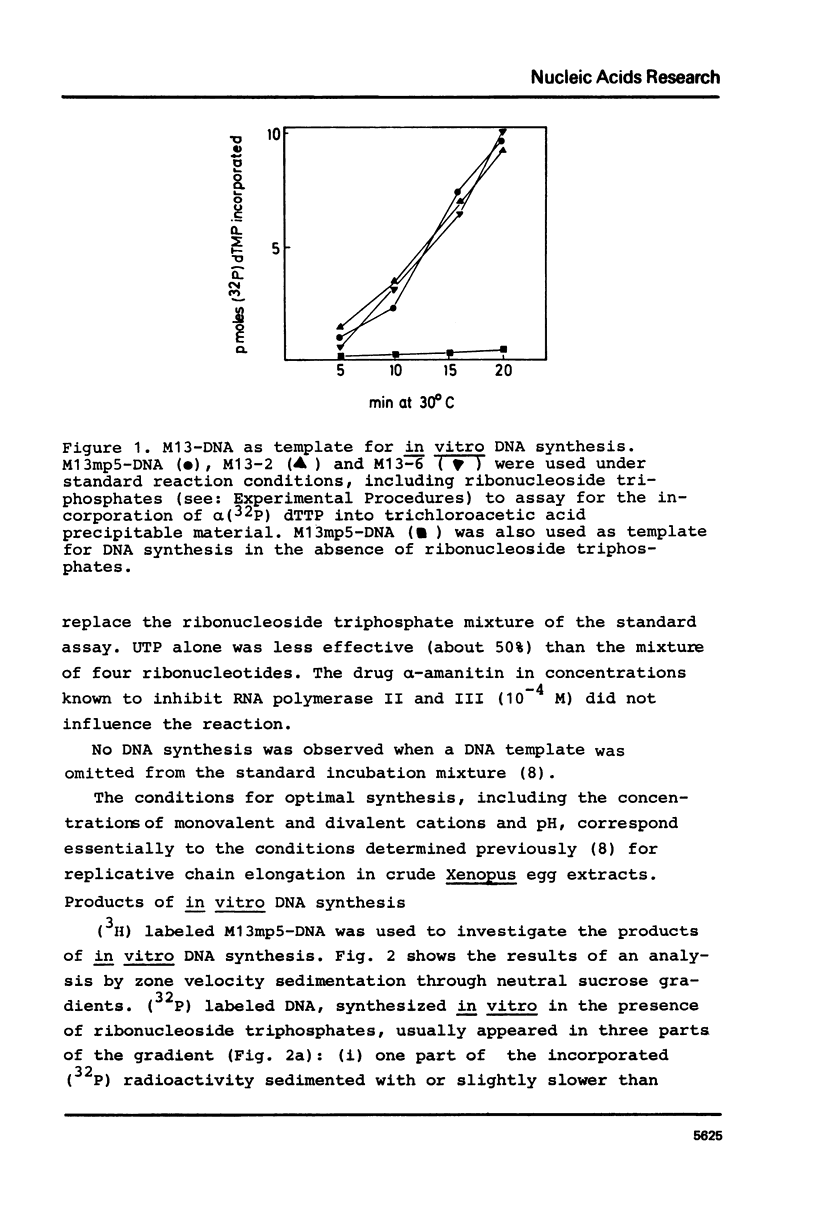
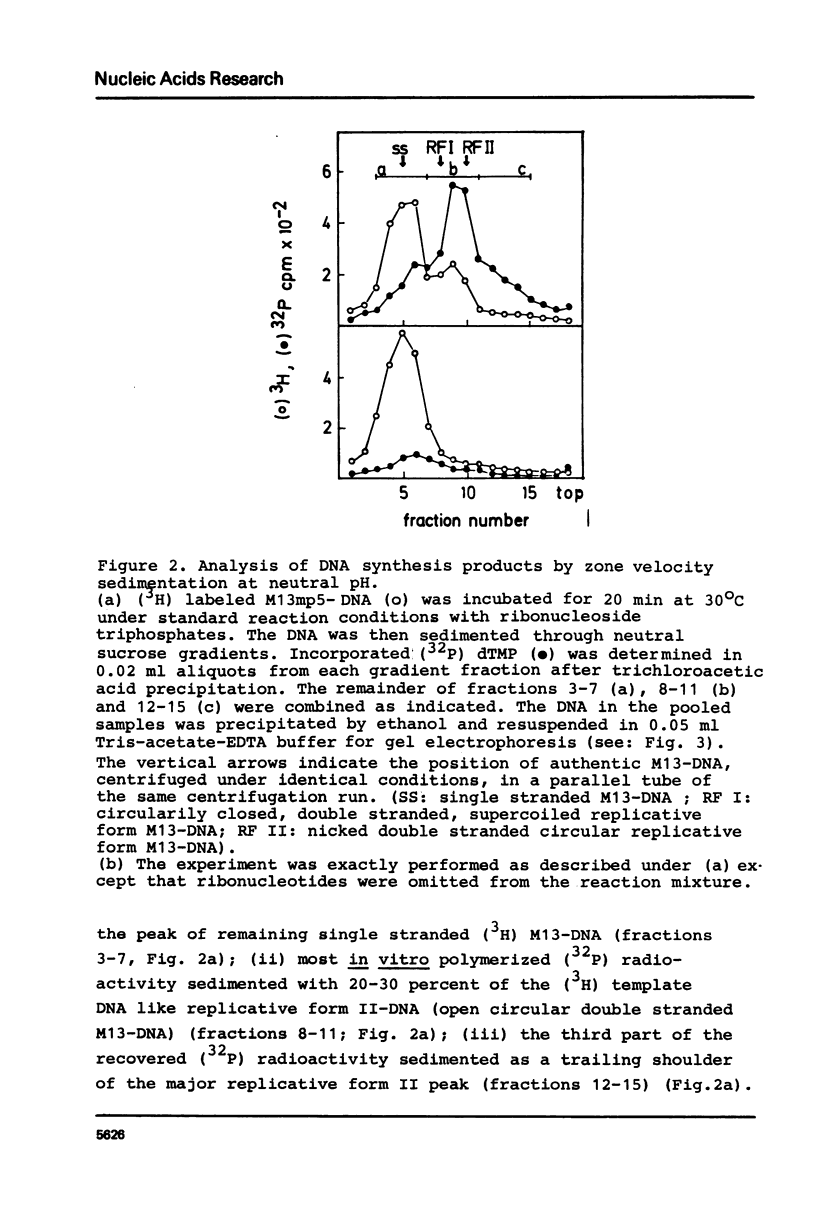
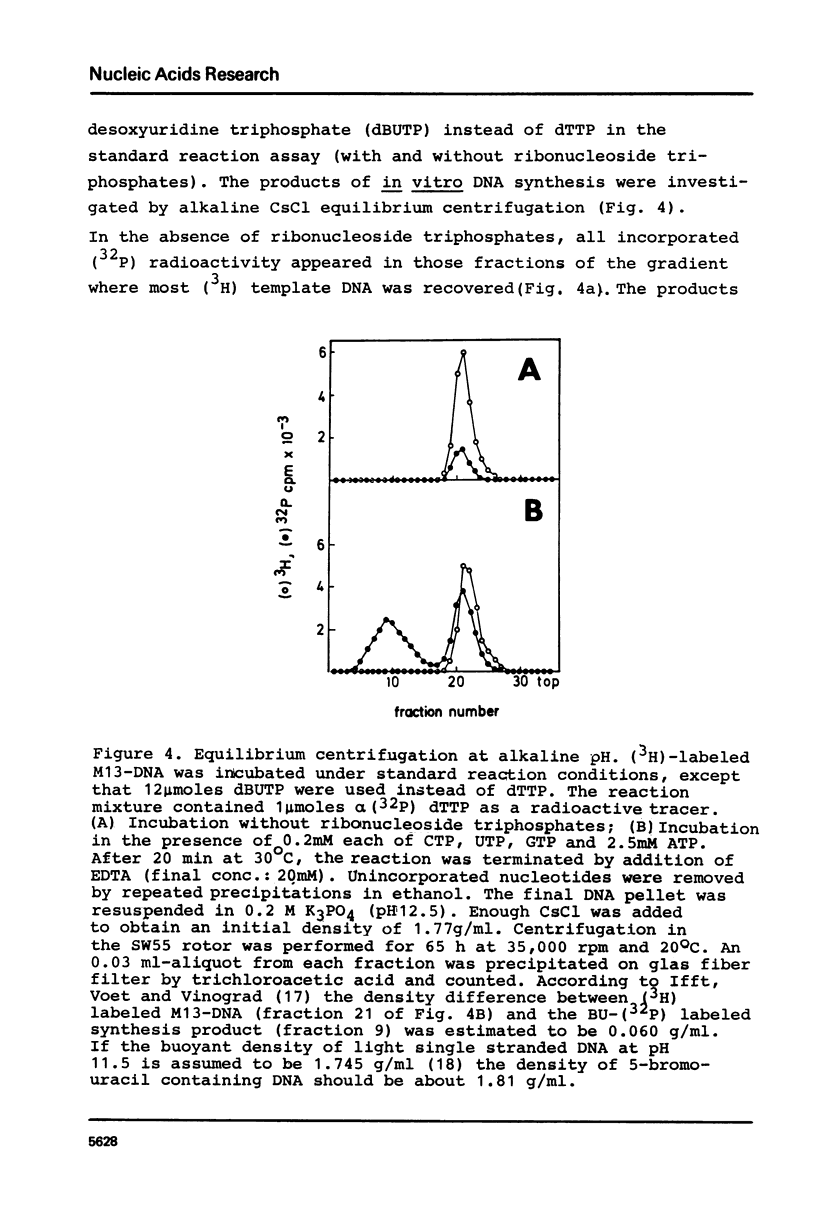
Abstract
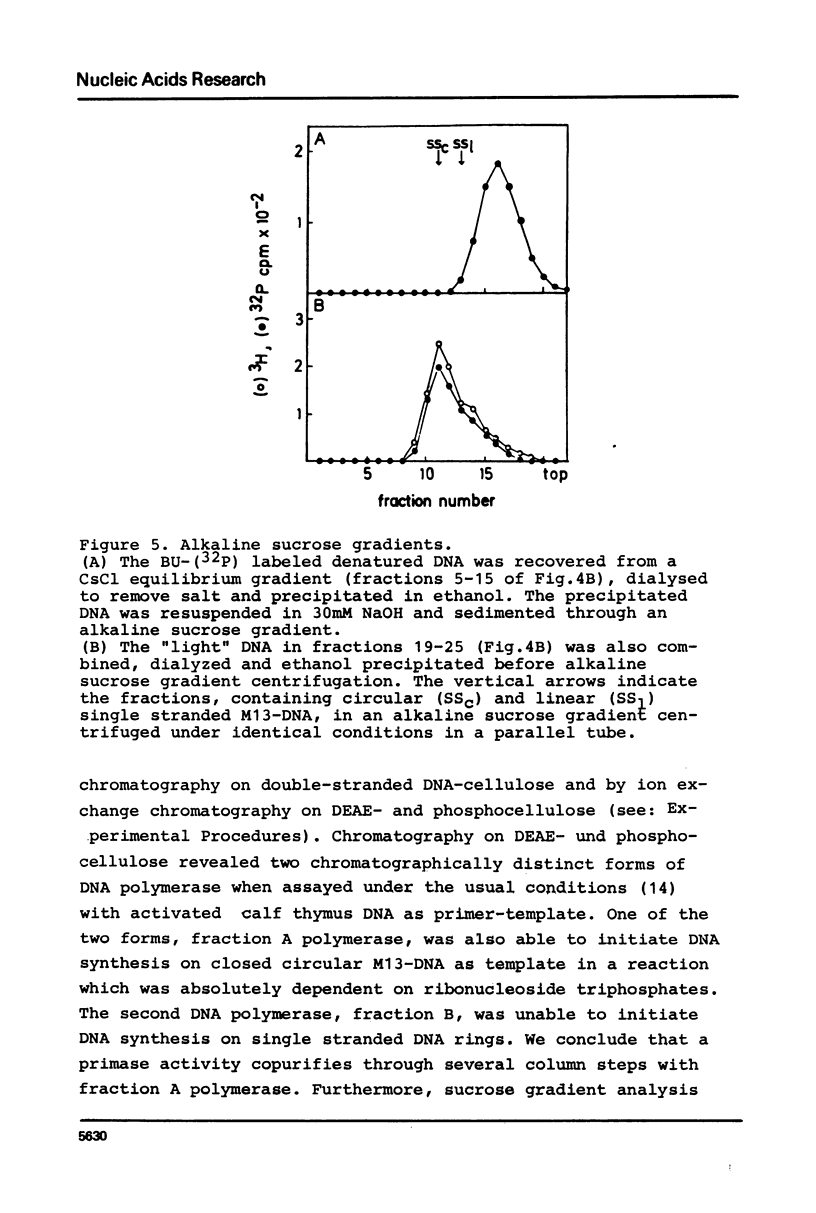
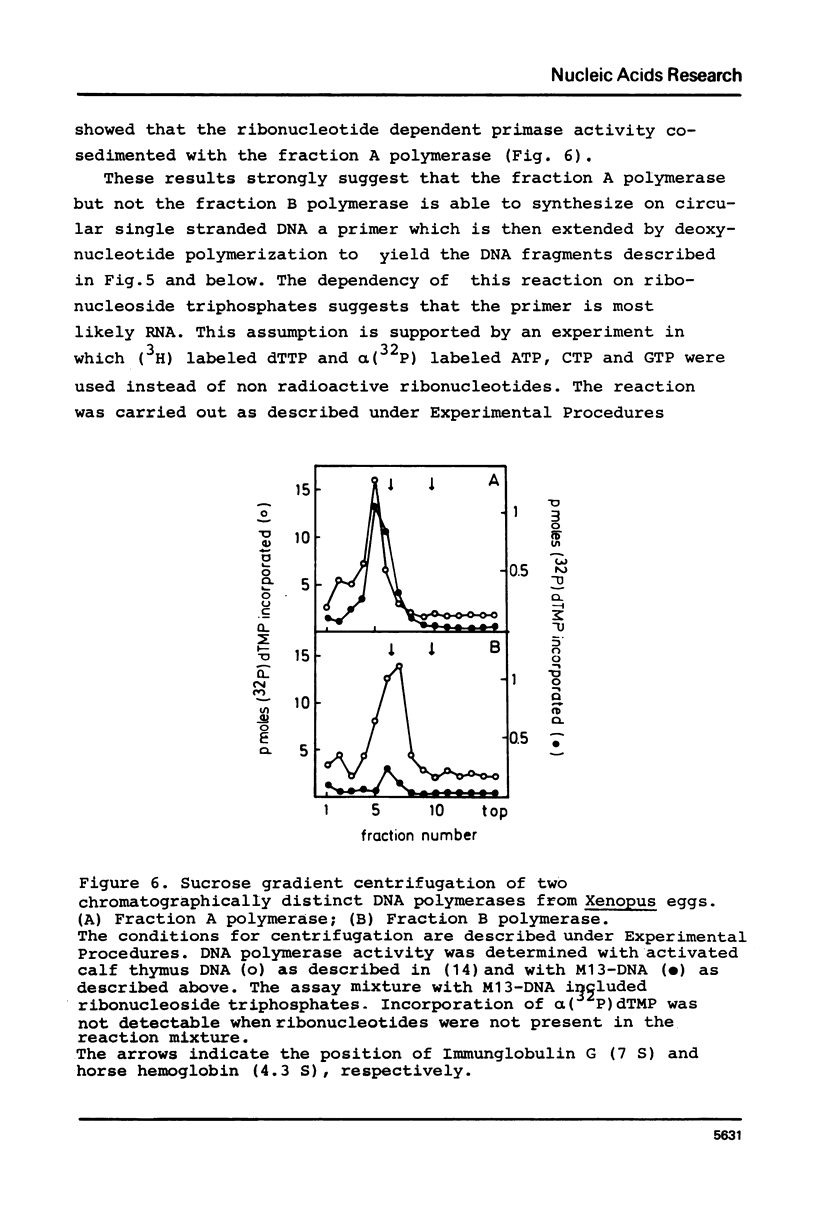
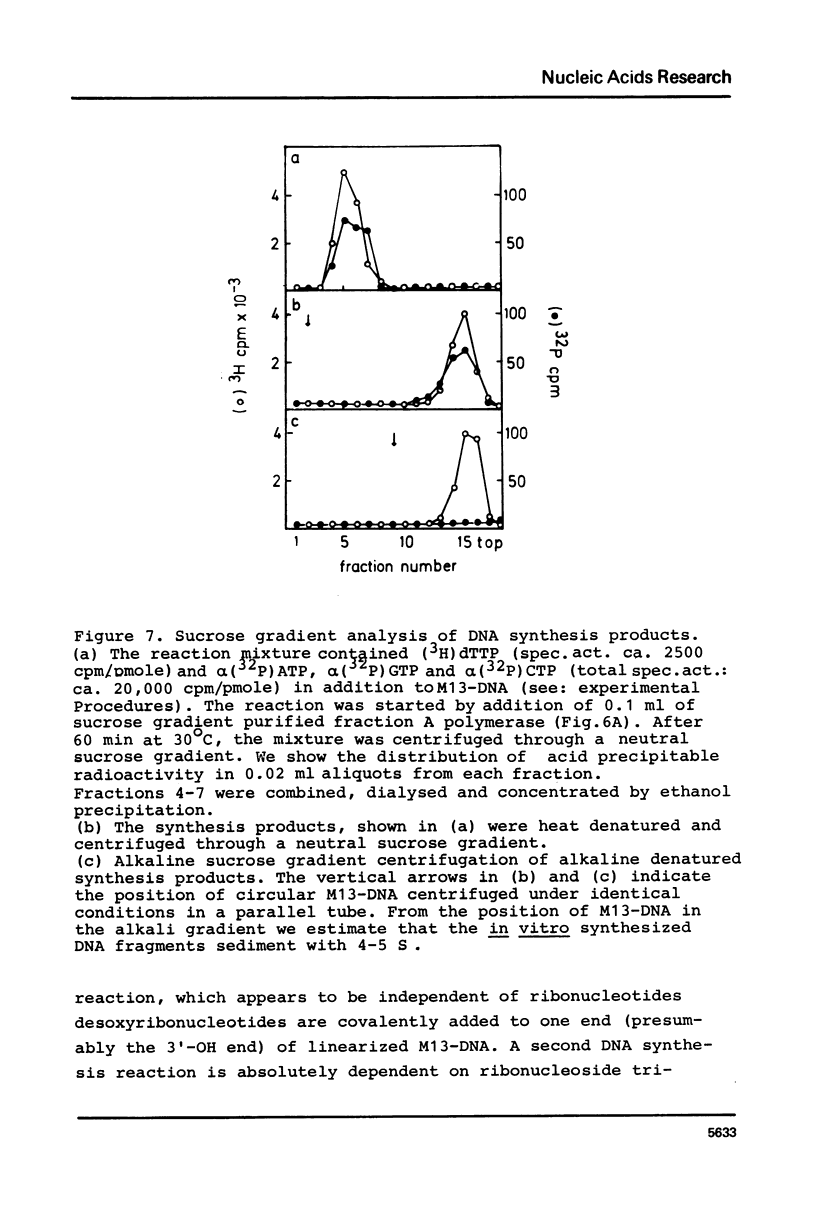
Unfractionated protein extracts from activated Xenopus laevis eggs contain all functions required for the chain elongation reactions in replicative DNA synthesis (A.Richter, B.Otto and R.Knippers, 1981, Nucl.Ac.Res. 9, 3793-3807). In order to further explore the DNA synthesizing capacity of this in vitro system and to obtain information on the DNA priming activity in these extracts single stranded phage M13-DNA was used as template for in vitro DNA synthesis. The main results of this investigation are: (i) single stranded circular template DNA is converted to a double stranded DNA form in an alpha-amanitin-insensitive reaction which is absolutely dependent on ribonucleoside triphosphates; (ii) the in vitro synthesized complementary strands are DNA fragments of 1000-2000 nucleotides lengths; (iii) the DNA primase activity copurifies through several column steps and sucrose gradient centrifugation with a DNA polymerase alpha. These activities may therefore be closely associated in a quarternary enzyme complex.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDWIN R. L., SHOOTER E. M. THE ALKALINE TRANSITION OF BU-CONTAINING DNA AND ITS BEARING ON THE REPLICATION OF DNA. J Mol Biol. 1963 Nov;7:511–526. doi: 10.1016/s0022-2836(63)80098-4. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Krauss M. R., Reeder R. H. DNA synthesis in a multi-enzyme system from Xenopus laevis eggs. Cell. 1978 Feb;13(2):307–318. doi: 10.1016/0092-8674(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Carrara G., Gattoni S., Mercanti D., Tocchini-Valentini G. P. Purification of a DNA-binding protein from Xenopus laevis unfertilized eggs. Nucleic Acids Res. 1977 Aug;4(8):2855–2870. doi: 10.1093/nar/4.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. M., Breaux C. B., Benbow R. M. Intracellular localization of DNA polymerase activities within large oocytes of the frog, Xenopus laevis. Dev Biol. 1980 Nov;80(1):79–95. doi: 10.1016/0012-1606(80)90500-x. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Birnstiel M. L., Speight V. A. The replication of purified DNA introduced into living egg cytoplasm. Biochim Biophys Acta. 1969 Feb 18;174(2):614–628. doi: 10.1016/0005-2787(69)90291-3. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Laskey R. A. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980 Oct;21(3):761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Knippers R., Hoffmann-Berling H. A coat protein from bacteriophage fd. I. Hydrodynamic measurements and biological characterization. J Mol Biol. 1966 Nov 14;21(2):281–292. doi: 10.1016/0022-2836(66)90099-4. [DOI] [PubMed] [Google Scholar]
- Knippers R., Razin A., Davis R., Sinsheimer R. L. The process of infection with Bacteriophage phi-X174. XXIX. In vivo studies on the synthesis of the single-stranded DNA of progeny phi-X174 bacteriophage. J Mol Biol. 1969 Oct 28;45(2):237–263. doi: 10.1016/0022-2836(69)90103-x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Gurdon J. B. Induction of polyoma DNA synthesis by injection into frog-egg cytoplasm. Eur J Biochem. 1973 Sep 3;37(3):467–471. doi: 10.1111/j.1432-1033.1973.tb03007.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Mattoccia E., Attardi D. G., Tocchini-Valentini G. P. DNA-relaxing activity and endonuclease activity in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4551–4554. doi: 10.1073/pnas.73.12.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyczka N., Poland R. L., Bessman M. J. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J Biol Chem. 1972 Nov 25;247(22):7116–7122. [PubMed] [Google Scholar]
- Ogawa T., Okazaki T. Discontinuous DNA replication. Annu Rev Biochem. 1980;49:421–457. doi: 10.1146/annurev.bi.49.070180.002225. [DOI] [PubMed] [Google Scholar]
- Otto B., Baynes M., Knippers R. A single-strand-specific DNA-binding protein from mouse cells that stimulates DNA polymerase. Eur J Biochem. 1977 Feb 15;73(1):17–24. doi: 10.1111/j.1432-1033.1977.tb11287.x. [DOI] [PubMed] [Google Scholar]
- Richter A., Otto B., Knippers R. Replication of SV40 chromatin in extracts from eggs of Xenopus laevis. Nucleic Acids Res. 1981 Aug 11;9(15):3793–3807. doi: 10.1093/nar/9.15.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Scheu R., Otto B. Replication of simian virus 40 chromatin in vitro depends on the amount of DNA polymerase alpha associated with replicating chromatin. Eur J Biochem. 1980 Aug;109(1):67–73. doi: 10.1111/j.1432-1033.1980.tb04768.x. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA polymerase accompanied by a novel RNA polymerase activity: purification and partial characterization. J Biochem. 1982 Feb;91(2):607–618. doi: 10.1093/oxfordjournals.jbchem.a133732. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]