Abstract
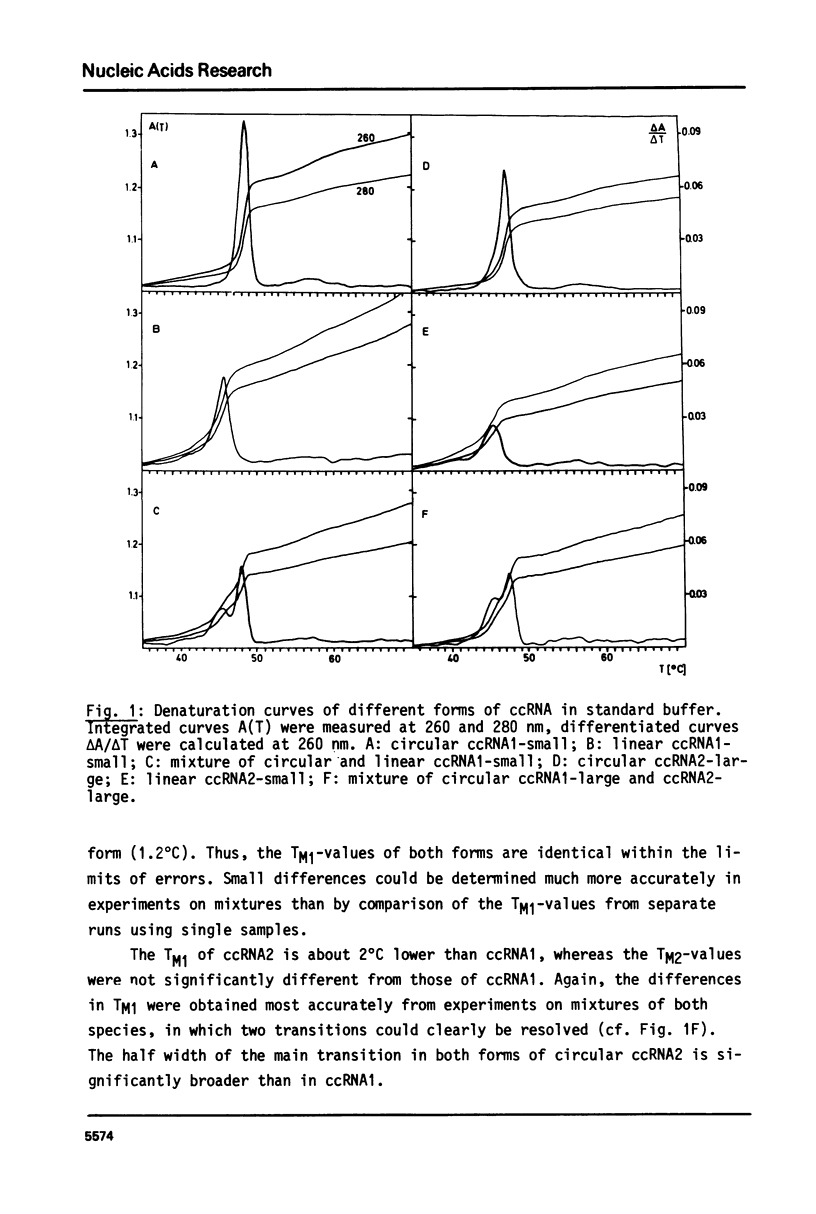
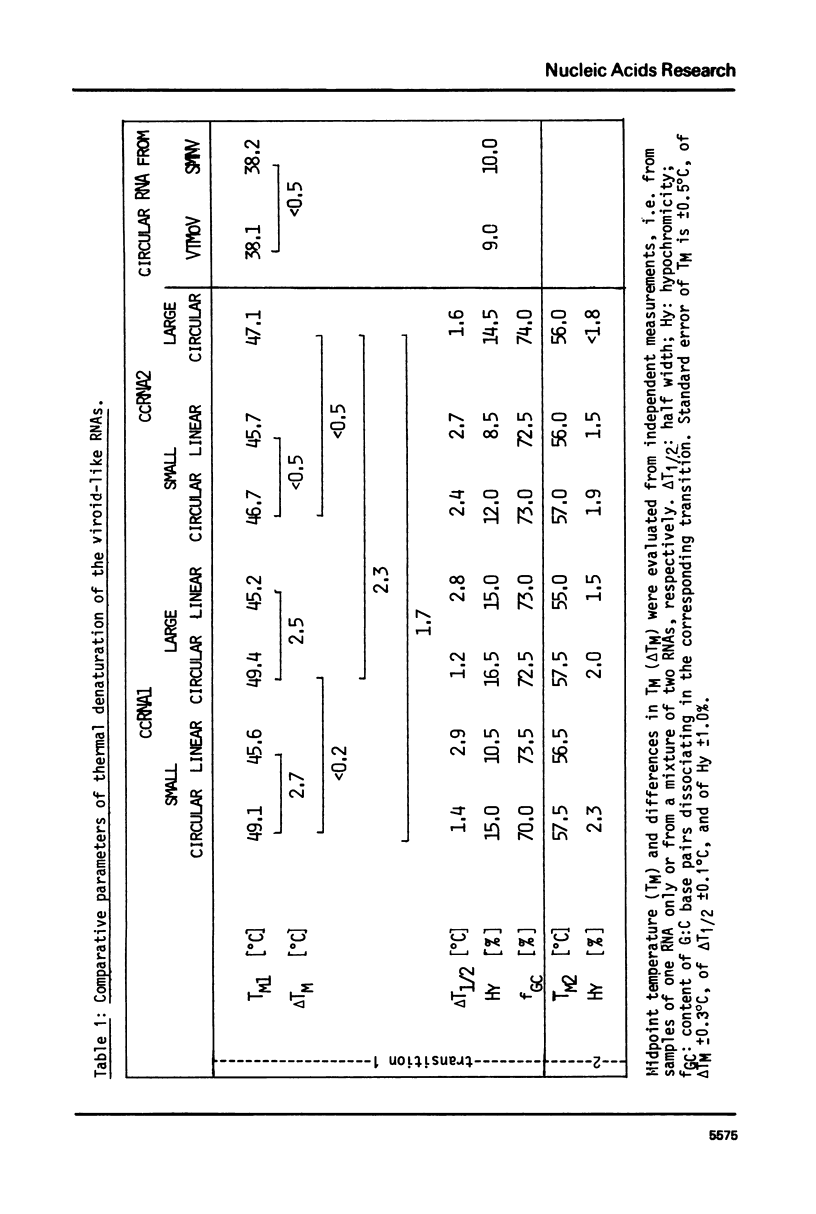
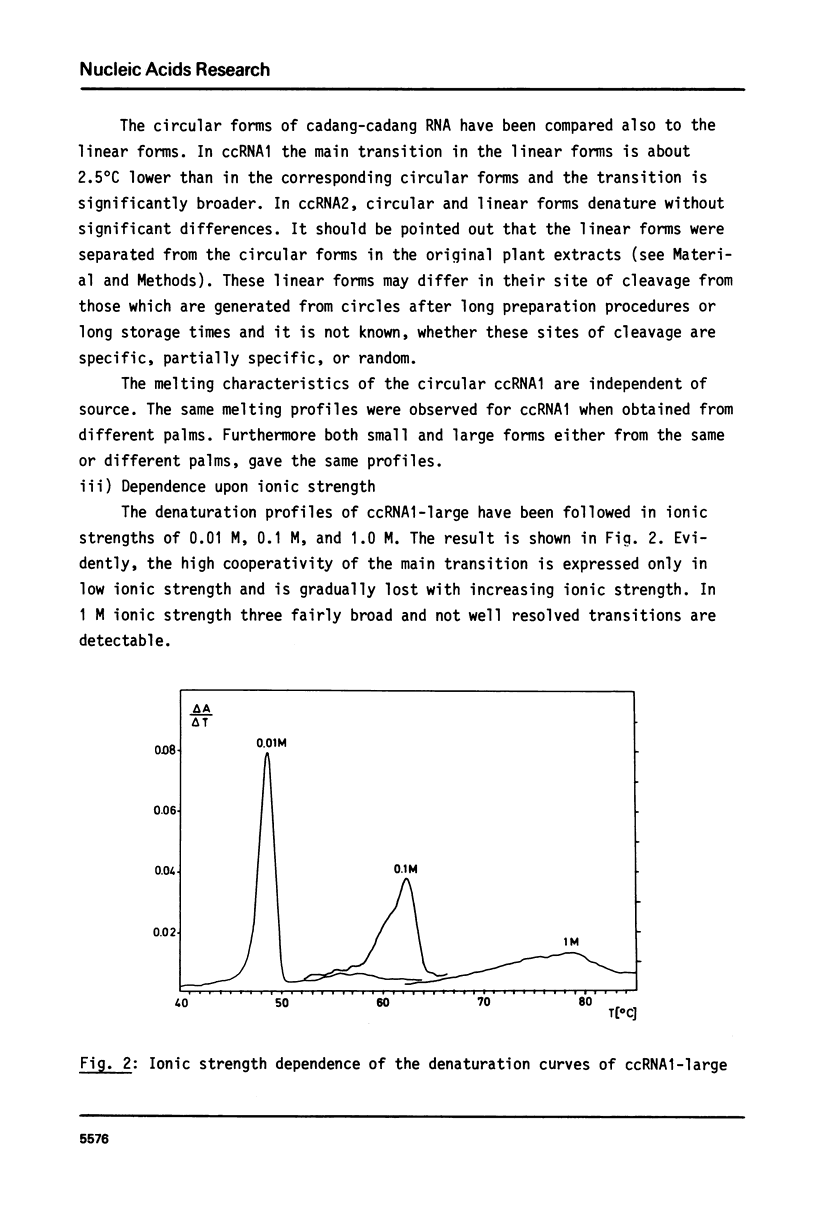
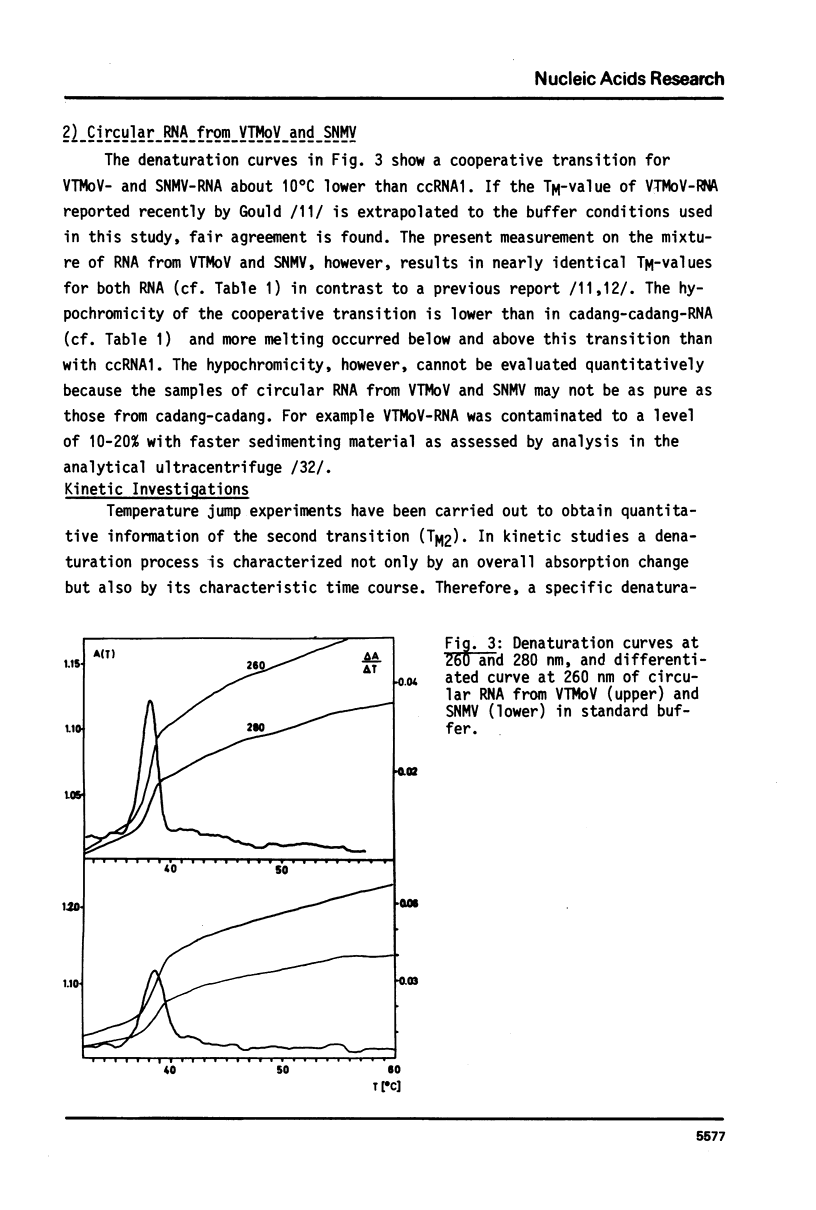
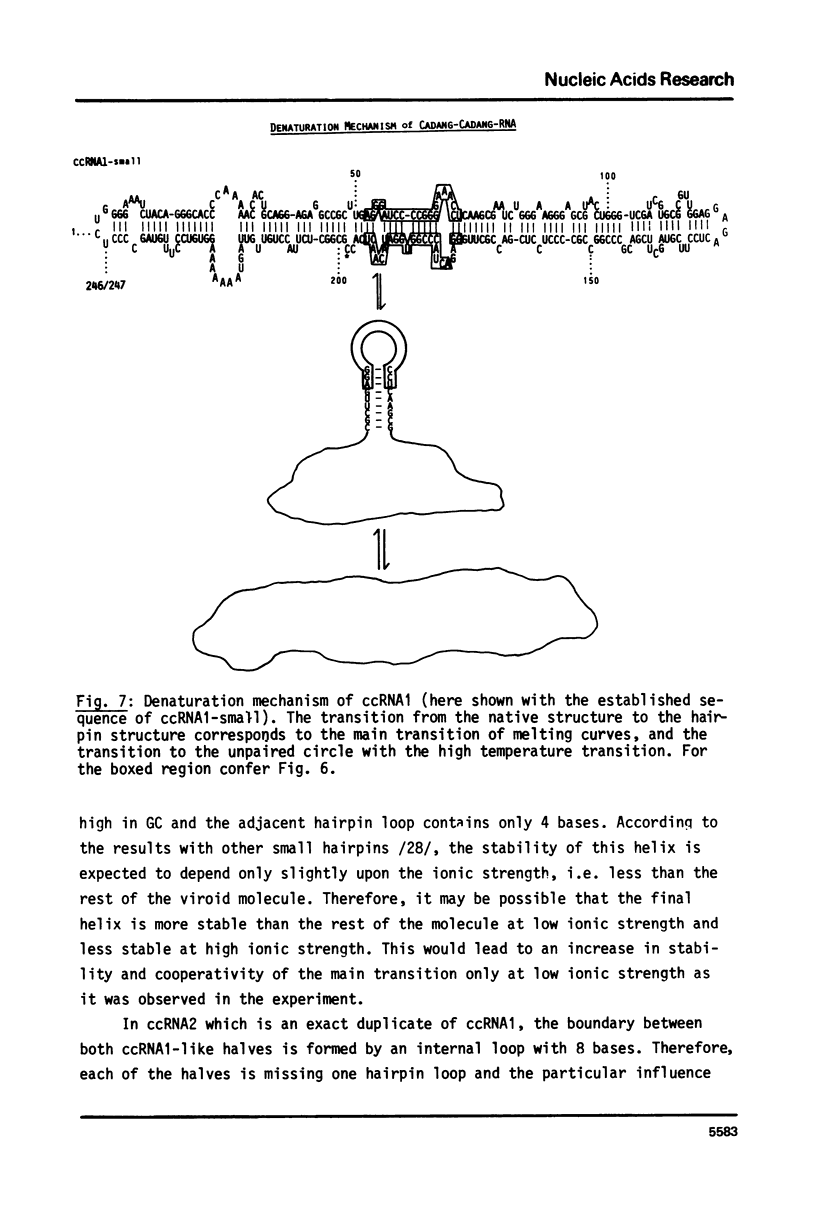
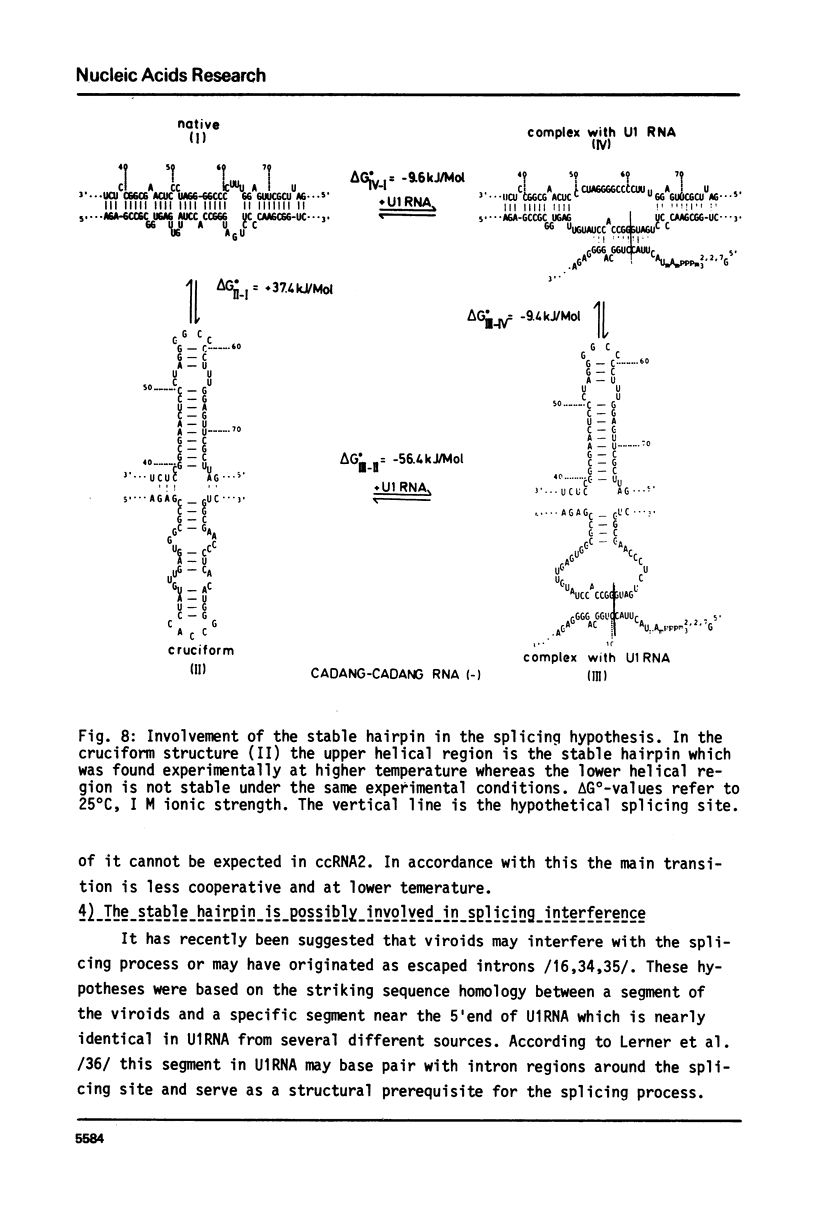
The conformational transitions of viroid-like RNAs associated with cadang-cadang disease, velvet tobacco mottle virus, and solanum nodiflorum mottle virus were studied by melting analysis and fast temperature jump technique in 1 mM sodium-cacodylate, 10 mM NaCl, 0.1 mM EDTA, pH 6.8. The 4 circular RNAs of cadang-cadang show a highly cooperative transition between 45 and 49 degrees C, respectively, and a second transition of less hypochromicity at about 10 degrees C higher temperatures. The data are interpreted quantitatively on the basis of the sequences and secondary structure models. A very similar scheme for the structure and structural transitions as derived earlier for other viroids applies to the cadang-cadang RNAs. In the main transition the total native secondary structure is disrupted and a stable hairpin consisting of 9 base pairs is newly formed which dissociates in the second transition. The thermal denaturation of the circular RNAs from the viruses mentioned above is clearly distinct from viroid RNA in respect to stability and cooperativity. The results on cadang-cadang RNA are discussed in the light of recent hypotheses about the interference of viroids with the splicing process of the host cell.
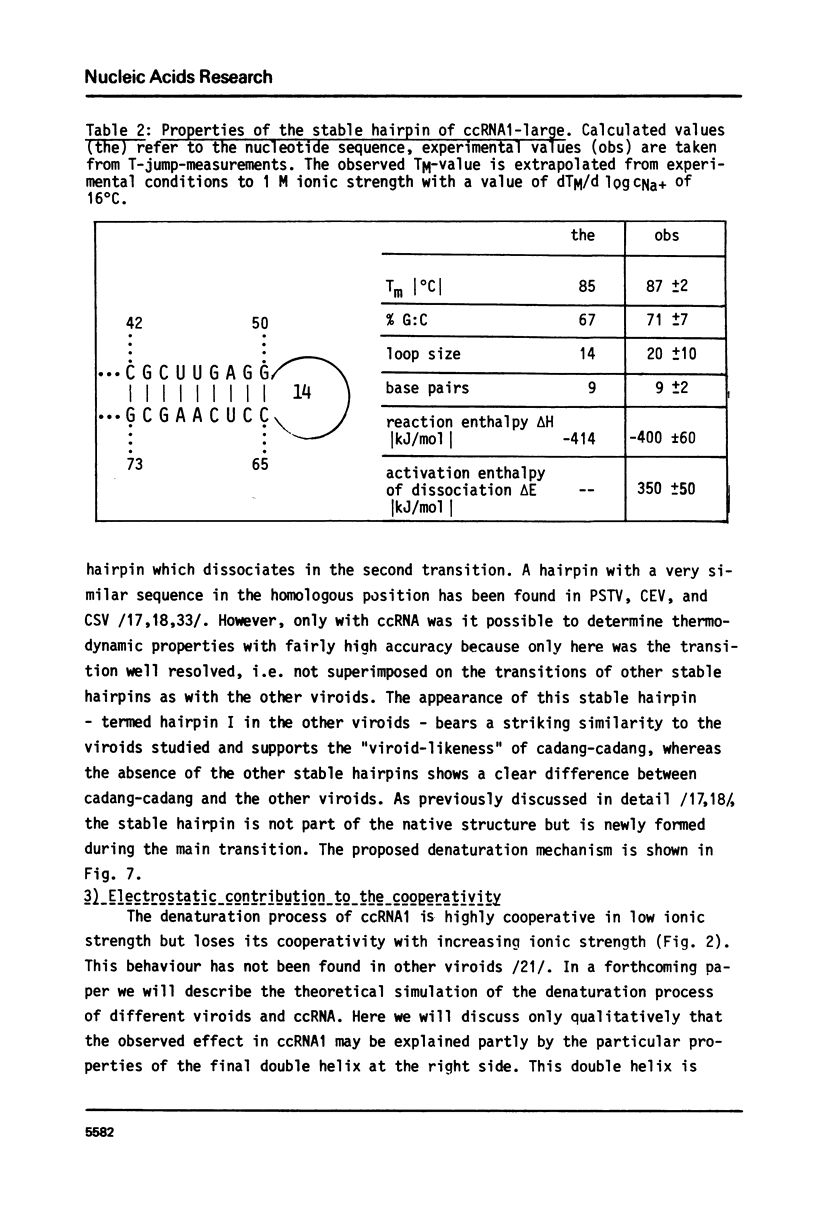
Full text
PDF




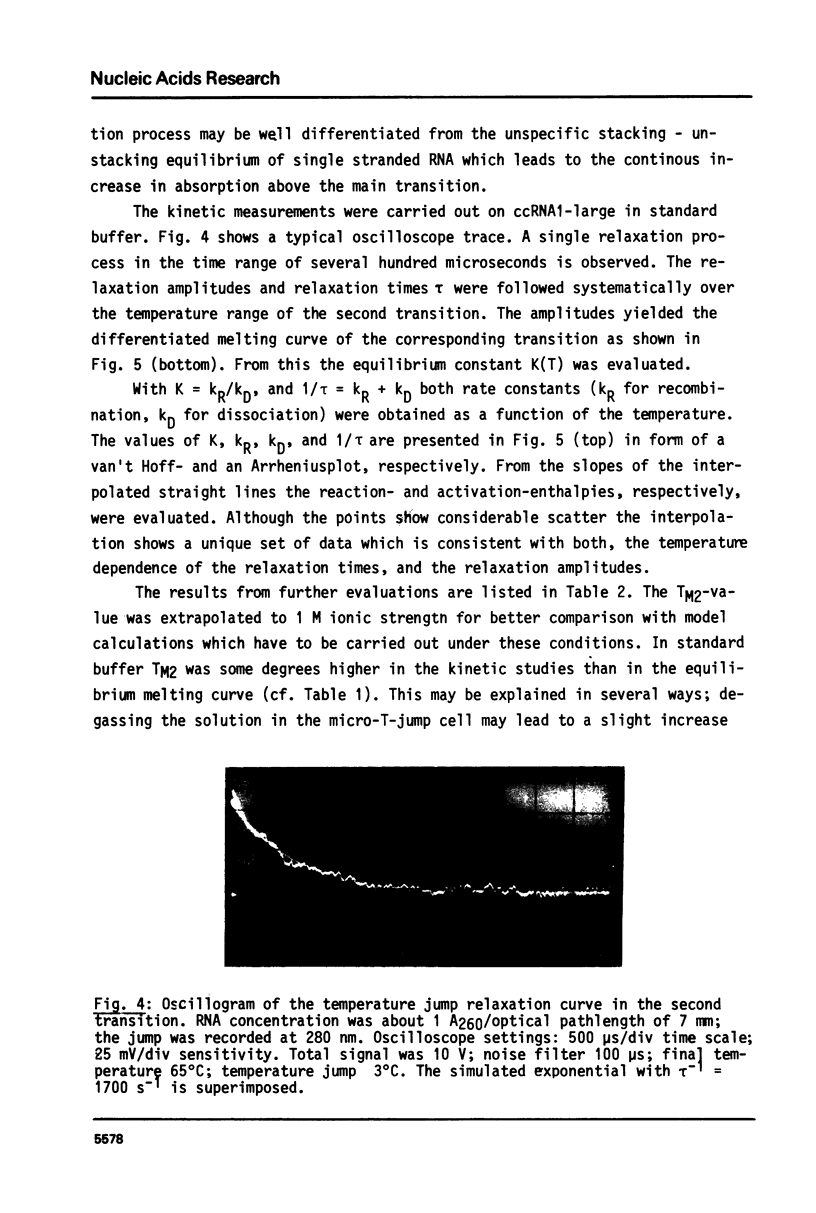



Images in this article
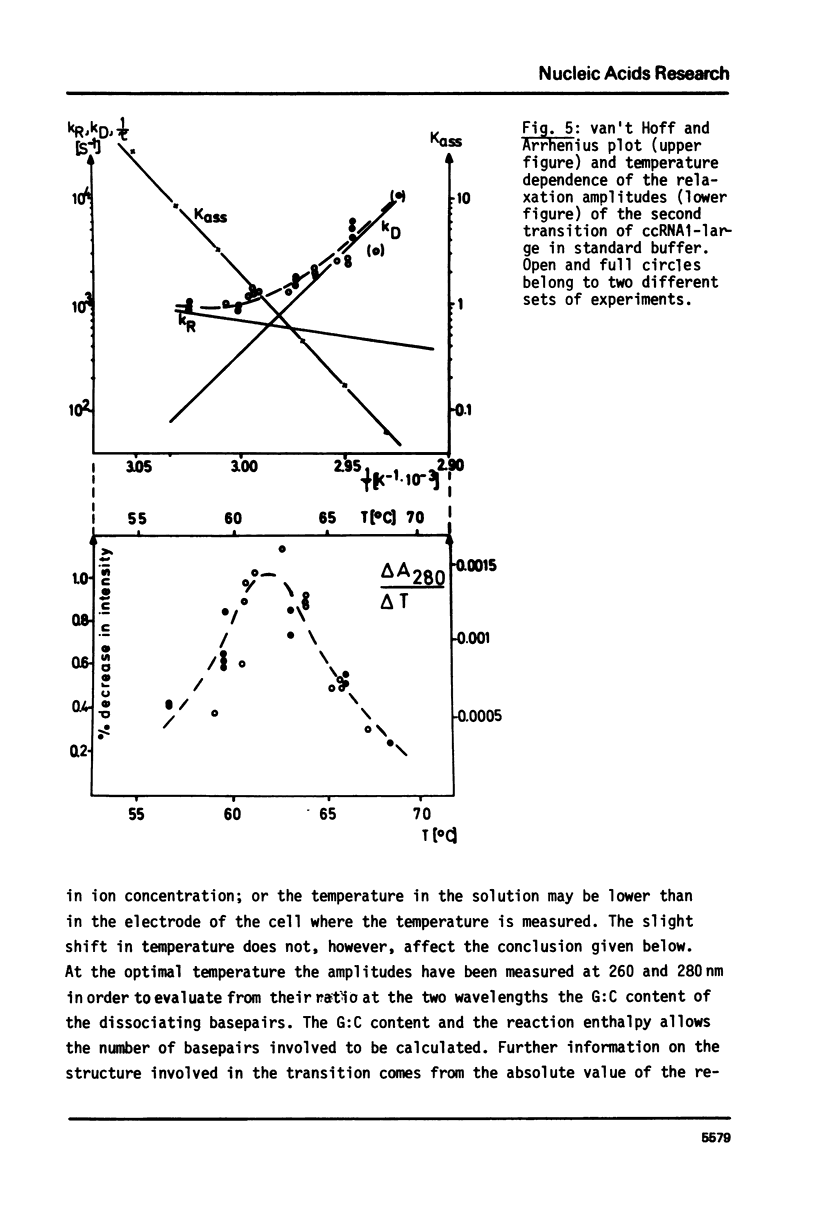
Selected References
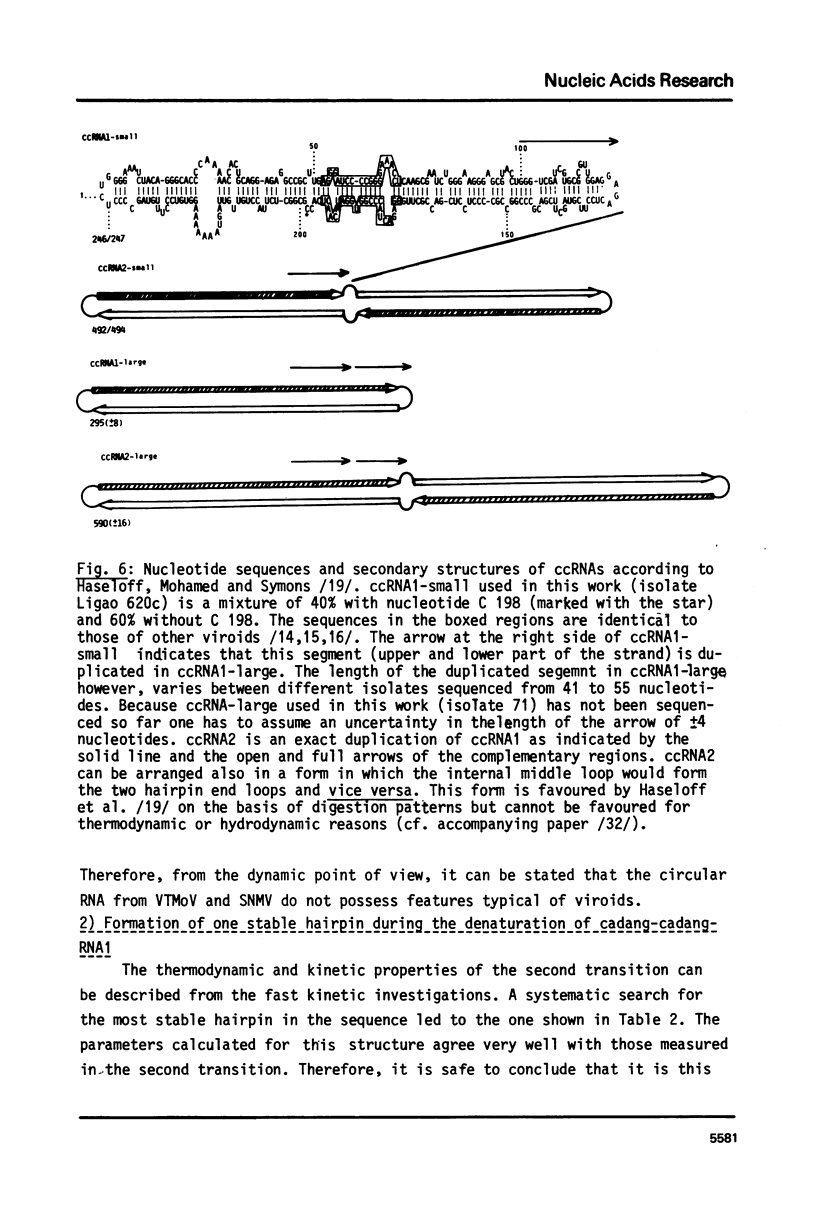
These references are in PubMed. This may not be the complete list of references from this article.
- Coutts S. M., Riesner D., Römer R., Rabl C. R., Maass G. Kinetics of conformational changes in tRNA Phe (yeast) as studied by the fluorescence of the Y-base and of formycin substituted for the 3'-terminal adenine. Biophys Chem. 1975 Oct;3(4):275–289. doi: 10.1016/0301-4622(75)80020-2. [DOI] [PubMed] [Google Scholar]
- Coutts S. M. Thermodynamics and kinetics of G-C base pairing in the isolated extra arm of serine-specific transfer RNA from yeast. Biochim Biophys Acta. 1971 Feb 25;232(1):94–106. doi: 10.1016/0005-2787(71)90494-1. [DOI] [PubMed] [Google Scholar]
- Dickson E. A model for the involvement of viroids in RNA splicing. Virology. 1981 Nov;115(1):216–221. doi: 10.1016/0042-6822(81)90104-5. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Are viroids escaped introns? Proc Natl Acad Sci U S A. 1981 Aug;78(8):5014–5015. doi: 10.1073/pnas.78.8.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Krupp G., Domdey H., Raba M., Jank P., Lossow C., Alberty H., Ramm K., Sänger H. L. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem. 1982 Jan;121(2):249–257. doi: 10.1111/j.1432-1033.1982.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Chrysanthemum stunt viroid: primary sequence and secondary structure. Nucleic Acids Res. 1981 Jun 25;9(12):2741–2752. doi: 10.1093/nar/9.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Comparative sequence and structure of viroid-like RNAs of two plant viruses. Nucleic Acids Res. 1982 Jun 25;10(12):3681–3691. doi: 10.1093/nar/10.12.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henco K., Steger G., Riesner D. Melting curves on less than 1 microgram of nucleic acid. Anal Biochem. 1980 Jan 1;101(1):225–229. doi: 10.1016/0003-2697(80)90065-2. [DOI] [PubMed] [Google Scholar]
- Henco K., Sänger H. L., Riesner D. Fine structure melting of viroids as studied by kinetic methods. Nucleic Acids Res. 1979 Jul 11;6(9):3041–3059. doi: 10.1093/nar/6.9.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- RALPH R. K., BELLAMY A. R. ISOLATION AND PURIFICATION OF UNDEGRADED RIBONUCLEIC ACIDS. Biochim Biophys Acta. 1964 May 18;87:9–16. doi: 10.1016/0926-6550(64)90041-6. [DOI] [PubMed] [Google Scholar]
- Randles J. W., Rillo E. P., Diener T. O. The viroidlike structure and cellular location of anomalous RNA associated with the cadang-cadang disease. Virology. 1976 Oct 1;74(1):128–139. doi: 10.1016/0042-6822(76)90135-5. [DOI] [PubMed] [Google Scholar]
- Riesner D., Colpan M., Randles J. W. A microcell for the temperature-jump technique. Anal Biochem. 1982 Mar 15;121(1):186–189. doi: 10.1016/0003-2697(82)90574-7. [DOI] [PubMed] [Google Scholar]
- Riesner D., Henco K., Rokohl U., Klotz G., Kleinschmidt A. K., Domdey H., Jank P., Gross H. J., Sänger H. L. Structure and structure formation of viroids. J Mol Biol. 1979 Sep 5;133(1):85–115. doi: 10.1016/0022-2836(79)90252-3. [DOI] [PubMed] [Google Scholar]
- Steger G., Müller H., Riesner D. Helix-coil transitions in double-stranded viral RNA. Fine resolution melting and ionic strength dependence. Biochim Biophys Acta. 1980 Feb 29;606(2):274–284. doi: 10.1016/0005-2787(80)90037-4. [DOI] [PubMed] [Google Scholar]




