Abstract
GSK1322322 is a novel peptide deformylase inhibitor in the early phase of development for treatment of complicated bacterial skin and skin structure infection and hospitalized community-acquired pneumonia. This quality control (QC) study was performed to establish broth microdilution and disk diffusion QC ranges for strains Staphylococcus aureus ATCC 29213 (MIC range, 1 to 4 μg/ml), Haemophilus influenzae ATCC 49247 (MIC and disk diffusion zone diameter ranges, 0.5 to 4 μg/ml and 20 to 28 mm, respectively), Streptococcus pneumoniae ATCC 49619 (MIC and disk diffusion zone diameter ranges, 0.12 to 0.5 μg/ml and 23 to 30 mm, respectively), and S. aureus ATCC 25923 (disk diffusion zone diameter range, 18 to 26 mm). These ranges are crucial for evaluating GSK1322322 potency as it progresses through clinical trials.
TEXT
A novel peptide deformylase inhibitor (PDF), GSK1322322, is being developed by GlaxoSmithKline (GSK; Collegeville, PA) for treatment of complicated bacterial skin and skin structure infection (CaBSSSI) and hospitalized community-acquired pneumonia. This novel drug demonstrates in vitro activity against multidrug-resistant (MDR) respiratory and skin infection pathogens, including MDR Streptococcus pneumoniae (MDRSP) and methicillin-resistant Staphylococcus aureus (MRSA) (1, 6, 7). As the prevalence of resistance in staphylococcal strains increases, GSK1322322 could warrant further investigation as a potential treatment option. This report describes the results from a multilaboratory quality control (QC) trial designed (4) to determine QC ranges for GSK1322322 according to broth microdilution MICs and disk diffusion zone diameters (measured in millimeters) following the methods published in Clinical and Laboratory Standards Institute (CLSI) documents (2, 3).
Eight laboratories were used in the MIC and disk diffusion studies; however, only seven laboratories are required to establish a QC range according to CLSI document M23-A3 (4). The technicians in those laboratories were experienced clinical microbiology researchers and followed the CLSI methods for disk diffusion and broth microdilution testing (2, 3). The sites and researchers included in the studies were as follows: Massachusetts General Hospital, Boston, MA (M. J. Ferraro; disk diffusion study only); Duke University Medical Center, Durham, NC (L. B. Reller; disk diffusion study only); Wheaton Franciscan Laboratory, Wauwatosa, WI (E. Munson); JMI Laboratories, North Liberty, IA (R. N. Jones and H. S. Sader); TREK Diagnostics, Cleveland, OH (C. Knapp); University of Alberta, Edmonton, Alberta, Canada (R. Rennie); University of Washington, Seattle, WA (S. Swanzy); Robert Wood Johnson Medical School, New Brunswick, NJ (M. Weinstein); University of Texas Medical Center, Houston, TX (A. Wanger; MIC study only); and the Cleveland Clinic Foundation, Cleveland, OH (G. Hall; MIC study only).
Reference broth microdilution panels were prepared by TREK Diagnostics according to GMP guidelines and shipped frozen to all participants. Panels contained four lots of cation-adjusted Mueller-Hinton broth (one lot each from Oxoid, Hampshire, United Kingdom, and BBL, Sparks, MA, and two lots from Difco, Detroit, MI). Also, panels containing four lots of Haemophilus test medium (HTM) and four lots of Mueller-Hinton broth supplemented with 2 to 5% lysed horse blood were provided as four identical broth lots by the same vendor. Levofloxacin, linezolid, and azithromycin were utilized as QC agents (5). For broth microdilution testing, each laboratory tested replicates of S. aureus ATCC 29213, Haemophilus influenzae ATCC 49247, and Streptococcus pneumoniae ATCC 49619. Colony counts were performed on drug-free agar media and resulted in the following average counts: S. aureus ATCC 29213, 3.8 × 105 CFU/ml; H. influenzae ATCC 49247, 5.0 ×105 CFU/ml; and S. pneumoniae ATCC 49619, 2.2 × 105 CFU/ml.
For disk diffusion, two different lots of 20-μg disks were manufactured by two companies, the MAST Group, Merseyside, United Kingdom (Mast Group lot no. 261822), and BD (BD lot no. 0117541). Single lots of levofloxacin (BD lot no. 9194392) (5 μg), linezolid (BD lot no. 9225172) (30 μg), and azithromycin (BD lot no. 0027437) (15 μg) comparator disks from BD were used. Three manufacturers (Hardy Diagnostics, Santa Maria, CA; Remel, Lenexa, KS; and BBL) were used to produce lots for Mueller-Hinton agar (lot no. H11-10175P, -911489, and -0154058), Haemophilus test medium (lot no. H07-10175P, -914032, and -0140073), and Mueller-Hinton agar with 5% sheep blood (lot no. H21-10175P, -910860, and -0140072).
The GSK1322322 MIC results are summarized as proposed ranges in Table 1. There was a frequent occurrence of a one-doubling-dilution trailing endpoint in reading the MIC endpoints for S. aureus ATCC 29213. All participant laboratories were instructed to read the endpoint at 100% inhibition of growth. Laboratories were also instructed to read the panels at two time points (16 to 20 h [the standard CLSI incubation time] and 24 h). For S. aureus ATCC 29213 at the 16-to-20-h endpoint, GSK1322322 produced a three-doubling-dilution QC range of 1 to 4 μg/ml, which included 95.6% of reported MIC values. The extended incubation time produced more defined endpoints but still produced an overall QC range of 1 to 4 μg/ml; therefore, the standard CLSI incubation time of 16 to 20 h is recommended. A slight variation in media occurred with one lot compared to the other two media, for which the geometric mean MIC was slightly lower (1.3 versus 1.8 μg/ml).
Table 1.
Proposed disk diffusion and broth microdilution test QC ranges for GSK1322322 (CLSI, 2009 [2, 3])a
| QC organism | Disk diffusion zone |
Broth microdilution MIC |
||
|---|---|---|---|---|
| Proposed range (mm diam) | % in range | Proposed range (μg/ml) | % in range | |
| S. aureus ATCC 25923 | 18–26 | 99.0 | ||
| S. aureus ATCC 21213 | 1–4 | 95.6 | ||
| H. influenzae ATCC 49247 | 20–28 (20–29) | 99.0 (99.2) | 0.5–4 (0.5–2) | 100.0 |
| S. pneumoniae ATCC 49619 | 23–30b (22–32) | 96.4 (98.3) | 0.12–0.5 | 99.4 |
Range Finder results are shown in parentheses if different from proposed range.
Laboratory B (outlier) was excluded from the analysis.
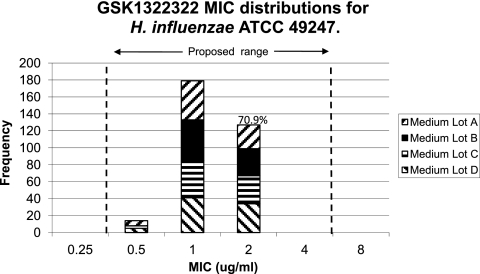
H. influenzae ATCC 49247 tested with GSK1322322 (Fig. 1) produced a four-dilution QC range of 0.5 to 4 μg/ml. A “shoulder” at 2 μg/ml, which included 70.9% (n = 127) of the MIC values compared to the modal occurrences at 1 μg/ml (n = 179), indicated the need for a four-doubling-dilution range. The Range Finder program (8) proposed a range of 0.5 to 2 μg/ml, which still included all of the reported participant results. Lastly, S. pneumoniae ATCC 49619 tested with GSK1322322 has a proposed MIC QC range of 0.12 to 0.5 μg/ml, which includes 99.4% of reported MIC values. MIC results for the control agents (levofloxacin, linezolid, and azithromycin) were within CLSI ranges (5), with the exception of six linezolid values (6/960 [0.6%], all contributed by laboratory G).
Fig. 1.
GSK1322322 MIC distributions for H. influenzae ATCC 49247.
Over 1,400 GSK1322322 zone diameters were reported for the disk diffusion method; the results are summarized in Table 1. Using CLSI M23-A3 criteria (4), a 9-mm GSK1322322 range of 18 to 26 mm for S. aureus ATCC 25923 was proposed; that range included 99.0% of the zone diameters reported (Table 2). A “target” or “bullseye” zone diameter was observed in reading S. aureus ATCC 25923 results. Laboratories were instructed to read the inner-zone diameter that represented complete inhibition of growth (2) The H. influenzae ATCC 49247 results suggested a QC range of 20 to 28 mm, which included 99.0% of observed values. The Range Finder method suggested a wider alternative range of 20 to 29 mm. A range of 23 to 31 mm was calculated by eight laboratories for S. pneumoniae ATCC 49619 and included 94.6% of participant zone diameters. Laboratory B was found to be an outlier laboratory (8) and was excluded from analysis to provide a QC range of 23 to 30 mm, which included 96.4% of all results from the remaining seven laboratories. The Range Finder program again calculated a wider QC range of 22 to 32 mm, which included 98.3% of the zone diameter values.
Table 2.
Inter- and intralaboratory comparisons of GSK1322322 inner-zone-diameter test results for Staphylococcus aureus ATCC 25923
| Zone diam (mm) | No. of samples with indicated zone diam |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disk lot |
Occurrences by medium lot |
Laboratory |
Total | |||||||||||
| A | B | A | B | C | A | B | C | D | E | F | G | H | ||
| 15 | ||||||||||||||
| 16 | ||||||||||||||
| 17 | 2 | 2 | 2 | 2 | ||||||||||
| 18 | 9 | 2 | 9 | 2 | 7 | 4 | 11a | |||||||
| 19 | 16 | 7 | 15 | 8 | 9 | 2 | 8 | 4 | 23a | |||||
| 20 | 32 | 16 | 25 | 15 | 8 | 5 | 5 | 11 | 9 | 15 | 3 | 48a | ||
| 21 | 46 | 36 | 26 | 36 | 20 | 16 | 4 | 11 | 10 | 24 | 11 | 3 | 3 | 82a |
| 22 | 40 | 45 | 29 | 29 | 27 | 10 | 5 | 15 | 15 | 8 | 6 | 18 | 8 | 85a |
| 23 | 49 | 64 | 33 | 37 | 43 | 6 | 12 | 20 | 15 | 7 | 8 | 23 | 22 | 113a |
| 24 | 29 | 33 | 13 | 19 | 30 | 1 | 17 | 6 | 4 | 9 | 7 | 18 | 62a | |
| 25 | 6 | 27 | 4 | 7 | 22 | 2 | 10 | 3 | 3 | 3 | 6 | 6 | 33a | |
| 26 | 7 | 9 | 3 | 4 | 9 | 9 | 4 | 3 | 16a | |||||
| 27 | 3 | 2 | 1 | 3 | 3 | |||||||||
| 28 | ||||||||||||||
| Median | 22 | 23 | 21 | 22 | 23 | 21 | 24 | 22 | 22 | 21 | 21 | 23 | 23 | 22 |
| Total | 239 | 239 | 159 | 159 | 160 | 58 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 478 |
99.0% of qualified results were in the proposed QC range of 18 to 26 mm.
Levofloxacin, linezolid, and azithromycin comparator disk zones provided valid internal controls, with 95.8 to 100.0% of zones within the CLSI published ranges (4). Laboratory B recorded 10 results outside the published azithromycin range for S. pneumoniae ATCC 49619. That laboratory was excluded from analysis for the S. pneumoniae strain (see above). There was minimal variance between the two lots of disks (1 mm of difference in median zones) and ≤2 mm of differences between the median zones for the medium lots across all organisms.
The results from this GSK1322322 QC study are intended to provide the initial ranges for routine susceptibility testing performed using disk diffusion and reference broth microdilution methods (2, 3, 5) as this PDF-class agent progresses through human clinical trials. All proposed QC ranges for GSK1322322 (Table 1) were presented to the CLSI Subcommittee on Antimicrobial Susceptibility Testing and were approved for publication in meetings held in June 2010 (broth microdilution) and January 2011 (disk diffusion).
Acknowledgments
This study was funded by an educational/research grant provided to JMI Laboratories by GlaxoSmithKline (Collegeville, PA).
Footnotes
Published ahead of print on 14 September 2011.
REFERENCES
- 1. Bouchillon S. K., Hackel M., Hoban D. J., Zalazain M., Butler D. 2010. In vitro activity of GSK1322322, a novel peptide deformylase inhibitor, against 4,836 pathogens from skin and soft tissue infections and respiratory tract infections, abstr. F1-2112. Proceedings of the 50th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, September 12–15, 2010 American Society for Microbiology, Washington, DC [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2009. M02-A10. Performance standards for antimicrobial disk susceptibility tests; approved standard, 10th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2009. M07-A8. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2008. M23-A3. Development of in vitro susceptibility testing criteria and quality control parameters, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2011. M100-S21. Performance standards for antimicrobial susceptibility testing, 21st informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Lewandowski T., Demarsh P., Peters T., Kulkarni S. 2010. Potent activity of GSK1322322, a novel peptide deformylase inhibitor, after oral dosing in a murine multi-drug resistant Staphylococcus aureus infection model, abstr. F1-2113. Proceedings of the 50th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, September 12–15, 2010 American Society for Microbiology, Washington, DC [Google Scholar]
- 7. Lewandowski T., Peters T., Simon N., Kulkarni S. 2010. Potent activity of GSK1322322, a novel peptide deformylase inhibitor, in a Haemophilus influenzae and Streptococcus pneumoniae respiratory tract infection model, abstr. F1-2115. Proceedings of the 50th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, September 12–15, 2010 American Society for Microbiology, Washington, DC [Google Scholar]
- 8. Turnidge J., Bordash G. 2007. Statistical methods for establishing quality control ranges for antibacterial agents in Clinical and Laboratory Standards Institute susceptibility testing. Antimicrob. Agents Chemother. 51:2483–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]