Abstract
The Mycobacterium tuberculosis complex (MTBC) consists of a group of closely related species that differ in their epidemiological profiles, host ranges, pathogenicities, geographic distributions, and drug resistances. Identification of members in the MTBC is essential for monitoring the epidemiology of tuberculosis (TB) and implementing appropriate public health control measures. In this study, 188 consecutive MTBC clinical isolates from 2007 to 2010 were evaluated to determine the prevalence of MTBC species in Turkey. PCR and restriction fragment length polymorphism analysis (PCR-RFLP) of the gyrB gene were used, and results for species other than M. tuberculosis were confirmed using the GenoType MTBC assay (Hain Lifescience, Nehren, Germany). Most of the strains were found to be M. tuberculosis (94.1%). The prevalences of M. bovis and M. caprae were 4.3% and 1.6%, respectively. Only one M. bovis BCG strain was identified. Overall, the frequency of bovine tuberculosis in humans was 5.3%. We had assumed that bovine TB infection was under control in animal herds, but primary M. bovis infections in humans caused by transmission from infected animals are still an issue in Turkey. Our results indicate that the frequent identification of M. bovis in routine mycobacteriological laboratory work has further importance due to the well-known resistance of this species to pyrazinamide.
INTRODUCTION
The Mycobacterium tuberculosis complex (MTBC) consists of a group of closely related Mycobacterium species, including M. tuberculosis, M. bovis, M. africanum, M. microti, and M. caprae. These species are the primary cause of tuberculosis in humans, and they also infect wild and domesticated animals. The close interrelatedness of these species has been demonstrated by DNA-DNA hybridization, multilocus enzyme electrophoresis, and sequencing of the 16S rRNA genes and the 16S-23S rRNA gene internal transcribed spacers (12, 16).
However, the epidemiology, host range, pathogenicity, geographic distribution, and drug resistance of these species are quite different (12, 16, 22). Different species display different phenotypic characteristics according to conventional biochemical tests. Because differentiation of species in the MTBC by these methods is labor-intensive, time-consuming, and dependent on sufficient bacterial growth, these tests may not be performed routinely in every laboratory (19, 23). Therefore, various molecular methods have been used for differentiation, such as DNA sequencing (i.e., gyrB and hsp65) and PCR amplification of regions of differences (RDs) or spoligotyping (9, 11, 26, 29, 30). Although they are rapid and more accurate than the conventional methods, such procedures usually require specialized molecular laboratories and technical experience (22, 26, 29).
In this study, our goal was to determine the distribution of consecutive MTBC isolates at the species level from 2007 to 2010 in one of the largest research and training hospitals in Istanbul. We also wished to investigate the current status of bovine tuberculosis in Turkey. To our knowledge, this is the first study conducted in Turkey to define the prevalences of different species in the MTBC. We also attempted to determine the suitability of gyrB gene amplification with M. tuberculosis primers followed by restriction fragment length polymorphism (RFLP) analysis, as described by Niemann et al. (16), for routine use in mycobacteriology laboratories to identify species in the MTBC.
MATERIALS AND METHODS
Strains analyzed.
A total of 188 consecutive MTBC strains isolated from 2007 to 2010 at Sisli Etfal Research and Training Hospital were included in the study. During that period, 3,828 samples from 2,436 patients were sent to the Clinical Microbiology Laboratory of the hospital for acid-fast staining and mycobacterium culture. Specimens were inoculated into mycobacterial-growth indicator tubes (MGIT) and Lowenstein-Jensen (LJ) medium after appropriate homogenization and decontamination procedures. Only one isolate per patient was included in the study. Patients whose strains were included resided in Turkey and did not have a history of travel outside the country.
Extraction of DNA.
From each MGIT, 500 μl of medium containing mycobacteria was transferred into a 1.5-ml microcentrifuge tube, and organisms were inactivated by incubation at 80°C for 10 min. Then 250 μl of 10% Chelex100 was added to the tube; the mixture was vortexed and incubated at 60°C for 10 min. The suspension was vortexed again and incubated at 100°C for a further 15 min. Lastly, the tube was centrifuged at 14,000 rpm for 3 min, and the supernatant was kept at −70°C and used as the DNA template in PCRs.
hsp65 PCR-RFLP.
All isolates were identified using the hsp65 PCR-RFLP method as described by Telenti et al. (31) with some modifications. Briefly, the hsp65 gene was amplified in a master mix containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate (dNTP) (Promega), 50 pmol of each primer (Tb11 [5′-ACC AAC GAT GGT GTG TCC AT-3′] and Tb12 [CTT GTC GAA CCG CAT ACC CT-3′]), 1 U of Taq DNA polymerase (Promega), and 5 μl of template DNA in a final volume of 50 μl. PCR amplifications were performed in a MyCycler (Bio-Rad) according to the following protocol: 94°C for 5 min; 30 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min; 72°C for 10 min. The 441-bp amplicon was digested with BstEII and HaeIII, and strains showing banding patterns of 231 bp, 116 bp, 79 bp, 152 bp, 127 bp, and 69 bp in agarose gel electrophoresis were accepted as MTBC strains. The ϕX174 DNA/HinfI marker (Fermentas) was used in gel electrophoresis as a size standard.
gyrB PCR-RFLP.
gyrB PCR allows amplification of a part of the gyrB gene specific to MTBC species, so it can be used for identification of MTBC isolates (16). Furthermore, DNA sequence polymorphisms in a 1,020-bp fragment of the gyrB gene were used in the PCR-RFLP assay for rapid differentiation of the MTBC strains. RsaI, TaqI, and SacII, which target the discriminative regions at positions 675/756, 1450 and 1410/1311, respectively, of the gyrB gene, were exploited for this purpose. The primers MTUB-f (5′-TCGGACGCCTATGCGATATC-3′) and MTUB-r (5′-ACATACAGTTCGGACTTGCG-3′) were used for amplification of a 1,020-bp fragment of the gyrB gene (16). Each 50-μl reaction mixture contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 M MgCl2, 200 μM (each) deoxynucleoside triphosphate (dNTP) (Promega), 20 pmol of each primer, 1 U of Taq DNA polymerase (Promega) and 5 μl of template DNA. PCR amplifications were performed in a MyCycler (Bio-Rad) according to the following protocol: 80°C for 5 min; 30 cycles of 94°C for 1 min, 65°C for 1 min, and 72°C for 1 min; 72°C for 10 min. DNA polymorphisms in the 1,020-bp gyrB fragment amplified with the primers MTUB-f and MTUB-r were analyzed after digestion with restriction enzymes RsaI, SacII, and TaqI as recommended by the manufacturer (Promega). The DNA digest was separated in a 2% agarose gel by electrophoresis at a constant voltage of 90 V for 1 h, and the gels were visualized under a UV transilluminator. A 100-bp DNA ladder was used as a size standard.
Interpretation of the results was based on the association of species designations with certain banding patterns; certain modifications were made due to changes in the nomenclature of the MTBC (Table 1). M. africanum subtype II is no longer considered a different species; it is a phenotypic variant of M. tuberculosis and has been reclassified as M. tuberculosis sensu stricto. M. africanum subtype I was renamed M. africanum. It is not possible to differentiate “Mycobacterium canettii” from M. tuberculosis with gyrB PCR-RFLP. However, use of this method in non-African populations with a low frequency of M. canettii was reported not to cause significant errors due to failure of this differentiation (5, 15, 26).
Table 1.
RFLP patterns for differentiation of members of the MTBC using RsaI, TaqI, and SacI to digest a 1,020-bp fragment of gyrB
| Enzyme | Banding pattern | Identification |
|---|---|---|
| RsaI | 360 and 560 bp | M. tuberculosis/M. africanum |
| 360 and 480 bp | M. bovis/M. caprae | |
| 360 and 660 bp | M. microti | |
| TaqI | 250 and 440 bp | M. tuberculosis |
| 440 bp | M. africanum | |
| SacII | No digestion | M. bovis |
| 280 and 740 bp | M. caprae |
GenoType MTBC assay.
The GenoType MTBC assay (Hain Lifescience, Nehren, Germany) was performed according to the manufacturer's instructions. Thirty-five microliters of a primer-nucleotide mixture (provided with the kit), an amplification buffer containing 2.5 mM MgCl2 and 1.25 U of Hot Start Taq polymerase (Qiagen, Hilden, Germany), and 5 μl of DNA in a final volume of 50 μl were used for amplification. The amplification protocol consisted of 15 min of denaturation at 95°C; 10 cycles of 30 s at 95°C and 2 min at 58°C; 20 cycles of 25 s at 95°C, 40 s at 53°C, and 40 s at 70°C; and 70°C for 8 min. Hybridization and detection were performed manually according to the kit insert with strips containing 13 probes in plastic wells. The hybridization procedure was performed at 45°C for 0.5 h followed by colorimetric detection of hybridized amplicons on strips, which takes 1 h. Amplification and hybridization controls are incorporated in the assay to verify the test procedure. A template sheet showing the positions of the lines and the interpretation table, both provided with the kit, were used for interpretation of the test results. Discrimination of M. bovis BCG and confirmation of M. bovis and M. caprae strains were performed with the GenoType MTBC assay.
RESULTS
A total of 192 Mycobacterium strains were isolated during the study period, 4 of which were mycobacteria other than M. tuberculosis (MOTT) (one M. abscessus strain and three M. avium complex strains) and 188 of which were identified as MTBC by hsp65 PCR-RFLP. It was determined that 54% of the clinical specimens came from patients with pulmonary tuberculosis. The distribution of specimens was as follows; sputum, 65; gastric lavage fluids, 34; biopsy specimens, 29; abscess, 27; body fluids, 25 (cerebrospinal, 12; pleural, 4; peritoneal, 9); urine, 5; bronchoalveolar lavage fluid, 2; and tracheal aspirate, 1.
The RsaI digestion patterns of the MTBC are shown in Fig. 1, and the TaqI digestion patterns are shown in Fig. 2. Of 188 MTBC strains, 177 (94.1%) were determined to be M. tuberculosis, 8 (4.3%) were M. bovis, and 3 (1.6%) were M. caprae. One sample was determined to be M. bovis BCG by the GenoType MTBC assay (Hain Lifescience, Nehren, Germany). The frequency of bovine tuberculosis (TB) in humans was found to be 5.3%. The characteristics of patients from whom M. bovis or M. caprae strains were isolated are summarized in Table 2. Patients identified as having M. bovis or M. caprae infections either were living in or regularly visited rural areas. Moreover, 2 of them (patients 5 and 10) had a history of working in the stockbreeding industry, and 2 of them (patients 2 and 11) were children from families that had cattle herds. The patients with M. caprae infections lived in the European part of Turkey, and the patients with M. bovis infections were from the Asian part. Neither M. africanum nor M. microti strains were detected during the 4-year period.
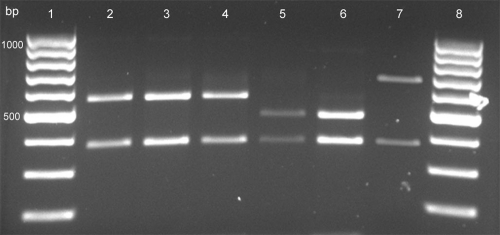
Fig. 1.
RFLP patterns of gyrB PCR products obtained by digestion with RsaI. Lanes 1 and 8, 100-bp DNA ladder; lanes 2 and 3, M. tuberculosis (360- and 560-bp bands); lane 4, M. africanum (360- and 560-bp bands); lane 5, M. bovis (360- and 480-bp bands); lane 6, M. caprae (360- and 480-bp bands); lane 7, M. microti (360- and 660-bp bands).
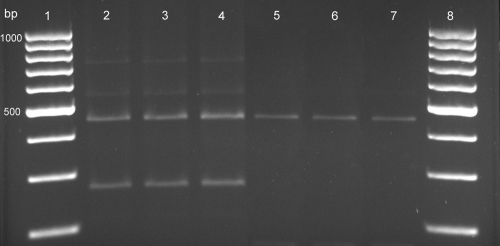
Fig. 2.
RFLP patterns of gyrB PCR products obtained by digestion with TaqI. Lanes 1 and 8, 100-bp DNA ladder; lanes 2 to 4, M. tuberculosis (250- and 440-bp bands); lanes 5 to 7; M. africanum (440-bp band).
Table 2.
Characteristics of M. bovis and M. caprae tuberculosis patients
| Patient no. | Age (yr) | Sex | Clinic | Material sampled | Diagnosis | Species | Predisposing factors |
|---|---|---|---|---|---|---|---|
| 1 | 12 | F | Pediatrics | Biopsy | Intestinal TB | M. bovis | Crohn's disease |
| 2 | 5 | F | Pediatrics | Pleural fluid | Disseminated TB | M. bovis | Chronic renal failure |
| 3 | 27 | F | Orthopedics | Biopsy | Pott disease | M. bovis | NDa |
| 4 | 56 | F | Otolaryngology | Biopsy | Lymphadenitis | M. bovis | End-stage renal failure |
| 5 | 72 | M | Orthopedics | Abscess | Vertebral osteomyelitis | M. bovis | ND |
| 6 | 49 | F | Otolaryngology | Biopsy | Lymphadenitis | M. bovis | ND |
| 7 | 39 | F | Otolaryngology | Biopsy | Lymphadenitis | M. bovis | ND |
| 8 | 1 | F | Pediatrics | Abscess | Cutaneous TB | M. bovis BCG | BCG vaccination at 8 months of age |
| 9 | 27 | F | Nephrology | Peritoneal fluid | Peritoneal TB | M. caprae | End-stage renal failure |
| 10 | 25 | M | Nephrology | Urine | Urinary TB | M. caprae | Polycystic kidney disease, chronic renal failure |
| 11 | 6 | M | Pediatrics | Biopsy | Lymphadenitis | M. caprae | ND |
ND, not determined.
DISCUSSION
The MTBC remains the most important group within the genus Mycobacterium from global and clinical perspectives because it includes the causative agent of the worldwide TB epidemic. In low-burden countries, a resurgence of tuberculosis has occurred due to the emergence of HIV in the 1980s, and it has been considered a disease of specific populations, including immunodeficient patients, immigrants, migrant workers, homeless people, and residents of long-term-care facilities (33). However, Turkey is a country with an intermediate epidemic load, and tuberculosis is still a disease of young persons, which indicates the need for improved tuberculosis control (13). Therefore, it was not surprising for us to find that of all the positive mycobacterial cultures in our study population, only four were MOTT, indicating that TB remains an issue. Similarly, it was reported recently that MOTT strains are rarely isolated as the causative agents of tuberculosis in Turkey (3). In contrast, the prevalence of MOTT species is reported to have risen in Canada and several European countries. Among MOTT species, the Mycobacterium avium complex (MAC) is the most frequently isolated (25). In addition, the rate of isolation of MAC strains equals or surpasses the rate of isolation of MTBC strains in some parts of the United States due to the HIV epidemic (33).
Of species in the MTBC, M. tuberculosis is the most common cause of tuberculosis in humans worldwide, except in parts of Africa where M. africanum is widely distributed (5, 8, 10, 22). Because tuberculosis caused by other species in the MTBC is rare, identification of those species is not usually performed in routine mycobacteriological laboratory work (22). However, those species have different host preferences, pathogenicity, geographic distribution, and drug resistance, which may have distinct public health implications and may affect the selection of appropriate treatment regimens (12, 16, 17, 22).
To our knowledge, this is the first study to identify the prevalences of species in the MTBC in Turkey. M. tuberculosis was found to be the major causative agent of human tuberculosis in our study population, as was expected. The prevalences of M. tuberculosis, M. bovis, and M. caprae were found to be 94.1%, 4.3%, and 1.6%, respectively, within the 4-year period. Although isolation of M. microti and M. africanum strains in Turkey had been reported previously (1), we did not detect M. africanum or M. microti strains. Our data show that M. bovis is the second most common MTBC species that causes tuberculosis in Turkey. We also isolated three M. caprae strains, which used to be regarded as exceptional strains of M. bovis because of their susceptibility to pyrazinamide (PZA) (14). The host preference of M. caprae and its transmission to humans are the same as those of M. bovis. Human tuberculosis cases caused by M. caprae are rarely seen worldwide. However, M. caprae was found to be the major agent of bovine TB in both animals and humans in central Europe (14, 23, 24). The prevalence of M. bovis and M. caprae infections in humans in Turkey is not yet known. The prevalences found in this study reflect the situation in Istanbul, where our hospital is located, but comparison of Istanbul with other regions in Turkey is impossible due to a lack of data. It has been reported that M. bovis infection in humans accounts for 0.5 to 7.2% of human tuberculosis cases in industrialized nations, and it is estimated to be responsible for 10 to 15% of new cases in the developing world (6). In a study conducted by the Spain National Mycobacterial Reference Laboratory of all isolates obtained between 2004 and 2007, the prevalences of M. bovis and M. caprae were reported to be 1.9% and 0.3%, respectively (27).
The frequency of bovine TB in humans was found to be 5.3% in this study. Primary bovine TB infections in humans indicate ongoing transmission of the disease from infected animals to humans. Humans are infected via consumption of raw milk, inhalation, or contact with the meat or blood of infected animals (4, 6, 7, 32). Most of the M. bovis isolates in this study came from patients with extrapulmonary infections, including cervical lymphadenitis and bone and intestinal tuberculosis, which is consistent with the transmission of bacilli via consumption of contaminated milk (4). Patients identified as having M. bovis or M. caprae infections in our study either were living in or regularly visited rural areas, where they had close contact with animals and/or consumed raw milk. Although it is not possible to distinguish M. bovis from M. caprae clinically, our findings suggest that the geographic distributions of M. bovis and M. caprae are different from each other in Turkey: the M. caprae cases occurred in the European part of the region, while the M. bovis cases were in the Asian part. Nevertheless, this hypothesis is of limited value because the number of the cases is small, indicating a need for broader epidemiological studies in both humans and animals.
The tuberculosis control system for livestock in Turkey includes the tuberculin test, laboratory testing, slaughtering of infected animals (with compensation), and quarantine measures (18). In a study conducted on cattle in a slaughterhouse in the southern region of the country, the lungs were reported to be the organs most commonly infected. Respiratory transmission of the disease was common due to the use of intensive breeding and closed barn systems (21). Other factors, such as the small-scale structure of animal rearing, overstocking or poor animal nutrition due to grazing livestock on public lands, difficulty controlling animals' movements across the eastern and southeastern borders of the country, and the existence of disease reservoirs in isolated rural areas, impede appropriate monitoring and control (20). Based on an agreement with the European Union, new regulations requiring tuberculosis-free cattle herds were put into practice, and the frequency of animal inspection was increased over the years. Implementation of tighter control measures will further decrease the number of human cases in Turkey. Identification of M. bovis and M. caprae infections in humans might also help detect the animal source of the infection.
We isolated one M. bovis BCG strain during the study period from a 1-year-old infant who had cutaneous tuberculosis, an infection that was attributed to receiving the BCG vaccine at 8 months of age. Predisposing factors that may lead to BCG infection were investigated, but no deficiencies in the infant's humoral or cell-mediated immunity were found (data not shown). Cutaneous tuberculosis due to BCG vaccination in an otherwise healthy baby was also reported by Okazaki et al. (19).
An assay capable of distinguishing between species in the MTBC is needed in clinical laboratories (4, 22). Moreover, the assay needs to be rapid, simple, and affordable. In the present study, MTBC species were differentiated by using the gyrB PCR-RFLP method. The gyrB PCR-RFLP assay is an in-house method, and results can be obtained in 9 h, but it is inadequate for discriminating between M. bovis BCG and other M. bovis strains (16). However, the GenoType MTBC assay (Hain Lifescience, Nehren, Germany) is a commercial kit, and therefore it requires less expertise and takes less time (results available in about 5 h) than the gyrB PCR-RFLP assay. It can also discriminate between BCG strains and other M. bovis isolates by identifying the deletion of the RD1 region (26, 28). This feature is important for the detection of M. bovis BCG infections caused by vaccination or intravesicular instillation of BCG for treatment of superficial transitional cell carcinomas of the bladder (2, 17, 19). However, the high cost of the GenoType MTBC assay might limit its use in routine laboratory practice, especially in countries with limited resources. The use of the GenoType MTBC assay in cases where M. bovis and BCG discrimination is crucial would be a cost-effective approach.
Identification of unusual members of the MTBC, such as M. bovis and M. caprae, is essential to understanding the epidemiology and clinical significance of those members. Moreover, because of speciation, it has become possible to exclude PZA from the treatment of M. bovis infections. Identification of MTBC strains at the species level should be performed in clinical microbiology laboratories, and those data ought to be made available for national reference centers to map the distribution of tuberculosis species according to region. Collection of data in reference centers could be useful for developing more effective prevention and control measures for both human and animal diseases.
Footnotes
Published ahead of print on 21 September 2011.
REFERENCES
- 1. Agacayak A., Bulut Y., Seyrek A. 2007. Detection of Mycobacterium species distribution in the sputum samplesof tuberculosis patients by PCR-RFLP method in Elazığ province. Microbiol. Bült. 41:203–209 [PubMed] [Google Scholar]
- 2. Aljada I. S., Crane J. K., Corriere N., Wagle D. G., Amsterdam 1999. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott's disease) following intravesical BCG therapy. J. Clin. Microbiol. 37:2106–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bicmen C., et al. 2010. Nontuberculous mycobacteria isolated from pulmonary specimens between 2004 and 2009: causative agent or not? New Microbiol. 33:399–403 [PubMed] [Google Scholar]
- 4. Cicero R., Olivera H., Hernandez-Solis A., Ramirez-Casanova E., Escobar-Gutierrez A. 2009. Frequency of Mycobacterium bovis as an etiologic agent in extrapulmonary tuberculosis in HIV-positive and -negative Mexican patients. Eur. J. Clin. Microbiol. Infect. Dis. 28:455–460 [DOI] [PubMed] [Google Scholar]
- 5. de Jong B. C., Antonio M., Gagneux S. 2010. Mycobacterium africanum—review of an important cause of human tuberculosis in West Africa. PLoS Neglected Trop. Dis. 4:e744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de la Rua-Domenech R. 2006. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis 86:77–109 [DOI] [PubMed] [Google Scholar]
- 7. Gibson A. L., et al. 2004. Molecular epidemiology of disease due to Mycobacterium bovis in humans in the United Kingdom. J. Clin. Microbiol. 42:431–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grange J.M., Yates M. D. 1989. Incidence and nature of human tuberculosis due to Mycobacterium africanum in south-east England: 1977–87. Epidemiol. Infect. 103:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haddad N., et al. 2001. Spoligotype diversity of Mycobacterium bovis strains isolated in France from 1979 to 2000. J. Clin. Microbiol. 39:3623–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huard R. C., Lazzarini L. C., Butler W. R., Van Soolingen D., Ho J. L. 2003. PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions. J. Clin. Microbiol. 41:1637–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamerbeek J., et al. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kasai H., Ezaki T., Harayama S. 2000. Differentiation of phylogenetically related slowly growing mycobacteria by their gyrB sequences. J. Clin. Microbiol. 38:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kılıcaslan Z. 2007. Tuberculosis in the world and Turkey. Ankem Derg 21:76–80 [Google Scholar]
- 14. Kubica T., Rüsch-Gerdes S., Niemann S. 2003. Mycobacterium bovis subsp. caprae caused one-third of human M. bovis-associated tuberculosis cases reported in Germany between 1999 and 2001. J. Clin. Microbiol. 41:3070–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mostowy S., et al. 2004. Genomic analysis distinguishes Mycobacterium africanum. J. Clin. Microbiol. 42:3594–3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Niemann S., Harmsen D., Rüsch-Gerdes S., Richter E. 2000. Differentiation of clinical Mycobacterium tuberculosis complex isolates by gyrB DNA sequence polymorphism analysis. J. Clin. Microbiol. 38:3231–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nikaido T., et al. 2007. Mycobacterium bovis BCG vertebral osteomyelitis after intravesical BCG therapy, diagnosed by PCR-based genomic deletion analysis. J. Clin. Microbiol. 45:4085–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. OIE 2004. World animal health report; 2004 country report on Turkey, p. 343–347 OIE, Paris, France [Google Scholar]
- 19. Okazaki T., et al. 2005. Multiplex PCR-identified cutaneous tuberculosis evoked by Mycobacterium bovis BCG vaccination in a healthy baby. J. Clin. Microbiol. 43:523–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oskam A., et al. 2004. Turkey in the European Union: consequences for agriculture, food, rural areas and structural policy—final report. Wageningen University and Research Centre (UR), Wageningen, The Netherlands [Google Scholar]
- 21. Ozmen O., Kursun O., Ozcelik M. 2005. Bovine tuberculosis in Burdur, southern Turkey: epidemiological, pathological and economic study. Int. J. Tuberc. Lung Dis. 9:1398–1402 [PubMed] [Google Scholar]
- 22. Pinsky B. A, Banaei N. 2008. Multiplex real-time PCR assay for rapid identification of Mycobacterium tuberculosis complex members to the species level. J. Clin. Microbiol. 46:2241–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prodinger W. M., et al. 2005. Characterization of Mycobacterium caprae isolates from Europe by mycobacterial interspersed repetitive unit genotyping. J. Clin. Microbiol. 43:4984–4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prodinger W. M., Eigentler A., Allerberger F., Schönbauer M., Glawischnig W. 2002. Infection of red deer, cattle, and humans with Mycobacterium bovis subsp. caprae in western Austria. J. Clin. Microbiol. 40:2270–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richter E., Brown-Elliot B. A., Wallace R. J., Jr 2011. Mycobacterium: laboratory characteristics of slowly growing mycobacteria, p. 503–524 In Versalovic J., Caroll K. C., Funke G., Jorgensen J. H., Landry M. L., Warnock D. W. (ed.), Manual of clinical microbiology, 10th ed. ASM Press, Washington, DC [Google Scholar]
- 26. Richter E., Weizenegger M., Rüsch-Gerdes S., Niemann S. 2003. Evaluation of Genotype MTBC assay for differentiation of clinical Mycobacterium tuberculosis complex isolates. J. Clin. Microbiol. 41:2672–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez E., et al. 2009. Human tuberculosis due to Mycobacterium bovis and M. caprae in Spain, 2004-2007. Int. J. Tuberc. Lung. Dis. 13:1536–1541 [PubMed] [Google Scholar]
- 28. Romero Gomez M. P., Herrera-Leon L., Jimenez M. S., Garcia Rodriguez J. 2007. Comparison of GenoType MTBC with RFLP-PCR and multiplex PCR to identify Mycobacterium tuberculosis complex species. Eur. J. Clin. Microbiol. Infect. Dis. 26:63–66 [DOI] [PubMed] [Google Scholar]
- 29. Somoskovi A., et al. 2007. Sequencing of the pncA gene in members of the Mycobacterium tuberculosis complex has important diagnostic applications: identification of a species-specific pncA mutation in “Mycobacterium canettii” and the reliable and rapid predictor of pyrazinamide resistance. J. Clin. Microbiol. 45:595–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Somoskovi A., et al. 2008. Direct comparison of the GenoType MTBC and genomic deletion assays in terms of ability to distinguish between members of the Mycobacterium tuberculosis complex isolates in clinical isolates and in clinical specimens. J. Clin. Microbiol. 46:1854–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Telenti A., et al. 1993. Rapid identification of mycobacteria to species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilkins E. G. L., Griffiths R. J., Roberts C. 1986. Pulmonary tuberculosis due to Mycobacterium bovis. Thorax 41:685–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woods G. L. 2007. Mycobacteria, p. 1074-1085 In McPherson R. A., Pincus M. R. (ed.) Henry's clinical diagnosis and management by laboratory methods, 21st ed. Elsevier, Philadelphia, PA [Google Scholar]