Abstract
The osmotic activation of sigma B (σB) in Listeria monocytogenes was studied by monitoring expression of four known σB-dependent genes, opuCA, lmo2230, lmo2085, and sigB. Activation was found to be rapid, transient, and proportional to the magnitude of the osmotic stress applied, features that underpin the adaptability of this pathogen.
TEXT
Listeria monocytogenes is a Gram-positive bacterium that is ubiquitous in the environment and is a facultative intracellular pathogen of humans (3). Infections arise primarily in immunocompromised individuals following the ingestion of contaminated food and are associated with high (typically 25 to 30%) mortality rates (19). The remarkable adaptability of L. monocytogenes to different physical and chemical stresses underpins its ability to survive and grow in wide range of different environments. It can grow at temperatures as low as −0.4°C (32), it can survive over a wide pH range (31), it is extremely tolerant to bile (14, 33), and it can grow in the presence of salt concentrations as high as 2 M (10). In recent years it has become clear that many of these traits are partly under the control of the stress-inducible sigma factor SigB (σB) (35, 36).
Several recent studies have highlighted the role of σB in allowing L. monocytogenes to survive in the gastrointestinal tract, which is a prerequisite for establishing successful infection in the host (27, 30). Mutants of L. monocytogenes lacking sigB display decreased virulence in guinea pigs infected orally but not intravenously (12). These mutants also display reduced rates of epithelial cell invasion, a finding that is explained by the involvement of σB in the transcription of the inlAB operon (18, 21), which encodes internalin (InlA) and InlB, the surface proteins responsible for host cell invasion. Indeed, PrfA, the central regulator of virulence gene expression in L. monocytogenes, is itself transcribed in a manner that partly depends on the presence of σB (23). Further compelling evidence of the role for σB in the early stages of a listeriosis infection comes from a transcriptomics study that found expression of numerous genes induced in the gastrointestinal tract to be under σB control (30).
Although the importance of σB in stress adaptation and virulence is now well established, very little is known about how the activity of σB is regulated in this pathogen. Based on homology with the σB regulatory apparatus in Bacillus subtilis, it is likely that regulation is achieved primarily at the posttranslational level through an interaction with RsbW, an anti-sigma factor (9, 15). In order to develop an understanding of the kinetics and extent of σB activation in L. monocytogenes, we have investigated the effects of osmotic stress on the expression of four genes and loci (opuCA, lmo2230, lmo2085, and the sigB gene itself) already known to be under σB control in this pathogen. The opuCA gene encodes a component of the OpuC system that is involved in osmo- and cryotolerance (7, 11, 34) and also plays an important role in survival during the intestinal phase of infection (28), probably because it contributes to bile resistance (33). Its transcription is under σB control, and the promoter has been mapped to a position 58 bp upstream from the start codon of opuCA (11, 17). The lmo2230 gene locus encodes a putative arsenate reductase, and its σB promoter has been mapped to a position 143 bp upstream from the start codon (17). This gene belongs to a category of genes that are under both σB and PrfA control (8, 22). The lmo2085 gene encodes a putative peptidoglycan bound protein that has no homologue in the nonpathogenic species L. innocua (6). This gene has been shown in several studies to be expressed in a highly σB-dependent manner (1, 13, 17, 24); in recent gene microarray experiments we found it to be the gene most affected in a ΔsigB background (E. Starr and C. P. O'Byrne, unpublished data). The sigB gene itself is positively autoregulated; mutants lacking σB fail to induce the 4-gene sigB operon (consisting of rsbV, rsbW, sigB, and rsbX) in response to stress stimuli. The σB-dependent promoter is located upstream from the rsbV gene (4).
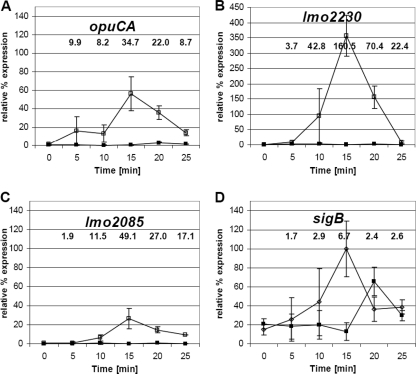
We first investigated the kinetics of σB activation by following σB-dependent transcription after the sudden imposition of an osmotic stress. Cultures of L. monocytogenes wild-type EGD-e and an isogenic ΔsigB mutant derivative (5) were grown to the mid-exponential phase (optical density at 600 nm [OD600] of 0.6) in brain heart infusion (BHI) broth at 37°C, and then salt (0.5 M NaCl [in the form of solid salt crystals]) was added to the medium. RNA was prepared at several time intervals (0, 5, 10, 15, 20, and 25 min) and collected from cultures kept at 37°C and vigorously shaken. Real-time reverse transcription-PCR (RT-PCR) was then used to measure transcript levels over the indicated time period for each of the four σB-dependent genes studied (opuCA, lmo2085, lmo2230, and sigB) (Fig. 1; Table 1; see also Table S1 in the supplemental material). Rapid induction of each of the four genes was recorded following a sudden osmotic upshock, with lmo2230 showing the highest level of induction (160-fold at 15 min relative to time zero). For all four genes, the maximum transcript level was observed at the 15-min time interval, with a steady decrease in transcript levels thereafter, suggesting a transient activation of transcription in response to osmotic upshock. In the ΔsigB mutant there was no significant increase in transcription recorded for opuCA, lmo2085, and lmo2230 following osmotic upshock (Fig. 1A, B, and C), confirming the σB-dependent transcription of these genes and indicating that σB activation occurs transiently in response to osmotic upshock. A small delay in induction of sigB transcription was observed with the ΔsigB mutant (detected with primers directed against the undeleted part of the sigB gene), presumably resulting from the activation of σB-independent promoter (Fig. 1D). At 25 min after the addition of NaCl, transcription of each gene had returned to a level similar to that detected when cells were subjected to continuous osmotic stress during balanced growth (compare fold change values in Fig. 1 at the 25-min time point with those indicated in Fig. S1 in the supplemental material), a level that was still significantly higher than that detected in the absence of NaCl (Fig. 1; P < 0.05 [Student's t test]). These data suggest that σB is activated rapidly and transiently following sudden exposure to osmotic stress. Furthermore, the extent of σB activation following osmotic upshock was, for a short time, much greater than that observed during balanced growth when the same concentration of salt was present.
Fig. 1.
Activation of σB by osmotic upshock occurs rapidly and transiently. Relative transcript levels of sigB and three other σB-dependent genes (opuCA, lmo2230, and lmo2085) were measured in exponential-phase cells in wild-type (open symbols) and ΔsigB (closed symbols) backgrounds grown in BHI broth at 37°C. RNA extracts were prepared either immediately before the addition of 0.5 M NaCl (0 min) or 5 min, 10 min, 15 min, 20 min, or 25 min after osmotic upshock. Real-time determination of gene transcription levels was carried out as previously described (16). All transcript levels were first normalized to the corresponding 16S RNA reference gene levels (internal control) (29), with an efficiency correction included for each primer pair as previously described (25), and then expressed as a percentage of the maximal level of sigB transcript detected in the experiment (thereby allowing the relative transcript levels of the 4 genes to be compared). The values presented on the graphs represent the means of the results of three independent experiments, and error bars indicate the standard deviations (n = 3). Numbers shown above the graphs indicate statistically significant differences in relative gene expression levels (severalfold change) for the wild type between the results seen under stressed (NaCl added at each time point) and nonstressed (time point 0 before adding NaCl) conditions (P < 0.05 by Student's t test). Note that the plot in panel B uses a different scale on the y axis.
Table 1.
Primers used in the study
| Target | Forward (5′–3′) | Reverse (5′–3′) | Product size (bp) | Efficiency (E)c |
|---|---|---|---|---|
| 16S rRNAa | TGGGGAGCAAACAGGATTAG | TAAGGTTCTTCGCGTTGCTT | 213 | 2.27 |
| sigBb | CTATATTGGATTGCCGCTTAC | CAAACGTTGCATCATATCTTC | 191 | 1.98 |
| opuCAb | GGATGAAGCGATTAAACTG | CCGCTGTAATAGAGACTGG | 206 | 2.19 |
| lmo2230b | CATATTCGAAGTGCCATTGC | CTGAACTAGGTGAATAAGACAAAC | 169 | 2.12 |
| lmo2085b | GGTAATGGATATAATGTGCCTGC | GGGTGAACCAATAAGCAAATC | 177 | 2.27 |
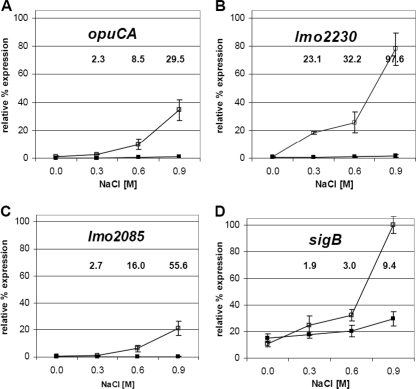
When the transcript levels of the four selected genes (opuCA, lmo2085, lmo2230, and sigB) were investigated during exponential growth at 37°C (RNA extraction performed when cells reached an OD600 of 0.6) with a range of salt concentrations (0 to 0.9 M NaCl), a dramatic and proportional increase in the transcript levels occurred in each case as the salt concentration increased (Fig. 2; see also Table S1 in the supplemental material). The NaCl-induced increase in the transcript levels was not observed for any of the four genes in a background lacking σB. The most substantial induction of transcription for each of the four genes was seen with 0.9 M salt. Transcription of opuCA at 0.9 M salt in the wild type increased dramatically (∼29 times higher than that seen with the 0 M NaCl control), while lmo2230 and lmo2085 showed transcription ∼98 and ∼56 times higher at that salt concentration, respectively. No increase in transcription was detected in the ΔsigB mutant. Although the induction of sigB transcription resulting from osmotic stress was largely dependent on the presence of σB, a significant amount of sigB transcription was observed in the mutant background (Fig. 2D). This suggests that a baseline of σB-independent transcription of the sigB operon is maintained under all growth conditions, which is consistent with the requirement for a rapid transcriptional response when stress is encountered. Together, these results indicate that σB-dependent transcriptional activity is strongly stimulated by the presence of salt in a manner that is directly proportional to the extent of the stress, suggesting that σB activity can be finely tuned according to the environment encountered.
Fig. 2.
Osmotic activation of σB is proportional to the magnitude of the stress. Relative transcript levels of three σB-dependent genes (opuCA, lmo2230, and lmo2085) and sigB in the wild type (open symbols) and the ΔsigB mutant (closed symbols) grown in BHI broth at 37°C to the exponential phase (OD600 of 0.6) under a range of NaCl concentrations (0 M, 0.3 M, 0.6 M, or 0.9 M) are shown. RNA extracts and cDNA were prepared as previously described (25). All transcript levels were first normalized as described in Fig. 1 and then expressed as a percentage of the maximal level of sigB transcript detected in the experiment (thereby allowing the relative transcript levels of the 4 genes to be compared). The values presented on the graphs represent the means of the results of three independent experiments, and error bars indicate the standard deviations (n = 3). Numbers shown above the graphs indicate statistically significant differences in relative gene expression levels (severalfold change) between the results seen under stressed (0.3, 0.6, or 0.9 M NaCl added at each time point) and nonstressed (0 M NaCl) conditions (P < 0.05 by Student's t test).
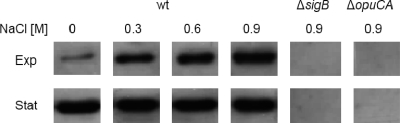
The data presented above suggest that measuring the transcription of opuC, lmo2230, or lmo2085 is useful for measuring σB activity in L. monocytogenes. We investigated whether proportional expression of one of these genes, opuCA, in response to osmotic stress could also be detected at the protein level. To detect OpuCA protein levels, polyclonal antibodies against the purified OpuCA protein (ATPase subunit of the OpuC transporter) were raised in chickens as described in Fig. S2 in the supplemental material. Cultures of the wild type (EGD-e) and of ΔsigB and ΔopuCA mutant derivatives were grown at 37°C with vigorous shaking in BHI broth supplemented with 0, 0.3, 0.6, or 0.9 M NaCl. Crude cell extracts were prepared from each culture as described previously (2) during either the exponential phase (OD600 of 0.6) or the stationary phase (16 h of culture), and the levels of OpuC expression were determined by Western blotting. For all OpuCA comparisons, the total protein concentrations were normalized to 5 mg ml−1, with 10 μl loaded onto gels. A clear band was detected by Western blotting for purified OpuCA; this band was absent from the ΔopuCA mutant blot, indicating that the antibodies produced were specific for OpuCA (see Fig. S2 in the supplemental material). OpuCA was not detected in the ΔsigB mutant under any of the described conditions (data are shown for the highest NaCl concentration only; Fig. 3), confirming the dependence of OpuC expression on σB. Strikingly, the highest level of OpuCA in the wild type occurred with the most severe osmotic challenge, specifically, 0.9 M NaCl. The levels of OpuCA were found to increase gradually and at a rate approximately in proportion to the salt concentration in the medium during exponential growth. These results correlate well with what was observed at the transcriptional level (Fig. 2) and suggest that OpuC levels reflect the extent of σB activation during balanced growth. Thus, the availability of antibodies against OpuCA should generally prove useful in measuring σB activity in L. monocytogenes. It is worth noting that the levels of OpuCA remained high over the whole range of salt concentrations tested in the stationary phase (Fig. 3), suggesting that expression was fully induced in the stationary phase regardless of whether or not salt was added. This result suggests that σB might be maximally active in the stationary phase, presumably because the entire cellular pool of σB is associated with RNA polymerase.
Fig. 3.
OpuCA expression is induced in proportion to the osmotic stress. The OpuCA protein was detected in three biological replicates of crude cell extracts by Western blotting using anti-OpuCA-His polyclonal antibodies as described in the legend to Fig. S2 in the supplemental material. Crude cell extracts were prepared from exponential-phase cells (Exp) or stationary-phase cells (Stat) of the wild-type strain (wt) or ΔsigB and ΔopuCA mutant strains grown at 37°C in BHI broth over a range of NaCl concentrations (0, 0.3, 0.6, and 0.9). Total protein concentrations were normalized to 5 mg ml−1, and 10 μl of each protein extract was loaded. Western blotting was carried out using semidry transfer with incubations and washing steps followed by chemiluminescent light detection as described in the legend to Fig. S2 in the supplemental material.
Overall, the findings of this study suggest that σB activation resulting from osmotic stress is proportional to magnitude of the stress, with a wide dynamic range of activities apparent. The kinetics of transcriptional activation in response to osmotic upshock are extremely fast, with a peak of activity occurring 15 min after upshock. Together, these data show that σB activation is carefully calibrated to meet the precise conditions encountered, a feature that is likely to contribute to the remarkable adaptability of this pathogen.
Supplementary Material
Acknowledgments
We thank our colleagues in the Bacterial Stress Response Group for useful comments and discussions. The ΔsigB strains used in this study were generously supplied by Kathryn Boor (Cornell University) and Cormac Gahan (University College Cork).
This work was supported by an Irish Research Council for Science, Engineering and Technology EMBARK grant together with Thomas Crawford Hayes funding awards to M.U. and by a Science Foundation Ireland Research Frontiers Programme grant (05/RFP/Gen0044).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 2 September 2011.
REFERENCES
- 1. Abram F., et al. 2008. Identification of components of the sigma B regulon in Listeria monocytogenes that contribute to acid and salt tolerance. Appl. Environ. Microbiol. 74:6848–6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abram F., et al. 2008. Proteomic analyses of a Listeria monocytogenes mutant lacking σB identify new components of the σB regulon and highlight a role for σB in the utilization of glycerol. Appl. Environ. Microbiol. 74:594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barbuddhe S. B., Chakraborty T. 2009. Listeria as an enteroinvasive gastrointestinal pathogen. Curr. Top. Microbiol. Immunol. 337:173–195 [DOI] [PubMed] [Google Scholar]
- 4. Becker L. A., Cetin M. S., Hutkins R. W., Benson A. K. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J. Bacteriol. 180:4547–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Begley M., Sleator R. D., Gahan C. G., Hill C. 2005. Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect. Immun. 73:894–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calvo E., et al. 2005. Analysis of the Listeria cell wall proteome by two-dimensional nanoliquid chromatography coupled to mass spectrometry. Proteomics 5:433–443 [DOI] [PubMed] [Google Scholar]
- 7. Chan Y. C., Boor K. J., Wiedmann M. 2007. σB-dependent and σB-independent mechanisms contribute to transcription of Listeria monocytogenes cold stress genes during cold shock and cold growth. Appl. Environ. Microbiol. 73:6019–6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chatterjee S. S., et al. 2006. Intracellular gene expression profile of Listeria monocytogenes. Infect. Immun. 74:1323–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaturongakul S., Boor K. J. 2004. RsbT and RsbV contribute to σB-dependent survival under environmental, energy, and intracellular stress conditions in Listeria monocytogenes. Appl. Environ. Microbiol. 70:5349–5356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cole M. B., Jones M. V., Holyoak C. 1990. The effect of pH, salt concentration and temperature on the survival and growth of Listeria monocytogenes. J. Appl. Bacteriol. 69:63–72 [DOI] [PubMed] [Google Scholar]
- 11. Fraser K. R., Harvie D., Coote P. J., O'Byrne C. P. 2000. Identification and characterization of an ATP binding cassette l-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garner M. R., James K. E., Callahan M. C., Wiedmann M., Boor K. J. 2006. Exposure to salt and organic acids increases the ability of Listeria monocytogenes to invade Caco-2 cells but decreases its ability to survive gastric stress. Appl. Environ. Microbiol. 72:5384–5395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hain T., et al. 2008. Temporal transcriptomic analysis of the Listeria monocytogenes EGD-e σB regulon. BMC Microbiol. 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hardy J., et al. 2004. Extracellular replication of Listeria monocytogenes in the murine gall bladder. Science 303:851–853 [DOI] [PubMed] [Google Scholar]
- 15. Hecker M., Pane-Farre J., Volker U. 2007. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61:215–236 [DOI] [PubMed] [Google Scholar]
- 16. Karatzas K. A., Brennan O., Heavin S., Morrissey J., O'Byrne C. P. 2010. Intracellular accumulation of high levels of gamma-aminobutyrate by Listeria monocytogenes 10403S in response to low pH: uncoupling of gamma-aminobutyrate synthesis from efflux in a chemically defined medium. Appl. Environ. Microbiol. 76:3529–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kazmierczak M. J., Mithoe S. C., Boor K. J., Wiedmann M. 2003. Listeria monocytogenes sigma B regulates stress response and virulence functions. J. Bacteriol. 185:5722–5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim H., Boor K. J., Marquis H. 2004. Listeria monocytogenes σB contributes to invasion of human intestinal epithelial cells. Infect. Immun. 72:7374–7378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lorber B. 1997. Listeriosis. Clin. Infect. Dis. 24:1–9; quiz, 10-1 [DOI] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21. McGann P., Wiedmann M., Boor K. J. 2007. The alternative sigma factor σB and the virulence gene regulator PrfA both regulate transcription of Listeria monocytogenes internalins. Appl. Environ. Microbiol. 73:2919–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Milohanic E., et al. 2003. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol. Microbiol. 47:1613–1625 [DOI] [PubMed] [Google Scholar]
- 23. Nadon C. A., Bowen B. M., Wiedmann M., Boor K. J. 2002. Sigma B contributes to PrfA-mediated virulence in Listeria monocytogenes. Infect. Immun. 70:3948–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oliver H. F., et al. 2009. Deep RNA sequencing of L. monocytogenes reveals overlapping and extensive stationary phase and sigma B-dependent transcriptomes, including multiple highly transcribed noncoding RNAs. BMC Genomics 10:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pfaffl M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reference deleted.
- 27. Sleator R. D., Watson D., Hill C., Gahan C. G. 2009. The interaction between Listeria monocytogenes and the host gastrointestinal tract. Microbiology 155:2463–2475 [DOI] [PubMed] [Google Scholar]
- 28. Sleator R. D., Wouters J., Gahan C. G., Abee T., Hill C. 2001. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl. Environ. Microbiol. 67:2692–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tasara T., Stephan R. 2007. Evaluation of housekeeping genes in Listeria monocytogenes as potential internal control references for normalizing mRNA expression levels in stress adaptation models using real-time PCR. FEMS Microbiol. Lett. 269:265–272 [DOI] [PubMed] [Google Scholar]
- 30. Toledo-Arana A., et al. 2009. The Listeria transcriptional landscape from saprophytism to virulence. Nature 459:950–956 [DOI] [PubMed] [Google Scholar]
- 31. Vasseur C., Baverel L., Hebraud M., Labadie J. 1999. Effect of osmotic, alkaline, acid or thermal stresses on the growth and inhibition of Listeria monocytogenes. J. Appl. Microbiol. 86:469–476 [DOI] [PubMed] [Google Scholar]
- 32. Walker S. J., Archer P., Banks J. G. 1990. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 68:157–162 [DOI] [PubMed] [Google Scholar]
- 33. Watson D., Sleator R. D., Casey P. G., Hill C., Gahan C. G. 2009. Specific osmolyte transporters mediate bile tolerance in Listeria monocytogenes. Infect. Immun. 77:4895–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wemekamp-Kamphuis H. H., Sleator R. D., Wouters J. A., Hill C., Abee T. 2004. Molecular and physiological analysis of the role of osmolyte transporters BetL, Gbu, and OpuC in growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 70:2912–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wemekamp-Kamphuis H. H., et al. 2004. Identification of sigma factor σB-controlled genes and their impact on acid stress, high hydrostatic pressure, and freeze survival in Listeria monocytogenes EGD-e. Appl. Environ. Microbiol. 70:3457–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiedmann M., Arvik T. J., Hurley R. J., Boor K. J. 1998. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.