Abstract
Molecular analysis of Clostridium difficile (28 isolates) from children (n = 128) in Oxfordshire, United Kingdom, identified eight toxigenic genotypes. Six of these were isolated from 27% of concurrent adult C. difficile-associated infections studied (n = 83). No children carried hypervirulent PCR ribotype 027. Children could participate in the transmission of some adult disease-causing genotypes.
TEXT
Clostridium difficile is a major cause of adult nosocomial diarrhea, with a wide spectrum of disease severity (1). The symptoms of Clostridium difficile-associated infection (CDI) are attributed to the production of toxin A and/or toxin B encoded by the genes tcdA and tcdB within the pathogenicity locus (PaLoc) (15). However, both toxigenic and nontoxigenic strains may be carried asymptomatically (2, 13). Epidemic outbreaks reported in Canada, the United States, and Europe (9, 12, 22) have been particularly associated with PCR ribotype 027, which is described as hypervirulent (12).
Most studies of pediatric C. difficile (4, 7, 17) precede the adult 027-associated epidemic (12). Asymptomatic carriage rates range from 18 to 68%, with toxigenic isolates more likely to be recovered from hospitalized (18 to 29%) (4, 20) or symptomatic (46 to 100%) (8, 16) children. More recently, C. difficile has become one of the most commonly identified pathogens in pediatric nosocomial diarrhea (10), with 10 to 24% of C. difficile strains from symptomatic children in North America being PCR ribotype 027 (19, 21). Similar contemporary United Kingdom studies are lacking, and the potential overlap between pediatric C. difficile genotypes and those causing adult CDI in the same locality has yet to be investigated. This pilot study used an established multilocus sequence typing (MLST) scheme (6) and partial sequencing of the PaLoc-associated tcdB and tcdC loci to characterize and compare pediatric C. difficile isolates with contemporaneous adult CDI isolates.
One hundred twenty-eight anonymized fecal samples from children aged <30 months were included (1 November 2008 to 1 February 2009). Samples comprised (i) nondiarrheal samples from discarded diapers from healthy children attending a breastfeeding clinic, Oxford, United Kingdom, or living in the Oxford area (n = 80) and (ii) diarrheal samples from symptomatic children (n = 48), submitted to the John Radcliffe Hospital microbiology laboratory for routine enteropathogen testing. All contemporaneous adult (≥18 years) C. difficile enzyme immunoassay (EIA)-positive (Premier Toxins A&B enzyme immunoassay; Meridian Bioscience, Italy) diarrheal samples from Oxfordshire processed routinely by the same laboratory were cultured (n = 83; 68 from inpatients, 15 from outpatients). Samples from the same individual with the same sequence type (ST) within a 14-day period were excluded from analysis.
As no patient-identifiable data were collected and anonymized samples were discards from routine testing or the breastfeeding clinic or were voluntarily donated, this study was considered a pilot falling under local generic approval for anonymized microbiological surveillance studies. Approval for using adult and pediatric diarrheal samples without individual consent was also obtained within a larger Oxfordshire C. difficile study (Berkshire Research Ethics Committee; 10/H0505/83).
Samples underwent spore-selective “alcohol shock” and anaerobic culture on modified Brazier's agar (6). C. difficile DNA was obtained from clarified boiled cell suspensions (6), and MLST was performed (6). The presence of the PaLoc (3), tcdA, tcdB, tcdC, and binary toxin genes (cdtA and cdtB) was confirmed by PCR (5, 11, 18). Part of the tcdB receptor-binding domain (tcdB-RBD) and the tcdC gene were sequenced (5).
Statistical analysis was performed using Fisher's exact test (FET) with Stata 11.1.
C. difficile was cultured from 28/128 (22%) children, 16/80 (20%) of whom were asymptomatic and 12/48 (25%) of whom were symptomatic (FET, P = 0.52). For the adults, 84 isolates from 83 individuals were studied, since one culture yielded colonies of different morphologies and both of these were analyzed.
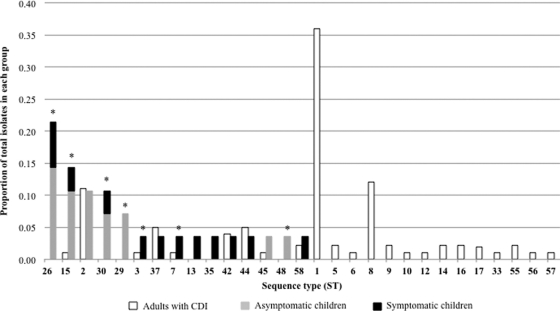
Fifteen STs were identified among 28 pediatric isolates, and 23 STs were identified among 84 adult isolates. Six STs were unique to children and 14 were unique to adults, whereas nine were shared (Fig. 1). Nontoxigenic ST-26 and ST-15 were the most common genotypes in children (n = 10/28; 36%). In contrast, 36% (n = 30/84) of adult isolates were toxigenic ST-1 (PCR ribotype 027), which was not found in children. The frequency distribution of specific STs in children compared to that in adults with CDI was significantly different (FET, P < 0.0001 for all isolates and P = 0.03 for toxigenic isolates only; Fig. 1).
Fig. 1.
Distribution of STs in children and adults with CDI as a proportion of the total number of isolates in each group (1 November 2008 to 1 February 2009). Asymptomatic or symptomatic refers to the absence or presence of diarrhea, respectively. Asterisks denote STs identified in children in this study that were nontoxigenic in this group. A− B+ isolates were exclusively ST-37.
Ten of 28 (32%) pediatric isolates were toxigenic (nine A+ B+, one A− B+). A negative lok1/lok3 PCR (3) indicated that these contained the PaLoc in the expected genomic position. Four asymptomatic (n = 4/80; 5%) and six symptomatic (n = 6/48; 13%) children carried toxigenic strains (FET, P = 0.19). Two isolates from adults with CDI were nontoxigenic (ST-7 and ST-15), indicating an inaccurate EIA result or coinfection with an uncultured toxigenic strain; four isolates were A− B+.
All pediatric isolates were negative for binary toxin genes. Thirty-two isolates from adults with CDI were binary toxin gene positive (ST-1 and ST-5; FET, P < 0.0001 for all isolates and P = 0.03 for toxigenic strains only).
Six of the eight toxigenic pediatric STs accounted for 23/84 (27%) of the adult isolates. The remaining two (ST-13 and ST-35) were not identified in adults in this study but have been found in a larger study of adult CDIs in Oxfordshire (5). There was no difference in tcdB-RBD and tcdC alleles of equivalent STs isolated from children and adults with CDI, and all pediatric isolates lacked the tcdC truncations described in hypervirulent strains (5).
Identifying reservoirs colonized by disease-causing C. difficile strains is essential for understanding transmission networks and developing effective infection control. Given that asymptomatic children are carriers of toxigenic C. difficile strains, they represent a potential reservoir of strains causing disease in adults. Although we did not demonstrate direct transmission events, our data confirmed that a significant proportion of our pediatric isolates shared the same STs and PaLoc variants as those isolates cocirculating in adults with CDI (n = 8/28; 28%), confirming that the potential for transmission exists. Notably, however, the two most common genotypes in adults with CDI (ST-1 and ST-8 [PCR ribotype 002]; n = 40 [48%]) were absent in children, suggesting that other possible reservoirs remain to be identified. Both observations are supported by a recent study identifying close contact with children <2 years as a risk factor for community-associated adult CDI (23) and by a study of French infants, which found no evidence of hypervirulent C. difficile strains in this group (14).
A limitation of this pilot study is the small sample size. In addition, larger studies are required to confirm whether toxigenic strains are causally associated with pediatric diarrhea or whether their isolation is coincidental. Extending sampling to hospitalized, symptomatic children could identify whether hypervirulent STs are present, as reported in the United States (21).
Comparative studies contemporaneously sampling multiple potential C. difficile reservoirs are best placed to accurately characterize chains of transmission. Longitudinal studies employing high-resolution genotyping of isolates from the food chain and animal and human subpopulations will enhance our understanding of the transmission networks of different genotypes.
Acknowledgments
This work was supported by the United Kingdom Clinical Research Collaboration (UKCRC) and the National Institute for Health Research (NIHR) Biomedical Research Centre, Oxford (OxBRC).
We acknowledge the help of Sally Inch, Infant Feeding Specialist, and staff at the Breastfeeding Clinic and all the staff in the Gastroenterology, Colorectal and Endoscopy Departments, John Radcliffe Hospital, Oxford, United Kingdom.
Footnotes
Published ahead of print on 21 September 2011.
REFERENCES
- 1. Bartlett J. G. 2006. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 145:758–764 [DOI] [PubMed] [Google Scholar]
- 2. Bolton R. P., Tait S. K., Dear P. R., Losowsky M. S. 1984. Asymptomatic neonatal colonisation by Clostridium difficile. Arch. Dis. Child. 59:466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braun V., Hundsberger T., Leukel P., Sauerborn M., von Eichel-Streiber C. 1996. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene 181:29–38 [DOI] [PubMed] [Google Scholar]
- 4. Delmee M., Verellen G., Avesani V., Francois G. 1988. Clostridium difficile in neonates: serogrouping and epidemiology. Eur. J. Pediatr. 147:36–40 [DOI] [PubMed] [Google Scholar]
- 5. Dingle K. E., et al. 2011. Clinical Clostridium difficile: clonality and pathogenicity locus diversity. PLoS One 6:e19993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffiths D., et al. 2010. Multilocus sequence typing of Clostridium difficile. J. Clin. Microbiol. 48:770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holst E., Helin I., Mardh P. A. 1981. Recovery of Clostridium difficile from children. Scand. J. Infect. Dis. 13:41–45 [DOI] [PubMed] [Google Scholar]
- 8. Kim K., DuPont H. L., Pickering L. K. 1983. Outbreaks of diarrhea associated with Clostridium difficile and its toxin in day-care centers: evidence of person-to-person spread. J. Pediatr. 102:376–382 [DOI] [PubMed] [Google Scholar]
- 9. Kuijper E. J., et al. 2007. Update of Clostridium difficile-associated disease due to PCR ribotype 027 in Europe. Euro Surveill. 12:E1–E2 [DOI] [PubMed] [Google Scholar]
- 10. Langley J. M., LeBlanc J. C., Hanakowski M., Goloubeva O. 2002. The role of Clostridium difficile and viruses as causes of nosocomial diarrhea in children. Infect. Control Hosp. Epidemiol. 23:660–664 [DOI] [PubMed] [Google Scholar]
- 11. Lemee L., et al. 2004. Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (toxin A), and tcdB (toxin B) genes for toxigenic culture of Clostridium difficile. J. Clin. Microbiol. 42:5710–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDonald L. C., et al. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433–2441 [DOI] [PubMed] [Google Scholar]
- 13. Riggs M. M., et al. 2007. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin. Infect. Dis. 45:992–998 [DOI] [PubMed] [Google Scholar]
- 14. Rousseau C., et al. 2011. Prevalence and diversity of Clostridium difficile strains in infants. J. Med. Microbiol. 60:1112–1118 [DOI] [PubMed] [Google Scholar]
- 15. Rupnik M., Wilcox M. H., Gerding D. N. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7:526–536 [DOI] [PubMed] [Google Scholar]
- 16. Schuller I., et al. 1995. Investigation and management of Clostridium difficile colonisation in a paediatric oncology unit. Arch. Dis. Child. 72:219–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stark P. L., Lee A., Parsonage B. D. 1982. Colonization of the large bowel by Clostridium difficile in healthy infants: quantitative study. Infect. Immun. 35:895–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stubbs S., et al. 2000. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol. Lett. 186:307–312 [DOI] [PubMed] [Google Scholar]
- 19. Suh K. N. G., et al. 2008. Clostridium difficile associated diarrhea in children admitted to acute care hospitals participating in the Canadian Nosocomial Infections Surveillance Program (CNISP), 2004-2005, abstr. no. 307. Soc. Healthcare Epidemiol. Am. 18th Annu. Sci. Meet., Orlando, FL. [Google Scholar]
- 20. Thompson C. M., Jr., Gilligan P. H., Fisher M. C., Long S. S. 1983. Clostridium difficile cytotoxin in a pediatric population. Am. J. Dis. Child. 137:271–274 [DOI] [PubMed] [Google Scholar]
- 21. Toltzis P., et al. 2009. Presence of the epidemic North American pulsed field type 1 Clostridium difficile strain in hospitalized children. J. Pediatr. 154:607–608 [DOI] [PubMed] [Google Scholar]
- 22. Warny M., et al. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079–1084 [DOI] [PubMed] [Google Scholar]
- 23. Wilcox M. H., Mooney L., Bendall R., Settle C. D., Fawley W. N. 2008. A case-control study of community-associated Clostridium difficile infection. J. Antimicrob. Chemother. 62:388–396 [DOI] [PubMed] [Google Scholar]