Abstract
The role of virulent bacteriophages in staphylococcal colonization of the human anterior nares is not known. This report of lytic bacteriophages against Staphylococcus epidermidis in the anterior nares of 5.5% of human subjects (n = 202) suggests their potential role in modulating staphylococcal colonization in this ecological niche.
TEXT
Microbial colonization of the anterior nares of humans is expected to be a dynamic process modulated by a variety of competing bacterial species, viruses, and health conditions (19). Limited reports (8, 25) have suggested that the anterior nares represent a complex ecological niche in which the microbial species interaction may well determine the colonization and prevalence of staphylococci, one of the most common flora in the human anterior nares. Staphylococcus epidermidis represents 8% to 43% of staphylococcal abundance in the nares (14), while Staphylococcus aureus represents about 25 to 30% (15). The presence of S. epidermidis in the anterior nares has been shown to reduce S. aureus colonization (14, 18). S. aureus colonization of the anterior nares is additionally influenced by the host, bacterial diversity, and abiotic factors (20, 22). The role of lytic phages in this setting, if any, has not been described.
Virulent phages, which lyse their host bacterium as a normal part of their life cycle, have been isolated from several human microenvironments where the host bacterium is likely to be present, including the oral cavity (23, 3), the vagina (17), stool (12), and the gut (5, 6). As far as S. epidermidis phages are concerned, nearly all reports in the literature are linked to temperate phages, which integrate their genome into that of the bacterial chromosome (4, 16, 18, 21, 24, 11). A recent study reported the failure to isolate S. epidermidis phages from skin, mucous surface exudates, and breast milk (16). To our knowledge, virulent bacteriophages have never been reported from the anterior nares of humans. This study attempted to determine if virulent bacteriophages against S. aureus and S. epidermidis are found in the anterior nares and if they play a role in modulating staphylococcal colonization.
We screened for the presence of lytic phages against S. epidermidis and S. aureus in the anterior nares of ambulatory patients and health care environment workers of a Midwestern rural multispecialty clinic. The study design and methodological details are described elsewhere (2). Briefly, the anterior nares of 202 human subjects were swabbed with dry swabs. Each swab was suspended in 3 ml of Trypticase soy broth and incubated at room temperature for 20 min. Phages against S. epidermidis and S. aureus were detected by a modified plate assay technique (2) using S. epidermidis ATCC 35983 and S. aureus ATCC 29213, respectively, as host strains. A 30-μl aliquot of each of the nasal swab filtrate sample was spotted onto each strain (2). The 202 samples were also pooled into 8 groups, numbered I to VIII (Table 1), of 25 samples each, and 50-μl aliquots of each pool were rechecked for the presence of phages on Trypticase soy agar (TSA) plates incubated at 37°C for 48 h.
Table 1.
S. epidermidis virulent bacteriophages detected by plate plaque assay
| Samples | Individual sample(s) containing phagesa | Sample pool | Pooled samples with phagesb | Mean capsid (tail) length [width] ± SEM (nm) |
|
|---|---|---|---|---|---|
| Podoviridae | Siphoviridae | ||||
| 1–25 | None | I | Negative | ||
| 26–50 | None | II | Positive | 37 ± 2 (28 ± 2) | |
| 51–75 | 26, 60 | III | Positive | 36 ± 1 (24 ± 1) | |
| 76–100 | None | IV | Positive | 36 ± 1 (28 ± 1) | |
| 101–125 | 111 | V | Positive | 73 ± 2 (366 ± 4 [10 ± 1]) | |
| 126–150 | 129, 131, 146 | VI | Positive | 41 ± 1 (38 ± 1) | 69 ± 1 (362 ± 1 [12 ± 1]) |
| 151–175 | 154, 162 | VII | Positive | 37 ± 5 (30 ± 4) | 74 ± 1 (338 ± 1 [10 ± 1]) |
| 176–202 | 177, 192, 195 | VIII | Positive | 40 ± 1 (31 ± 2) | |
Individual samples tested for the presence of virulent phages by plaque assay.
Pooled samples tested for the presence of virulent phages.
Lysis zones were observed only on TSA plates containing S. epidermidis and not on those containing S. aureus. Putative S. epidermidis lytic phages were recovered from these lysis zones with a sterile truncated tip and placed into 500 μl of ammonium acetate (100 mM, pH 7.0). Plates of pooled samples exhibited large lysis zones rather than individual plaques, most likely because of the larger aliquot volume loaded. Phages were allowed to diffuse (60 min) at room temperature. Each sample was then centrifuged (13,000 × g, 2 min), and the supernatants were transferred to a fresh microcentrifuge tube and centrifuged again at 23,500 × g for 1 h at 4°C. The phage pellet was washed and observed with an electron microscope as described elsewhere (13). The specimens were observed at 80 kV using a JEOL 1230 transmission electron microscope located at the Pavillon Marchand of the Université Laval.
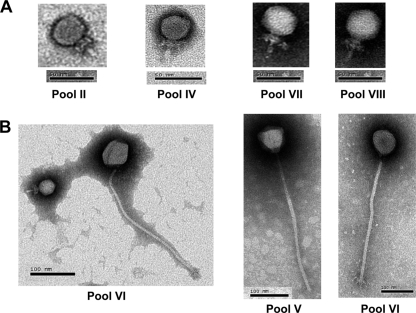
Overall, 11 individual samples out of 202 (5.4%) nasal swabs were positive for the presence of virulent bacteriophages against S. epidermidis ATCC 35983. The presence of phages was confirmed by the observation of clear phage plaques on TSA plates, as well as virion particles, with the electron microscope (Fig. 1). Figure 1 shows representative transmission electron micrographs of the seven bacteriophages: four Podoviridae phages (Fig. 1A) and three Siphoviridae phages (Fig. 1B). In two cases, pooled samples showed the presence of phages (pools II and IV, Table 1), while no phages were detected in the individual samples of those pools. This was probably due to the use of a larger aliquot of the pooled samples on the TSA plates for phage detection. Hurdles to the isolation of phages from individual samples from the anterior nares included low sample volumes (each was obtained from a single swab taken from the anterior nares of an individual patient) and low phage numbers. Since a lysis zone from pooled extracts was observed under an electron microscope, it was not surprising to see a mixture of different phages in some of the samples. Seven of the positive samples were from males, and four were from females. Coagulase-negative staphylococci were ubiquitous flora in these subjects (data not shown), while only 24% of the samples grew S. aureus (2). None of the samples yielded phages against S. aureus. The presence of virulent phages against S. epidermidis but not S. aureus was surprising but could be linked to the predominance of S. epidermidis host bacteria in the anterior nares of the subjects at the time of sampling.
Fig. 1.
Transmission electron micrographs of virulent bacteriophages against S. epidermidis ATCC 35983 isolated from the anterior nares of humans. Panels: A, Podoviridae; B, Siphoviridae. Roman numerals represent pooled samples (see Table 1 and text). Two pictures from pool VI are shown in panel B, one of a Siphoviridae phage alone and the other of a Siphoviridae and a Podoviridae phage.
All of the S. epidermidis phages found in the anterior nares possessed a tail and thus belong to the Caudovirales order (1). Phages belonging to the Podoviridae family (short tail, Fig. 1A) were more predominant, as they were found in all but one of the positive pools (II, III, IV, V, VI, VII, and VIII). Our PubMed searches failed to identify any reports of Podoviridae phages infecting S. epidermidis, and thus, this study may represent the first description of podophages for this staphylococcal species. The sizes of the phages observed are presented in Table 1.
Phages belonging to the Siphoviridae family (long noncontractile tail) (Fig. 1B) were also found but only in three positive pools (V, VI, and VII). Based on morphological criteria, pool VI contained the most diversified phages (Table 1). Of note, the S. epidermidis phages belonging to the Siphoviridae family observed here had a very long tail (>300 nm), twice the length of those of the five most recently characterized temperate S. epidermidis siphophages (10, 16), as well as that of the sole S. epidermidis phage available at the Félix d'Hérelle Reference Center for Bacterial Viruses (http://www.phage.ulaval.ca).
The lysis activity of diverse phages against S. epidermidis ATCC 35983, a known antibiotic-resistant, moderate slime-producing pathogen (7), suggests that they may be useful as antimicrobial agents (9) and that the human anterior nares may represent a reservoir of such novel natural antimicrobials. In conclusion, in this report, we describe the isolation of lytic phages from the anterior nares of humans against a very common human commensal and potential pathogen. We also document the lack of bacteriophages against S. aureus in our study. This study lays the foundation for future experiments to determine whether these phages play a modulating role in the competition between S. epidermidis and S. aureus in the microbial ecology of the anterior nares.
Acknowledgments
This study was supported by a grant (ASW10108) from the Marshfield Clinic to V.A. and funding from NSERC of Canada (Major Resources Support Program) and the Canadian Institutes of Health Research (Team Grant—Emerging: Novel Alternatives to Antibiotics) to S.M.
We thank Pravin Kaldhone and Jennifer Kislow for technical assistance, Po-Huang Chyou for statistical analysis, and MCRF research coordinators for collecting samples.
Footnotes
Published ahead of print on 16 September 2011.
REFERENCES
- 1. Ackermann H.-W. 2007. 5500 Phages examined in the electron microscope. Arch. Virol. 152:227–243 [DOI] [PubMed] [Google Scholar]
- 2. Aswani V. H., Shukla S. K. 2011. Prevalence of Staphylococcus aureus and lack of lytic bacteriophages in the anterior nares of patients and healthcare workers at a rural clinic. Clin. Med. Res. 9(2):75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bachrach G., Leizerovici-Zigmond M., Zlotkin A., Naor R., Steinberg D. 2003. Bacteriophage isolation from human saliva. Lett. Appl. Microbiol. 36:50–53 [DOI] [PubMed] [Google Scholar]
- 4. Bes M. 1994. Characterization of thirteen Staphylococcus epidermidis and S. saprophyticus bacteriophages. Res. Virol. 145:111–121 [DOI] [PubMed] [Google Scholar]
- 5. Breitbart M., et al. 2003. Metagenomic analyses of an uncultured viral community from human feces. J. Bacteriol. 185:6220–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chibani-Chennoufi S., et al. 2004. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: implications for phage therapy. Antimicrob. Agents Chemother. 48:2558–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christensen G. D., et al. 1982. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann. Intern. Med. 96:1–10 [DOI] [PubMed] [Google Scholar]
- 8. Costello E. K., et al. 2009. Bacterial community variation in human body habitats across space and time. Science 326:1694–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curtin J. J., Donlan R. M. 2006. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 50:1268–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daniel A., Bonnen P. E., Fischetti V. A. 2007. First complete genome sequence of two Staphylococcus epidermidis bacteriophages. J. Bacteriol. 189:2086–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dean B. A., Williams R. E., Hall F., Corse J. 1973. Phage typing of coagulase-negative staphylococci and micrococci. J. Hyg. (Lond.) 71:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Herelle F. 1917. Sur un microbe invisible antagoniste des bacilles dysénteriques. C. R. Acad. Sci. Paris 165:373–375 [Google Scholar]
- 13. Fortier L. C., Moineau S. 2007. Morphological and genetic diversity of temperate phages in Clostridium difficile. Appl. Environ. Microbiol. 73:7358–7366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frank D. N., et al. 2010. The human nasal microbiota and Staphylococcus aureus carriage. PLoS One 5:e10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gorwitz R. J., et al. 2008. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J. Infect. Dis. 197:1226–1234 [DOI] [PubMed] [Google Scholar]
- 16. Gutiérrez D., Martínez B., Rodríguez A., García P. 2010. Isolation and characterization of bacteriophages infecting Staphylococcus epidermidis. Curr. Microbiol. 61:601–608 [DOI] [PubMed] [Google Scholar]
- 17. Kiliç A. O., Pavlova S. I., Alpay S., Kiliç S. S., Tao L. 2001. Comparative study of vaginal Lactobacillus phages isolated from women in the United States and Turkey: prevalence, morphology, host range, and DNA homology. Clin. Diagn. Lab. Immunol. 8:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lina B., et al. 1993. Role of bacteriophages in genomic variability of related coagulase-negative staphylococci. FEMS Microbiol. Lett. 109:273–277 [DOI] [PubMed] [Google Scholar]
- 19. Lina G., et al. 2003. Bacterial competition for human nasal cavity colonization: role of staphylococcal agr alleles. Appl. Environ. Microbiol. 69:18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peacock S. J., de Silva I., Lowy F. D. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9:605–610 [DOI] [PubMed] [Google Scholar]
- 21. Rose D. L., McDonald J. S. 1973. Isolation and host range studies of Staphylococcus epidermidis and Micrococcus ssp. bacteriophage. Am. J. Vet. Res. 34:125–128 [PubMed] [Google Scholar]
- 22. Sivaraman K., Venkataraman N., Cole A. M. 2009. Staphylococcus aureus nasal carriage and its contributing factors. Future Microbiol. 4:999–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tylenda C. A., Calvert C., Kolenbrander P. E., Tylenda A. 1985. Isolation of Actinomyces bacteriophage from human dental plaque. Infect. Immun. 49:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verhoef J., Hoff A. J., Holtrigter B., Van der Drift A. C. 1971. Deoxyribonucleic acid base composition of Staphylococcus epidermidis and its phages. J. Gen. Microbiol. 69:279–283 [DOI] [PubMed] [Google Scholar]
- 25. Wos-Oxley M. L., et al. 2010. A poke into the diversity and associations within human anterior nare microbial communities. ISME J. 4:839–845 [DOI] [PubMed] [Google Scholar]