98.1 |
| Overall value | 1,473 | >58 | 97.4 | 98.2 | 98.4 |
Samples obtained from cattle from tuberculosis-free herds. AHVLA, Animal Health and Veterinary Laboratories Agency; UCD, University College Dublin; DAFF, Department of Agriculture, Fisheries and Food.
n, number of animals.
Samples from M. bovis-infected cattle in Colorado were obtained from the USDA, Animal Plant and Health Inspection Service (APHIS), Veterinary Services Tuberculosis Eradication Program staff. Upon depopulation, the M. bovis prevalence rate within this dairy herd was determined to be ∼10%. Animals within this herd were classified as infected by isolation of M. bovis through standard mycobacterial culture techniques or by detection of mycobacteriosis-compatible microscopic lesions that were IS6110 PCR positive, indicative of M. tuberculosis complex. Culture and histology were performed at the National Veterinary Services Laboratories, Ames, IA. Samples from M. bovis-infected cattle in Michigan were obtained from USDA, APHIS Veterinary Services, and the Michigan Department of Agriculture. A skin test had been applied to all animals in both the Colorado and Michigan herds at least 60 days prior to collection of serum.
Samples from M. bovis-infected cattle were also obtained from the USDA APHIS, tuberculosis serum bank (from Jeff Nelson), representing four states and 12 herds, and with confirmed M. bovis infection status. Forty-two serum samples (New Zealand Animal Health Board) were obtained from cattle from seven naturally infected herds in New Zealand. The animals had gross tuberculous lesions and/or were M. bovis culture positive. M. bovis was cultured from one or more animals from each herd. Serum samples were collected 2 to 4 weeks after caudal fold skin testing. In Ireland, serum or plasma was collected from defined cohorts of cattle with evidence of active M. bovis infection in the herd (herds with more than one standard single intradermal comparative cervical test [SICCT]-positive animal). In each of these herds, samples were collected, where feasible, from all SICCT reactors and from in-contact cohorts greater than 6 months of age. Samples were collected from affected herds as part of the Irish Department of Agriculture, Fisheries and Food (DAFF) bovine tuberculosis management program. The SICCT, ancillary testing (i.e., IFN-γ release assay [Bovigam; Prionics Ag, Schlieren, Switzerland]), and lesion status were available for all of these animals. Animals were initially classified as infected on the basis of their SICCT and/or Bovigam status, followed by examination for visible lesions. For Great Britain, samples from M. bovis-infected cattle originated from animals that had gross tuberculous lesions and/or that were M. bovis culture positive (sample set identified as AHVLA-1, where AHVLA is Animal Health and Veterinary Laboratories Agency). Mycobacterial culture was not performed on all tissues from animals with gross lesions originating from herds with known M. bovis-infected cattle (i.e., prior culture-confirmed tuberculosis). Skin test (SICCT) was applied to all animals within 1 month of serum collection. A second serum set (identified as AHVLA-2) was obtained from animals entering Welsh slaughterhouses from tuberculosis-positive herds. All animals were M. bovis culture positive. Both AHVLA-1 and AHVLA-2 serum sample sets were collected between 2 and 4 weeks after skin testing (SICCT) was applied. All animals in both sets were SICCT positive. Animals were of 13 different breeds (dairy and beef breeds).
For specificity studies, samples were obtained from noninfected cattle including diverse sources (designated tuberculosis-free by USDA or other regulatory organizations) or from herds with multiple official test-negative events over time (i.e., Great Britain and Ireland). For Ireland, samples were obtained from animals (>6 months of age) at their annual SICCT herd test and from herds without tuberculosis for at least 5 years. For samples from presumed noninfected cattle, positive serologic responses were considered false positives, and no attempts were made to retest animals. For Great Britain, samples were obtained from tuberculosis-free herds in regions where tuberculosis was nonendemic.
Serum Ig ELISA with M. bovis LAM-enriched antigen.
An ELISA with lipoarabinomannan (LAM)-enriched antigen was performed as described previously (18, 19). Briefly, Immulon II 96-well microtiter plates (Dynatech, Chantilly, Va.) were coated with LAM-enriched mycobacterial antigen (20 μg/ml, prepared as described previously [21]) or without antigen (coating buffer consisting of 0.05 M carbonate-bicarbonate coating buffer, pH 9.6, in a total volume of 100 μl; Sigma). The LAM-enriched antigen is a poorly characterized complex antigen preparation useful for the demonstration of antibody responses upon experimental M. bovis infection (18, 19, 21). Plates were washed three times with phosphate-buffered saline (PBS) with 0.05% Tween 20 (PBST; 200 μl/well), blocked with 0.1% gelatin diluted 1:20 in doubly deionized water, and added at 200 μl/well. After an incubation period of 1 h at 39°C, plates were washed nine times in PBST, and test serum was added to the wells (100 μl/well). Serum samples were diluted 1:100 in PBS containing 0.1% gelatin prior to addition to precoated and preblocked assay plates. The optimal dilution of test serum was determined beforehand by evaluating the reactivity of undiluted serum and serum diluted from 1:10 to 1:400. After 20 h at 4°C, plates were washed nine times with PBST. Plates were incubated for an additional 1 h at 39°C after addition of 100 μl of peroxidase-conjugated protein G (Sigma) diluted 1:2,000 in PBS containing 0.1% gelatin. Plates were subsequently washed nine times with PBST before addition of chromogenic substrate (100 μl/well SureBlue TMB Microwell Peroxidase Substrate [TMB is [3,3′,5,5′-tetramethylbenzidine]; KPL). Plates were read kinetically every minute for 15 min at 650 nm using an automated plate reader (Flex Station 3; Molecular Devices, Sunnyvale, CA). Acquired data were analyzed using Softmax Pro software (version 5.2; Molecular Devices). Using mean Vmax (milliunits/min) of duplicate wells, the Vmax was calculated by subtracting the mean Vmax of the no-antigen wells from the mean Vmax of antigen-coated wells. A cutoff value indicative of a positive response was not determined as this assay is used as a research tool only, not a diagnostic assay. Responses to the LAM-enriched antigen were used to demonstrate elicitation of antibody responses to Mycobacterium sp. infection/sensitization and for comparative purposes of responses to a complex mycobacterial antigen preparation (i.e., the LAM-enriched antigen) versus the IDEXX M. bovis ELISA (i.e., using M. bovis-specific antigens). Cross-reactive responses elicited by nontuberculous Mycobacterium spp. preclude the use of the LAM-enriched ELISA as a useful diagnostic test.
IDEXX M. bovis antibody ELISA.
Immulon I 96-well microtiter plates (Thermo Scientific, Hudson, NJ) were coated with a proprietary blend of mycobacterial antigens (MPB83 and MPB70) (24). For the assay, serum samples and kit controls were diluted 1:50 in the sample diluent provided in the kit. Diluted samples (100 μl/well) were added to the plates and allowed to incubate at room temperature (18 to 26°C) for 60 min. After four washes, 100 μl of a monoclonal anti-bovine IgG-horseradish peroxidase conjugate was added to each well and allowed to incubate for 30 min. After four washes, 100 μl of TMB substrate was added to each well. After a 15-min incubation at room temperature, color development in the wells was stopped by the addition of an acidified stop solution. Plates were read at 450 nm (Vmax; Molecular Devices, Sunnyvale, CA). Results are presented as sample-to-positive ratios (S/P) derived by subtracting the mean kit negative-control optical density (OD) from each sample and dividing this value by the corrected positive-control value (mean positive-control OD minus mean negative-control OD). Samples with S/P ratios greater than or equal to 0.30 were considered positive for M. bovis antibodies The ELISA cutoff of 0.30 was determined by receiver operating characteristic analysis using a specificity requirement of 97%, based on market analysis and feedback as well as on published reports (16) for the specificity of another blood-based M. bovis test (IFN-γ release assay; Bovigam [Prionics Ag, Schlieren, Switzerland]). All serum samples were tested at IDEXX Laboratories.
Milk test.
Preserved (Broad Spectrum Microtabs II; D&F Control Systems, Dublin, CA) bulk milk samples were centrifuged at 20,000 × g for 10 min, after which fat-free supernatant was diluted 1:1 with the kit-provided diluent. One-hundred-microliter volumes of diluted sample were tested according to the IDEXX M. bovis ELISA kit protocol described previously. Milk samples were also evaluated for the presence of M. bovis DNA by PCR. Briefly, 40-ml volumes of bulk tank milk were treated with Tween 20 solution (final sample concentration of 0.25%) and pelleted by centrifugation. The pellet was washed and suspended in PBS-EDTA and subjected to mechanical lysis, and the resultant free DNA was purified on commercially available silica columns (Qiagen, Valencia, CA). Each DNA sample was analyzed by TaqMan quantitative PCR (qPCR; Applied Biosystems, Carlsbad, CA) with IS1081 as the target sequence (3). ELISAs and PCR testing of milk samples were performed at Antel Biosystems.
Statistics.
Data were analyzed by analysis of variance repeated measures test followed by Tukey's multiple comparisons test or Student t test using a commercially available statistics program (Prism, version 4.0, for Macintosh; GraphPad Software, La Jolla, CA.).
RESULTS AND DISCUSSION
Assay development and experimental infection trials.
Aerosol inoculation of cattle with virulent M. bovis elicited antibody detectable by an LAM-enriched antigen ELISA (Fig. 1A) and IDEXX M. bovis ELISA (Fig. 1B). As described previously (17, 19), antibody responses were detectable immediately after injection of PPD for skin tests. Also, antibody responses were boosted by a second administration of PPD for skin tests at ∼175 days after challenge (Fig. 1B). With sera obtained from cattle inoculated with M. bovis via the intratracheal route, specific antibody was detectable by the IDEXX ELISA prior to the skin test (Fig. 1C). Regardless of the serum set (i.e., NADC versus AgResearch), M. bovis-specific antibody was detectable by the IDEXX ELISA at ∼90 to 100 days after inoculation.
Fig. 1.
Kinetics of serum antibody response to M. bovis infection. Sera were obtained from animals receiving 104 CFU of M. bovis by aerosol (NADC trial; n = 7) or 5 × 103 CFU of M. bovis by intratracheal inoculation (AgResearch trial; n = 10) at indicated time points relative to M. bovis challenge. Arrows indicate timing of intradermal administration of PPD for skin tests. *, P < 0.05 for difference prechallenge responses.
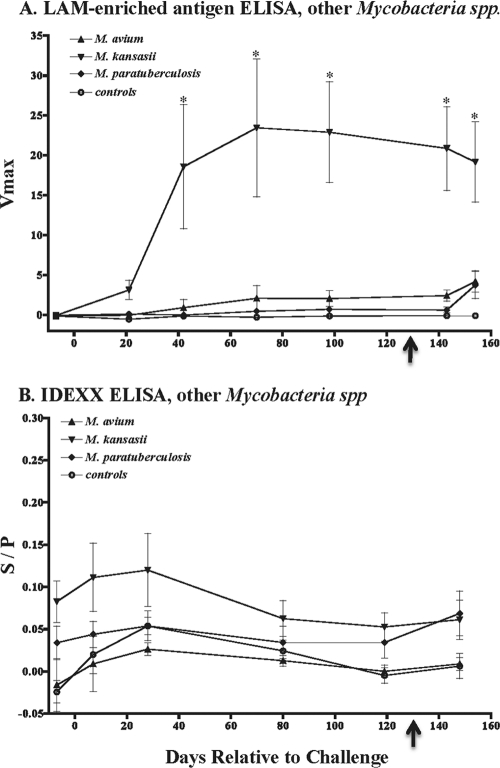
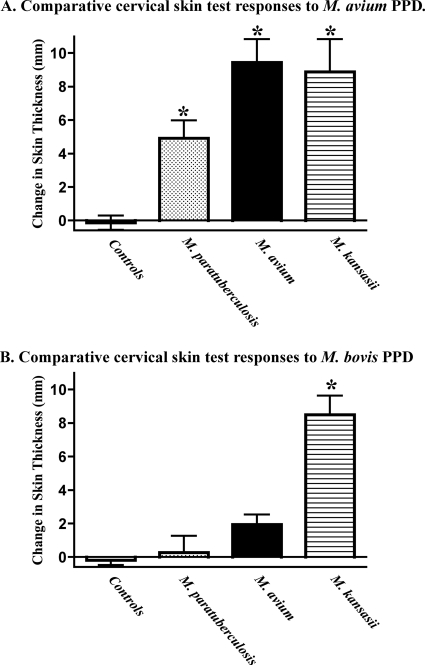
Determining the dose and timing of exposure to environmental nontuberculous Mycobacterium spp. via natural exposure is generally not possible. Thus, we utilized an experimental approach to model infection/sensitization to various nontuberculous Mycobacterium spp. (18, 20) and for collection of sera to determine the specificity of the IDEXX ELISA. Intratonsillar inoculation with M. kansasii elicited a sustained antibody response to M. bovis LAM-enriched antigen (Fig. 2A); however, antibodies generated by this response did not react with antigens in the IDEXX ELISA (i.e., MPB83 and MPB70) (Fig. 2B). Conversely, archived sera obtained from a prior study (18) with M. kansasii-inoculated cattle reacted (S/P ranging from 2.9 to 7.5; n = 4) with antigens within the IDEXX ELISA. The reason for the differential reactivity between these two studies is unclear. MPB70 and MPB83 are present in M. kansasii with an identity of 81% and 77%, respectively; thus, it is not surprising that the archived sera from M. kansasii-inoculated cattle reacted with antigens within the IDEXX ELISA. Antibody responses were not detected in either assay (i.e., LAM or IDEXX ELISA) with sera from noninoculated cattle or from M. avium- or M. avium subsp. paratuberculosis-inoculated cattle, despite significant delayed-type hypersensitive responses by M. avium- and M. avium subsp. paratuberculosis-inoculated cattle to M. avium PPD, which is indicative of sensitization (Fig. 3). Also, administration of PPD for skin tests did not elicit a response (i.e., with non-, M. avium-, or M. avium subsp. paratuberculosis-inoculated cattle) or boost the response (i.e., with LAM responses of M. kansasii-inoculated cattle) (Fig. 2). MPB70 and MPB83 are not present in M. avium or M. avium subsp. paratuberculosis. Delayed-type hypersensitive responses were also elicited by aerosol M. bovis infection (change in skin thickness in response to M. bovis PPD, 30.3 ± 6.52 mm) and intratracheal inoculation (change in skin thickness in response to M. bovis PPD, 22.5 ± 1.7 mm). Together, findings from the experimental infection trials demonstrate the potential for use of the IDEXX ELISA for the diagnosis of M. bovis infection, particularly after injection of PPD for skin tests.
Fig. 2.
Kinetics of serum antibody response to nontuberculous mycobacterial infections. Sera were obtained from animals receiving ∼108 CFU of M. avium subsp. paratuberculosis, ∼109 CFU of M. avium subsp. avium, or ∼108 CFU of M. kansasii by intratonsillar inoculation of sedated animals (n = 8 per group; inoculum was delivered in two equal doses at 2 weeks apart). (Note the very low y-axis scale compared to that of Fig. 1B and C). Arrows indicate timing of intradermal administration of PPD for skin tests. *, P < 0.05 for difference from prechallenge responses.
Fig. 3.
Delayed-type hypersensitivity responses by cattle inoculated with various nontuberculous mycobacteria. Cattle received either M. avium subsp. paratuberculosis (M. paratuberculosis) or M. avium subsp. avium (M. avium) or were not infected (controls). Delayed-type hypersensitivity to M. avium (A) and M. bovis (B) PPD was evaluated by comparative cervical skin test (1) ∼4.5 months after challenge. *, P < 0.05 for difference from controls (A) and from controls and M. avium and M. avium subsp. paratuberculosis treatment groups (B).
Field application and international evaluation.
To evaluate the accuracy of the IDEXX ELISA, serum samples were obtained from cattle naturally infected with M. bovis (Table 1), noninfected cattle (Table 2), or cattle naturally infected with M. avium subsp. paratuberculosis (Table 3) from numerous states within the United States and multiple countries. Three manufacturing scale lots were evaluated with each sample set. All samples were tested in single wells according to standard protocols; no repeat testing was performed. Prior to testing, sample sets were mixed (i.e., sequence of testing) to ensure representation of samples from both M. bovis-infected and noninfected cattle within plates, and technicians were blinded with respect to the sample sets being tested and corresponding M. bovis status. The positive-control sample for the assay consisted of pooled sera obtained from M. bovis-infected cattle (U.S. experimental infection trial) immediately prior to necropsy. Findings indicate that the accuracy of the test is ∼63% sensitivity (Table 1) and ∼98% specificity (Table 2), with no cross-reactivity to M. avium subsp. paratuberculosis-specific antibody (Table 3). As previously described (19), sensitivity values may have been boosted by application of skin tests. Low sensitivity values were noted with samples obtained from the United States and New Zealand, most likely due to early infection as these herds had been monitored closely prior to the detection of tuberculous animals.
Table 3.
Specificity of IDEXX M. bovis antibody ELISA with sera collected from cattle naturally infected with M. avium subsp. paratuberculosis
| Sample IDc |
M. avium subsp. paratuberculosis detection |
M. bovis ELISA (S/P)b |
|||
|---|---|---|---|---|---|
| Fecal culture | Antibody ELISA (S/P)a | Lot 1 | Lot 2 | Lot 3 | |
| 116 | Positive | 2.53 | −0.08 | −0.18 | −0.04 |
| 164 | Positive | 3.29 | −0.05 | −0.17 | −0.04 |
| 179 | Positive | 1.43 | −0.08 | −0.17 | −0.04 |
| 194 | Positive | 2.38 | 0.03 | −0.05 | −0.03 |
| 211 | Positive | 2.33 | −0.26 | −0.16 | −0.04 |
| 222 | Positive | 3.35 | −0.09 | −0.16 | −0.05 |
| 229 | Positive | 0.08 | −0.10 | −0.19 | −0.05 |
| 232 | Positive | 1.86 | 0.05 | −0.14 | 0.00 |
| 253 | Positive | 3.20 | −0.09 | −0.18 | −0.05 |
| 263 | Positive | 2.77 | −0.09 | −0.17 | −0.06 |
| 270 | Positive | 3.33 | −0.07 | −0.17 | −0.06 |
| 275 | Positive | 1.45 | −0.08 | −0.13 | −0.04 |
| 289 | Positive | 2.04 | −0.11 | −0.19 | −0.04 |
| 290 | Positive | 2.60 | 0.01 | −0.09 | 0.00 |
| 305 | Positive | 3.22 | −0.08 | −0.15 | −0.04 |
| 307 | Positive | 1.60 | −0.09 | −0.17 | −0.06 |
| 319 | Negative | 0.63 | −0.26 | −0.14 | −0.04 |
| 323 | Positive | 0.10 | −0.09 | −0.16 | −0.05 |
| 324 | Positive | NA | −0.10 | −0.19 | −0.06 |
M. paratuberculosis antibody ELISA was performed according to the manufacturer's instructions (IDEXX Laboratories). The antigen preparation in this kit is a complex protoplasmic antigen. All responses were considered positive except those for animals 229 and 323. NA, not available for testing.
All responses were considered negative.
ID, identification.
Application of IDEXX M. bovis ELISA with animals at various stages of disease.
The performance of the IDEXX ELISA was assessed in infected cattle in Ireland with respect to the absence or presence of detectable visible lesions at routine postmortem examination. Three manufacturing scale lots were evaluated with the following sample sets. In a group of animals (n = 50) that were positive by SICTT and/or the Bovigam test (Prionics Ag, Schlieren, Switzerland) but without visible lesions, the ELISA detected ∼46% of these infected animals. However, in another group of cattle (n = 50) that were positive by SICCT and/or the Bovigam test but had visible lesions, the sensitivity of the ELISA increased to ∼70%. In a group of animals (n = 30) that were SICCT and Bovigam test positive with visible lesions, the IDEXX ELISA was positive with ∼90% of the infected animals. These results are consistent with the serum antibody responses in the M. bovis experimentally infected cattle (Fig. 1), which showed that the antibody levels increased with the progression of infection and that sensitivity was highest when lesions were visible. The ELISA was then applied to a small group of animals (n = 10) that were negative by SICCT and Bovigam but had visible lesions. The IDEXX ELISA detected 2/10 (20%) of these infected animals. This result showed that the ELISA was capable of detecting infected animals missed by the conventional SICCT and Bovigam assay, demonstrating its potential as a supplementary test.
Intraplate reproducibility.
A positive sample approximating the potency of the kit positive control was tested in all wells of three plates from each of three lots; plates were selected from the beginning, middle, and end of the plate-coating step for this evaluation. The mean optical density (OD), standard deviation (SD), and coefficient of variation (CV) were calculated for each plate. Table S1 in the supplemental material summarizes the results from this evaluation. The CVs ranged from 5.7% to 10.3%.
Two weakly reactive samples, above and below the test cutoff, along with a negative sample were evaluated in a similar manner. Table S2 in the supplemental material summarizes the results from this evaluation. The CVs for the weakly reactive samples ranged from 8.7% to 11.4% and 9.6% to 11.9%, respectively, while the CVs for the negative sample ranged from 14.5% to 17.7%.
Repeatability.
As part of overall kit validation testing, five samples spanning the negative (TB-1) to equivocal (TB-5) to strong positive (TB-2, TB-3, and TB-4) S/P range were tested on three different lots of the M. bovis ELISA kit (see Table S3). Samples were tested in one random well by two operators over 5 days and included 23 to 25 plates (per lot), depending on the sample tested. Table S3 in the supplemental material summarizes the results from this evaluation. The negative sample (TB-1) was negative in all 71 wells tested. The CV for the equivocal sample (TB-5) ranged from 18% to 27% and was positive in 22 of 69 wells tested, consistent with its formulation at the very low end of the test. The CVs for the other positive samples ranged from 12% to 47%. For sample TB-2 on lot 2, there was one well with a very high S/P value (1.82) compared to expectations and beyond 3 SDs of the mean. The sample was an obvious outlier and when excluded from the analysis, the CV was 17%, more consistent with expectations.
Serum/plasma flexibility.
Paired serum and plasma samples (lithium heparin) were collected from M. bovis-infected animals (U.S. M. bovis experimental infection trial) immediately prior to necropsy. For negative samples, herds in Pennsylvania and Maine and NADC control animals (M. bovis study) were sampled using red-topped tubes for serum and either EDTA or sodium heparin tubes for plasma. Mean S/P values were similar (P > 0.05) with serum versus plasma for each animal on all serum sample sets, demonstrating the flexibility for use of serum or plasma as a test sample with this assay.
Milk test.
Another potential application of antibody-based tests is detection of M. bovis-specific antibody in milk samples. To assess this potential, bulk tank milk samples (all Holstein-Friesian) were obtained from TB-free (n = 185, Michigan) and TB-affected (n = 17, Mexico) dairies. All 185 of the milk samples from TB-free dairies were negative (S/P < 0.3), whereas 14/17 of the samples from TB-affected dairies were positive (S/P range, 0.31 to 5.11) by IDEXX M. bovis ELISA. All 17 of the bulk tank milk samples from the TB-affected herds were positive for M. bovis DNA using PCR for IS1081 (3, 14).
Conclusions.
Present findings indicate that the IDEXX M. bovis ELISA provides an apparent test sensitivity of 63% and specificity of 98% with samples from cattle naturally infected with M. bovis. The sensitivity of the IDEXX ELISA increases with disease severity; however, it can also detect infected animals that are SICCT and Bovigam test negative. Early infections, as seen in the present study with samples from the United States and New Zealand, may also contribute to reduced sensitivity in certain instances. With the IDEXX ELISA, responses were boosted by injection of PPD for skin tests. Thus, serologic assays would be particularly valuable when applied as a supplemental test to detect additional infected animals missed by cell-mediated response-based tests. Variable sensitivities of the assay with the different sample sets may have resulted from variations in disease severity, timing of injection of PPD for skin tests, or other environmental impacts such as parasite burden or exposure to nontuberculous Mycobacterium spp. The test platform is readily available and familiar to veterinary laboratory diagnosticians worldwide. Potential applications for the IDEXX M. bovis ELISA include the following: (i) a movement test to identify at-risk herds prior to shipment, (ii) a supplemental test to complement cell-mediated response-based assays (e.g., immediately after skin test to benefit from the boosting effect of PPD administration [19] or between the 3- to 5-year interval skin testing), (iii) a slaughter surveillance test, (iv) an import test, and (v) as a bulk tank/milk test to identify M. bovis-infected herds. Of particular interest, a 1-day, high-throughput ELISA could be used at borders to provide additional and relatively inexpensive assurance of tuberculosis test compliance by trading partners. Also, the IDEXX M. bovis ELISA may be applied in routinely monitored herds to prioritize reactors for disposal, especially in regions or situations in which skin testing procedures are not feasible. For each of these applications, interpretation parameters would need to be evaluated depending on the requirements of the test as a disease management or infection surveillance tool.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kristin Bass, Bart Olthof, Jessica Pollock, and Emma Frimml-Morgan for excellent technical support as well as the animal care staff at NADC for providing excellent animal care and handling. We also thank the Animal Health Board and AssureQuality in New Zealand; the Ireland Department of Agriculture, Fisheries, and Food; Todd Byrem, Antel Biosystems; and USDA, APHIS, Veterinary Service for collection of samples from naturally infected herds and assistance with these studies.
USDA is an equal-opportunity provider and employer.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Supplemental material for this article may be found at http://cvi.asm.org/.
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Animal Plant and Health Inspection Service. 15 April 2010, posting date Bovine tuberculosis (TB). U.S. Department of Agriculture, Ames, IA: http://www.aphis.usda.gov/newsroom/hot_issues/bovine_tuberculosis/bovine_tb.shtml [Google Scholar]
- 2. Buddle B. M., et al. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10–16 [DOI] [PubMed] [Google Scholar]
- 3. Collins D. M., Stephens D. M. 1991. Identification of an insertion sequence IS1081, in Mycobacterium bovis. FEMS Microbiol. Lett. 67:11–15 [DOI] [PubMed] [Google Scholar]
- 4. Corner L. A., Stevenson M. A., Collins D. M., Morris R. S. 2003. The re-emergence of Mycobacterium bovis infection in brushtail possums (Trichosurus vulpecula) after localised possum eradication. N. Z. Vet. J. 51:73–80 [DOI] [PubMed] [Google Scholar]
- 5. Donnelly C. A., et al. 2006. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 439:843–846 [DOI] [PubMed] [Google Scholar]
- 6. Green L. R., et al. 2009. Single-antigen serological testing for bovine tuberculosis. Clin. Vaccine Immunol. 16:1309–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gortazar C., et al. 8 March 2011. Progress in the control of bovine tuberculosis in Spanish wildlife. Vet. Microbiol. 151:170–178 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8. Lyashchenko K., et al. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72:2462–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. More S. J., Good M. 2006. The tuberculosis eradication programme in Ireland: a review of scientific and policy advances since 1988. Vet. Microbiol. 112:239–251 [DOI] [PubMed] [Google Scholar]
- 10. Palmer M. V., Waters W. R., Whipple D. L. 2003. Aerosol delivery of virulent Mycobacterium bovis to cattle. Tuberculosis (Edinb.) 82:275–282 [DOI] [PubMed] [Google Scholar]
- 11. Pollock J. M., Buddle B. M., Andersen P. 2001. Towards more accurate diagnosis of bovine tuberculosis using defined antigens. Tuberculosis (Edinb.) 81:65–69 [DOI] [PubMed] [Google Scholar]
- 12. Schiller I., et al. 2010. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 57:205–220 [DOI] [PubMed] [Google Scholar]
- 13. Schmitt S. M., O'Brien D. J., Bruning-Fann C. S., Fitzgerald S. D. 2002. Bovine tuberculosis in Michigan wildlife and livestock. Ann. N. Y. Acad. Sci. 969:262–268 [DOI] [PubMed] [Google Scholar]
- 14. Taylor G. M., Worth D. R., Palmer S., Jahans K., Hewinson R. G. 2007. Rapid detection of Mycobacterium bovis DNA in cattle lymph nodes with visible lesions using PCR. BMC Vet. Res. 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. USDA Animal Plant and Health Inspection Service. 2005. Bovine tuberculosis eradication uniform methods and rules (APHIS 91-45-011). U.S. Government Printing Office, Washington, DC [Google Scholar]
- 16. Vordermeier H. M., et al. 2006. The BOVIGAM assay as ancillary test to the tuberculin skin test. Gov. Vet. J. 16:72–80 [Google Scholar]
- 17. Waters W. R., et al. 2010. Immune responses in cattle inoculated with Mycobacterium bovis, Mycobacterium tuberculosis, or Mycobacterium kansasii. Clin. Vaccine Immunol. 17:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waters W. R., et al. 2006. Immune responses to defined antigens of Mycobacterium bovis in cattle experimentally infected with Mycobacterium kansasii. Clin. Vaccine Immunol. 13:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waters W. R., et al. 2006. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin. Vaccine Immunol. 13:648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waters W. R., et al. 2004. Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp. avium and M. avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 11:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waters W. R., Palmer M. V., Whipple D. L. 2002. Mycobacterium bovis-infected white-tailed deer (Odocoileus virginianus): detection of immunoglobulin specific to crude mycobacterial antigens by ELISA. J. Vet. Diagn. Invest. 14:470–475 [DOI] [PubMed] [Google Scholar]
- 22. Whelan C., et al. 2010. Performance of the Enferplex TB assay with cattle in Great Britain and assessment of its suitability as a test to distinguish infected and vaccinated animals. Clin. Vaccine Immunol. 17:813–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whelan C., et al. 2008. Multiplex immunoassay for serological diagnosis of Mycobacterium bovis infection in cattle. Clin. Vaccine Immunol. 15:1834–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiker H. G. 2009. MPB70 and MPB83—major antigens of Mycobacterium bovis. Scand. J. Immunol. 69:492–499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.